Abstract
The use of herbs in pharmaceutical preparation is ever increasing, and the demand for pesticides free material by the concern industries is on the rise. Consequently the need to grow disease-free plants using non-chemical fertilizers and pesticides is the need of the hour. Mentha arvensis cv. kosi is highly infested with Meloidogyne incognita (Kofoid and White) Chitwood, and severe oil yield loss occurs due to this nematode pest. Employing ecofriendly ways of nematode management, the mutualistic endophytes (Trichoderma harzianum strain Thu, Glomus intraradices) and plant growth promoting rhizobacteria (Bacillus megaterium and Pseudomonas fluorescens) were assessed individually and in combination on plant biomass, oil yield of menthol mint (M. arvensis cv. kosi), reproduction potential and population development of root knot nematode, M. incognita under glasshouse conditions. These microbes enhanced the plant biomass and percent oil yield both with and without M. incognita inoculation. Dual application of mutualistic fungal endophytes and Plant Growth Promoting Rhizobacteria (PGPRs) may be a wise option for enhancing the oil yield and tolerance of menthol mint against M. incognita infection.
Introduction
Menthol mint (Mentha arvensis L.), an important essential oil bearing herb, is a major source of natural menthol, menthyl acetate, menthone, and terpenes. These constituents of menthol mint oil are continuously used in pharmaceutical, perfumery, cosmetic, and food industries all over the world. Root knot nematode is one of the most important limiting factors for successful cultivation of menthol mint (Pandey Citation2005a). Although a large number of plant parasitic nematodes have been associated with menthol mint, the root knot nematode (Meloidogyne spp.) is a major pest highly prevalent in tropics and subtropics and causes more than US$100 billion loss/year worldwide (Luc et al. Citation2005). Menthol mint, a commercial crop in India, suffers severely with the infestation of root knot nematode, Meloidogyne incognita (Kofoid and White) Chitwood. Due to endoparasitic nature of this nematode the distribution is facilitated by infected suckers/runners/roots, which are used for propagation of this crop. This nematode species has become a major bottleneck for the successful cultivation of medicinal and aromatic plants in India (Pandey Citation2005b; Pandey and Kalra Citation2010). Chemical nematicides were the major weapons to fight against this nematode all around the world but due to their adverse effects on human health, environment, and on non-target organisms, its use has not only been restricted but also effective chemical nematicides have already been withdrawn from the world market. Because of this concern, there has been a worldwide swing toward the use of ecofriendly tactics which are bio-efficacious, economical, biodegradable, and environmentally safe and could be ideal for use as reliable agents to manage nematode diseases. The use of mutualistic fungi and plant growth promoting Rhizobacteria (PGPRs) to manage nematode disease offers an attractive alternative to synthetic nematicides. The growth or activity of pest and pathogen can be inhibited by several beneficial mutualistic endophytes and rhizobacteria (Bruehl Citation1987; Becker et al. Citation1988; Lynch Citation1990; Glick Citation1995; Pandey et al. Citation1997, Citation1999; Weller et al. Citation2002; Howell Citation2003; Lucy et al. Citation2004; Somers et al. Citation2004; Kloepper and Ryu Citation2006; Khan et al. Citation2008; Raaijmakers et al. Citation2009; Pandey Citation2010). Mutualistic endophytes and PGPRs are major occupants of rhizosphere, and their extensive microbial activity may provide major defense against nematode pest. Therefore, in the current experimentation the mutualistic fungi (Trichoderma harzianum, Glomus intraradices) and plant growth promoting bacteria (Bacillus megaterium, Pseudomonas fluorescens) and their different combinations were used to examine the impact on menthol mint biomass, oil yield, and population development of M. incognita.
Materials and methods
Nematode inoculums
Root knot nematode, M. incognita race-1 was obtained from pure cultures maintained on brinjal plant roots (Solanum melongena pusa purple long) using a single egg mass in glasshouse. Whenever inoculums were required, plants were uprooted and the entire root system was dipped in water to remove adhering soil. Egg masses were handpicked using forceps and placed in 9 cm diameter sieves of 1 mm pore size, which had been lined with cross-layered tissue paper. The sieves were placed in petri dishes with distilled water for hatching and incubated at 28°C. Five thousand freshly hatched second-stage juveniles were applied to each plant. Entire experiment was conducted in earthen pots (30 cm diameter) filled with autoclaved soil, sand, and compost mixture (7:2:1).
Mass culture of T. harzianum strain Thu
Pure culture of T. harzianum strain Thu (ATCC No. PTA 3701) was obtained from MTED, CIMAP, Lucknow, and maintained on potato dextrose agar (PDA) slants by sub-culturing periodically on fresh media at an interval of three months (Elad et al. Citation1981). For mass culture of T. harzianum, the mint distilled waste was air dried for 3–4 consecutive days, then ground to a fine powder. Powder of this distilled mint waste weighing 100 g each was filled into polypropylene bags to which 10 ml of sterilized distilled water was added. The bags were plugged with non-absorbent cotton. The medium was then autoclaved for 30 minutes at 121°C. Mycelial disc (3 mm diameter) from PDA slants was added to each polypropylene bag containing 100 g mint distilled waste with the help of inoculating needle under aseptic condition. The polypropylene bags were then incubated at 28±1°C for 7 days in an incubator for its multiplication.
Maintenance of bacterial culture and its multiplication
Pure cultures of P. fluorescens (ATCC No. 13525) and B. megaterium (ATCC No. 14581) were grown on King's B agar and nutrient agar media, respectively, and incubated at 30±1°C for 48 hours. The slants were then covered with sterilized mineral oil and preserved in refrigerator at 4°C for further use. For multiplication of P. fluorescens and B. megaterium, the actively growing cultures of P. fluorescens and B. megaterium were grown in nutrient broth for 48 hours in an incubated shaker at 30±1°C. The cultures were then centrifuged at 5500 RPM for 7 minutes, supernatant discarded, and the pellets containing bacterial cells were suspended in 100 ml of 0.01 M MgSO4 solution in sterile distilled water, and concentration of suspensions was adjusted to 108 CFU/ml. A quantity of 10 ml of bacterial suspension was used as inoculum per plant.
Maintenance, isolation, and observation of % infection of Glomus intraradices Schenck & Smith
The inoculum of G. intraradices (Gi) was originally obtained from MTED, CIMAP, Lucknow, and maintained in a glasshouse with palmarosa (Cymbopogon martinii, Roxb.JF Watson). A 30 g soil/pot containing 10 chlamydospore/g soil was placed along with suckers at the time of transplanting. The chlamydospores were extracted from the soil by wet sieving and decanting techniques (Gerdmann and Nicolson Citation1963). Rhizosphere soil samples with root from mycorrhizal treatments were collected. Roots were separated, washed and cut into 1–1.5 cm pieces, cleaned with KOH at 90°C for 1 hour in an oven and stained with trypan blue (Phillips and Haymann Citation1970; Giovannetti and Moss Citation1980). G. intraradices infection of each plant was determined by estimating the percent root colonization as described by Biermann and Lindermann (Citation1981). A minimum of 50 root segments were determined each time.
Isolation of nematode population from soil and roots
A 200 g sub-sample of well-mixed soil from each treatment was processed by Cobb's sieving and decanting method followed by Baermann's funnel extraction to extract the nematode population (Southey Citation1986). For estimating the number of juveniles, eggs, and females inside the roots, a 1 g sub-sample of roots was macerated in a Waring blender, and nematodes in the resulting suspension were counted. Nematodes counting in roots were carried out by multiplying the number of nematodes in 1 g of root by the total root fresh biomass.
Plants were inoculated with mutualistic endophytes viz. Glomus intraradices Schenk & Smith 40 g soil inoculum (containing 10 chlamydospores/g soil), T. harzianum Rifai isolate Thu 2 g waste materials (containing 2.0×108 CFU/g waste materials), and PGPRs (P. fluorescens, B. megaterium) 10 ml of each/plant (containing 108 CFU/ml at the time of transplanting menthol mint suckers. Five centimeter menthol mint suckers bearing single bud were sterilized with 0.01% mercuric chloride solution for one minute and rinsed three times with sterile distilled water and planted singly in each earthen pot. Carbofuran was incorporated at 0.0015 g a i/kg soil. In addition, an untreated-inoculated pot was kept as nematode control. There were five replicates for each treatment.
After two weeks of transplantation each pot was inoculated with 5000 freshly hatched second-stage larvae of M. incognita by pipetting nematode larvae into 1 cm holes around the base of plant and filling the holes with moist soil. After inoculation, pots were arranged in glasshouse in complete randomized complete block design. The plants were watered frequently for maintenance of proper moisture as the plant grows well in sufficient moisture levels. Daily observations were made for recording the development of symptoms. After 90 days of inoculation, the experiment was terminated, and data of different growth parameters viz. fresh and dry root/shoot weight, percent oil yield were determined by hydro distillation of shade dried herbs using Clevenger apparatus (Clevenger Citation1928), and nematode population in soil and root was isolated (Southey Citation1986). The root gall indices were calculated according to Krusberg and Neilson (Citation1958) on 0–4 scales, where 0 = no infection or no root galling, 1 = slight infection (1–25%), 2 = moderate infection (26–50%), 3 = severe infection (51–75%), and 4 = very severe infection (76–100%).
Experimental design
The experiment was set up in a complete randomized block design with 17 treatments. These treatments were tested in the presence and absence of M. incognita. Each treatment was replicated five times. The experiment was performed in a glasshouse and repeated twice. Data from both the experiments were almost identical, and therefore pooled.
Analysis of data
Data were analyzed by one way ANOVA and significant differences among the treatments were tested by critical difference (CD) test at the 5% probability level (Cochran and Cox Citation1957).
Results and discussion
Root knot disease caused by M. incognita on menthol mint was suppressed with application of mutualistic fungi and bacteria as soil applications. Root knot indices were much lower, thereby indicating the effectiveness of the treatments in inhibiting the development of M. incognita. Root knot nematode was substantially managed by individual and combined application of T. harzianum, B. megaterium, and G. intraradices. The combination of T. harzianum with G. intraradices, B. megaterium, and P. fluorescens caused 63.7%, 45.4%, and 54.6% reduction in root knot indices and 67.8%, 56.3%, and 60.4% reduction in total M. incognita population whereas the combined application of G. intraradices + B. megaterium and G. intraradices+P. fluorescens reduced only 52.7 and 54.0% of the total nematode population, respectively (). The combination of both the PGPRs also reduced the nematode population at significant level (48.5%). The individual application of T. harzianum, G. intraradices, B. megaterium, and P. fluorescens reduced root knot indices by 45.4%, 27.3%, 18.0%, and 27.3%, respectively, and total root knot nematode population reduction by 48.9%, 38.7, 20.1%, and 26.7% whereas the reduction of nematode population in carbofuran treated pots was 31.6% and root knot indices was 36.3%, respectively (). The total nematode population in roots of Carbofuran B. megaterium and P. fluorescens treated soils become high as compared to control-Mi inoculated treatment, may be due to enhancement in the total root biomass, whereas their population in soil is significantly reduced ().
Figure 1. Effect of different mutualistic fungi and PGPRs on percent reduction of root-knot indices in menthol mint.
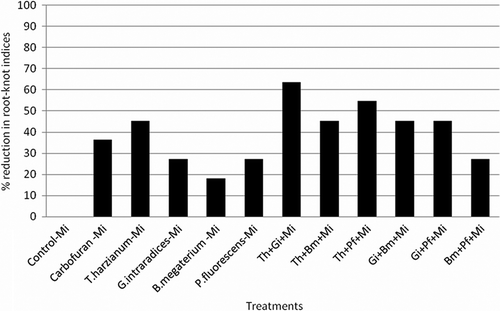
Figure 2. Effect of different mutualistic fungi and PGPRs on percent reduction in total nematode population development in menthol mint.
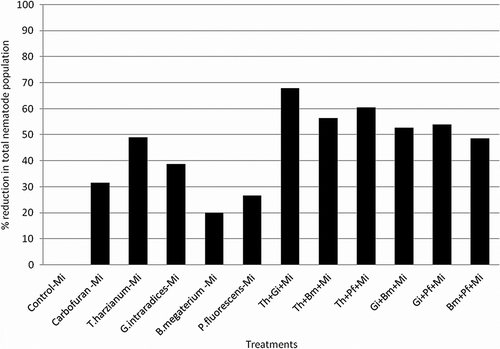
Table 1. Effect of mutualistic endophytes and PGPRs on growth/yield of menthol mint, root-knot, and mycorrhizal population development.
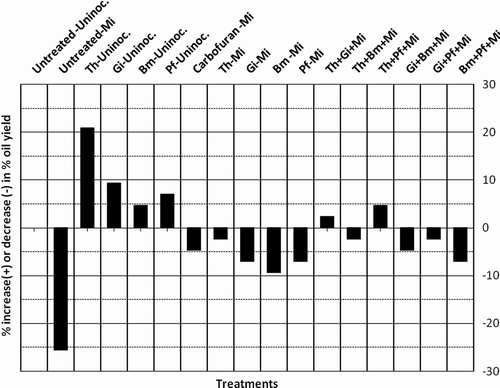
The maximum enhancement of plant dry weight was recorded in T. harzianum alone (23.8%) followed by T. harzianum+G. intraradices (20.0%), T. harzianum+P. fluorescens (14.4%), G. intraradices alone (10.0%) P. fluorescens alone (8.2%), G. intraradices+P. fluorescens (5.0%), G. intraradices+B. megaterium (1.2%), B. megaterium+P. fluorescens (1.5%), respectively, as compared to untreated-uninoculated control (, ). Tolerance of menthol mint against root knot nematode can be, therefore, enhanced via combined application of mutualistic fungi and PGPRs. These results show that mutualistic fungi and plant growth promoting bacteria were more effective when they were combined in different manner as compared to alone but could not bring about complete inhibition of nematode population development. As reported by various workers (Duffy et al. Citation1996; Larkin and Fravel Citation1998; Nemec et al. Citation2003; Kokalis-Burelle and Kloepper, Citation2004; Raj et al. Citation2005), the use of combinations of multiple antagonistic organisms may provide improved disease control over the use of single organism. Multiple organisms may enhance the level and consistency of control by providing multiple mechanisms of action, more stable rhizosphere community, and be effective over a wider range of environmental conditions (Li et al. Citation2006; Pandey Citation2010). The primary aim of the study was to identify effective mutualistic fungi and PGPRs which are able to reduce the severity of root knot disease. However, their performance in the fields would greatly depend on the natural microflora of the particular environment and their capability to survive and proliferate under such condition. This study perhaps is the first step toward developing microbial consortia for effective root knot disease management and higher plant productivity. However, the microbes must be tested under field conditions before finally arriving at a logical conclusion. The trend in% increase (+) or decrease (–) in plant dry weight and oil yield was found to be similar with little differences ( and ). In particular, combinations of fungi and bacteria may provide protection at different times or under various conditions, and occupy different or complementary niches. Such combinations may overcome inconsistencies in the performance of individual isolates. There are reports where production of metabolites by rhizospheric bacteria causes lysis of nematode eggs (Westcott and Kluepfel Citation1993), reduces egg hatching (Oostendorp and Sikora Citation1989), affects vitality of second-stage juveniles (Becker et al. Citation1988) and degrades specific root exudates resulting in reduced attraction and penetration of nematodes (Oostendorp and Sikora Citation1990; Pandey and Kalra Citation2010). Siddiqui and Shaukat (Citation2002) also reported that root colonization by rhizospheric bacteria reduced nematode invasion. The present study revealed that the tested strains of mutualistic fungi in combination with PGPRs had no adverse effect on plants but promoted significantly (p = 0.05)% oil yield of menthol mint (). Thus, the protective and nutritional properties of these microbes make them an ecofriendly useful tool for reducing deleterious impact of root knot disease caused by root knot nematode M. incognita in menthol mint.
Figure 3. Effect of different bio-inoculants on percent increase (+) or decrease (–) of total dry weight of menthol mint.
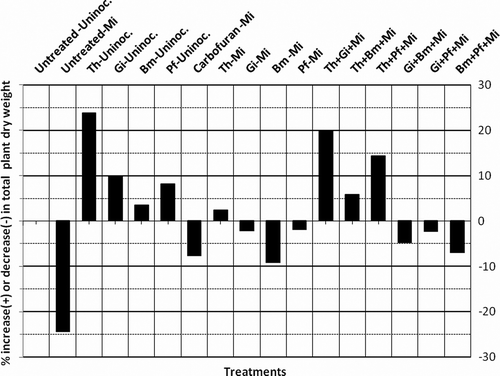
Figure 4. Effect of different bio-inoculants on percent increase (+) or decrease (–) in oil yield of menthol mint.
In conclusion, present results indicate that pre-inoculation with mutualistic fungi T. harzianum along with G. intraradices and P. fluorescens was highly beneficial for enhancing plant growth/yield by suppressing the development and reproduction of M. incognita. It is common practice in menthol mint growing areas of the world to transplant suckers and runners, and it is therefore feasible to incorporate different microbes during transplantation for obtaining higher oil yield/biomass of this commercial crop.
Acknowledgements
Authors are highly grateful to Prof. Ram Rajasekharan, Director, CIMAP, Lucknow, for his kind support and encouragement and to fellow scientists for their critical comments and suggestions.
References
- Becker , JO , Zavaleta-Mejia , E , Colbert , SF , Schroth , MN and Weinhold , AR. 1988 . Effect of rhizobacteria on root knot nematodes and gall formation . Phytopathology. , 78 : 1466 – 1469 .
- Biermann , B and Lindermann , RG. 1981 . Quantifying vesicular-arbuscular mycorrhizae, aproposed method towards standardization . New Phytol. , 87 : 63 – 67 .
- Bruehl , GW. 1987 . Soilborne plant pathogens , New York, NY : Macmillan .
- Clevenger JE. 1928 . Apparatus for the determination of volatile oil . J Am Pharma Assoc . 17 : 346 .
- Cochran WG , Cox GM. 1957 . Experimental designs. Vol. 2 . New York, NY : John Wiley and Sons .
- Duffy , BK , Simon , A and Weller , DM. 1996 . Combination of Trichoderma koningii with fluorescent pseudomonads for control of take-all on wheat . Phytopathology. , 86 : 188 – 194 .
- Elad , Y , Chet , I and Henis , Y. 1981 . A selective medium for improving quantitative isolation of Trichoderma spp. from soil . Phytoparasitica. , 9 : 59 – 67 .
- Gerdmann , JW and Nicolson , TH. 1963 . Spore of mycorrhizal Endogone species extracted from soil by wet sieving and decanting . Trans Brit Mycol Soc. , 46 : 235 – 244 .
- Giovannetti , M and Moss , B. 1980 . An evaluation of techniques for measuring VAM infection in roots . New Phytol. , 84 : 489 – 500 .
- Glick , BR. 1995 . The enhancement of plant growth by free living bacteria . Can J Microbiol. , 41 : 109 – 117 .
- Howell , CR. 2003 . Mechanisms employed by Trichoderma species in the biological control of plant diseases: the history and evolution of current concepts . Plant Dis. , 87 : 4 – 10 .
- Khan , Z , Kim , SG , Jeon , YH , Khan , HU , Son , SH and Kim , YH. 2008 . A plant growth promoting rhizobacterium, Paenibacillus polymyxa strain GBR-1, suppresses Root knot nematode . Bioresource Technol. , 99 : 3016 – 3023 .
- Kloepper JW , Ryu CM. 2006 . Bacterial endophytes as elicitors of induced systemic resistance . In: Schulz B , Boyle C , Siebern T Microbial root endophytes . Heidelberg : Springer-Verlag . p. 33 – 51 .
- Kokalis-Burelle N , Kloepper JW. 2004 . Soil ecosystem health and its role in plant disease suppression . In: Lartey RT , Caesar AM Emerging concepts in plant health management . Trivandrum : Research Signpost . p. 123 – 140 .
- Krusberg , LR and Neilson , LW. 1958 . Pathogenesis of root knot nematode to the Porto Rico variety of sweet potato . Phytopathology. , 48 : 36 – 39 .
- Larkin , RP and Fravel , DR. 1998 . Efficacy of various fungal and bacterial biocontrol organisms for control of Fusarium wilt of tomato . Plant Dis. , 82 : 1022 – 1028 .
- Li , HY , Yang , GD , Shu , HR , Yang , YT , Ye , BX , Nishida , I and Zheng , CC. 2006 . Colonization by the arbuscular mycorrhizal fungus Glomus versiforme induces a defense response against the root knot nematode Meloidogyne incognita in the grapevine (Vitis amurensis Rupr.), which includes transcriptional activation of the class III chitinase gene VCH3 . Plant Cell Physiol. , 47 : 154 – 163 .
- Luc , M , Sikora , RA and Bridge , J. 2005 . Plant parasitic nematodes in subtropical and tropical agriculture , 2nd ed , Egham, Surrey : CABI, Bioscience .
- Lucy , M , Reed , E and Glick , BR. 2004 . Applications of free living plant growth promoting rhizobacteria . Antonie van Leeuwenhoek. , 86 : 1 – 25 .
- Lynch , J. 1990 . The rhizosphere , London : Wiley .
- Nemec , S , Datnoff , LE and Strandberg , J. 2003 . Efficacy of biocontrol agents in planting mixes to colonize plant roots and control root diseases of vegetables and citrus . Crop Prot. , 15 ( 8 ) : 735 – 742 .
- Oostendorp , M and Sikora , RA. 1989 . Seed treatment with antagonistic rhizobacteria for suppression of Heterodera schachtii early root infection of sugar beet. Rev . Nematol. , 12 : 77 – 83 .
- Oostendorp , M and Sikora , RA. 1990 . In vitro interrelationship between rhizosphere bacteria and Heterodera schachtii . Rev Nematol. , 13 : 269 – 274 .
- Pandey , R. 2005a . Field application of bio-organics in the management of Meloidogyne incognita in Mentha arvensis . Nematologia Medit. , 33 ( 1 ) : 51 – 54 .
- Pandey , R. 2005b . Management of Meloidogyne incognita in Artemisia pallens with Bio-organics . Phytoparasitica. , 33 ( 3 ) : 304 – 308 .
- Pandey R. 2010 . Microbial versatility: towards plant growth and phytonematode management . In: Trivedi PC Plant diseases and its management . Jaipur : Pointer . p. 293 – 317 .
- Pandey , R and Kalra , A. 2010 . Inhibitory effects of vermicompost produced from agro-waste of medicinal and aromatic plants on egg hatching in Meloidogyne incognita (Kofoid and White) Chitwood . Current Science. , 98 ( 6 ) : 833 – 835 .
- Pandey , R , Gupta , ML , Singh , HB and Kumar , S. 1999 . Interaction potentialities of vesicular-arbuscular mycorrhizal fungi with root knot nematode, Meloidogyne incognita on black henbane . Bioresource Technol. , 69 : 275 – 278 .
- Pandey , R , Singh , HB and Gupta , ML. 1997 . Antagonistic impact of Vesicular-arbuscular (VAM) on Meloidogyne incognita population development in Japanese mint . Int J Trop Plant Dis. , 15 : 237 – 245 .
- Phillips , JM and Haymann , DS. 1970 . Improved procedure for cleaning roots and staining parasitic vesicular-arbuscular mycorrhizal fungi for rapid assessment infection . T Brit Mycol Soc. , 55 : 158 – 163 .
- Raaijmakers , JM , Timothy , C , Paulitz Christian , S , Claude , A and Yvan , ML. 2009 . The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms . Plant and Soil. , 321 : 341 – 361 .
- Raj , SN , Shetty , NP and Shetty , HS. 2005 . Synergistic effects of Trichoshield on enhancement of growth and resistance to downy mildew in pearl millet . Biocontrol. , 50 : 493 – 509 .
- Siddiqui , IA and Shaukat , SS. 2002 . Rhizobacteria-mediated induction of systemic resistance (ISR) in tomato against Meloidogyne javanica . J Phytopathol. , 150 : 469 – 473 .
- Somers , E , Vanderleyden , J and Srinivasan , M. 2004 . Rhizosphere bacterial signalling: a love parade beneath our feet . Crit Rev Microbiol. , 30 : 205 – 240 .
- Southey , JF . 1986 . Laboratory methods for work with plant and soil nematodes , London : Ministry of Agriculture, Fishery and Food, HMSO .
- Weller , DM , Raaijmakers , JM , McSpadden , BB and Thomashow , LS. 2002 . Microbial populations responsible for specific soil suppressiveness to plant pathogens . Annual Review of Phytopathology. , 40 : 309 – 348 .
- Westcott , SW and Kluepfel , DA. 1993 . Inhibition of Criconemella xenoplax egg hatch by Pseudomonas aureofaciens . Phytopathology. , 83 : 1245 – 1249 .