Abstract
Host plant resistance is one of the most economic and environment friendly method for pest management and, therefore, the present study was undertaken on induction of resistance against Helicoverpa armigera in three groundnut genotypes (ICGV 86699-resistant, NCAc 343-resistant, and TMV 2-susceptible). Observations were recorded on oxidative enzymes [peroxidase (POD) and polyphenol oxidase (PPO)], and on the amounts of other defensive components such as total phenols, hydrogen peroxide (H2O2), malondialdehyde (MDA), and proteins after 24, 48, 72, and 96 h following H. armigera infestation to understand the induced defense to H. armigera. Data were also recorded on leaf damage, larval survival, and larval weights. Increase in activities of POD and PPO and in the amounts of total phenols, H2O2, MDA, and proteins were observed in insect damaged plants as compared to uninfested control plants. In general, the induction was greater in the insect resistant genotypes than in the susceptible one. Leaf damage, larval survival, and larval weight were lower in resistant genotypes as compared to susceptible genotype. Therefore, induced resistance could be exploited in plant defense against insect pests for integrated pest management.
Abbreviations:
- analysis of variance, ANOVA
- bovine serum albumin, BSA
- ethylenediaminetetraacetic acid, EDTA
- fresh weight, FW
- gallic acid equivalents, GAE
- hydrogen peroxide, H2O2
- potassium iodide, KI
- lipoxygenase, LOX
- malondialdehyde, MDA
- sodium carbonate, Na2CO3
- peroxidase, POD
- polyphenol oxidase, PPO
- polyvinyl pyrolidone, PVP
- reactive oxygen species, ROS
- thiobarbituric acid, TBA
- thiobarbituric acid reactive substance, TBARS
- trichloroacetic acid, TCA
- Tris-hydrochloride, Tris-HCl
Introduction
Plants are always under heavy biotic stress due to the attack by insect pests, which are the main crop damaging agents worldwide. Plants have developed a wide range of physio-chemical mechanisms to defend themselves against herbivore attack (Rasmann and Agrawal Citation2009; Sharma et al. Citation2009; Usha Rani and Jyothsna Citation2010). This resistance can be constitutive, i.e. is present in the plant independent of any stress (Franceschi et al. Citation2005) and forms the first barrier to herbivore insects or it can be inducible, i.e. is activated only when the plant is attacked (Kessler and Baldwin Citation2002) thereby protects the plant from further damage. Induced resistance can be direct or indirect. Direct defense is aimed at the attacker and is mediated by the accumulation of substantial amounts of defensive compounds in plants to protect against herbivores or plants that produce noxious chemicals to reduce herbivore feeding, oviposition, growth and development, and so on (Hanley et al. Citation2007; Senthil-Nathan et al. Citation2009). Indirect defense is aimed at the attraction of natural enemies of the attacker, e.g. by the stimulating plant volatile emission (Bruinsma and Dicke Citation2008; Broze et al. Citation2010; Kappers et al. Citation2011). Direct and indirect defense mechanisms can function additively against the herbivores. Chemical compounds playing an effective role in plant defense are produced and stored in tissues of the plants that are consumed by the herbivores (Hanley et al. Citation2007). Herbivore stressed plants produce active defense responses at the site of tissue damage and also systemically in undamaged tissues (Bostock Citation2005). A slower herbivore growth also prolongs the time of the herbivore to be exposed to a predator or parasitoid (Bhonwong et al. Citation2009). This highly dynamic form of induced resistance has been documented in species throughout the plant kingdom (Wu and Baldwin Citation2010). Research on induced resistance has gained momentum worldwide due to its wide ranging nature as a cascade of biochemical changes occur in plants in response to different types of damage, e.g. in wheat against Sitobion avenae (Han et al. Citation2009; Zhao et al. Citation2009), rice against many insect pests (Usha Rani and Jyothsna Citation2010), cucumber against Bemisia tabaci (Zhang et al. Citation2008), and so on. Induced resistance is of higher energy utilization efficiency and more economic (Zhao et al. Citation2009). Although induced responses have some metabolic costs (Agrawal Citation2000), they play a potent role in plant defense when aimed at the stress of immediate concern (Miranda et al. Citation2007; Karban Citation2011). Moreover, induced resistance depends on wound-detection pathways, defense precursors, and storage vesicles that require energy and resources allocation away from growth and reproduction (Purrington Citation2000).
Herbivorous insects attack often leads to accumulation of defensive compounds through physiological, morphological, and chemical changes (Agrawal et al. Citation2009; Broze et al. Citation2010; Usha Rani and Jyothsna Citation2010). One of the important defensive responses of plants against insect attack is the accumulation of oxidative enzymes such as peroxidase (POD), polyphenol oxidase (PPO), lipoxygenase (LOX), catalase (CAT), and reactive oxygen species (ROS) (Zhang et al. Citation2008; Usha Rani and Jyothsna Citation2010). The potential role of these enzymes in the synthesis of defense compounds and/or in oxidative stress tolerance makes them an important weapon of plant resistance against insect herbivores. Peroxidase catalyzes lignin synthesis and thereby protects the plant tissues when threatened by herbivores and microorganisms (Han et al. Citation2009). Polyphenol oxidase plays an important role in plant defenses against insect herbivores as an antinutritional and antioxidative enzyme (Gatehouse Citation2002) and its role in wound healing, stress tolerance, and pathogen resistance has been thoroughly studied (Ramiro et al. Citation2006; Thipyapong et al. 2006). Plant phenolics, such as phenolic acids, flavonoids, isoflavonoids, tannins, lignins, etc., have been suggested to take part in plant defense as phytoanticipins, phytoalexins, and as structural barriers, modulators of pathogenicity, and activators of plant defense genes (Usha Rani and Jyothsna 2010). The H2O2 plays a central role in generation of defense response in plants (Boka et al. Citation2007) through signal transduction pathways that lead to the expression of defense-related genes (Orozco-Cardenas et al. Citation2001). Malondialdehyde (MDA) is a decomposition product of polyunsaturated fatty acid hydroperoxides and functions as a signal for the activation of defense system in plants (Zhang et al. Citation2008).
Groundnut (Arachis hypogaea L.) is one of the world's principal oilseed crops. Asia accounts for over 70% of the worlds’ groundnut production (Freeman et al. Citation1999). India is the largest groundnut producer in the world with an area of 6.40 million hectares, and the total production is estimated to be 7.21 million tons (Sharma et al. Citation2003). This crop is attacked by more than 350 species of insects in different parts of the world, among which, thrips, aphids, white grubs, leafhoppers, armyworm (Spodoptera litura), and cotton bollworm (Helicoverpa armigera) are the important pests in India (Sharma et al. Citation2003).
Helicoverpa armigera is one of the most important constraints to crop production in Asia, Africa, Australia, and Mediterranean Europe. It is a polyphagous pest and has been reported to attack more than 200 plant species, including cotton, groundnut, sorghum, maize, chickpea, pigeonpea, etc., and has developed resistance to most of the chemical insecticides (Sharma et al. Citation2005).
Screening of germplasm for resistance to insect pests has received considerable attention, however, there is limited progress in characterization of physiological and biochemical mechanisms conferring resistance to insects (Heng-Moss et al. Citation2004; Sharma et al. Citation2009). Although the response of groundnut to drought stress has been well studied (Sankar et al. Citation2007), studies on induced resistance in response to insect attack are less understood. Hence this study was undertaken to compare the biochemical responses of resistant and susceptible genotypes of groundnut to the damage by H. armigera. The study focused on the oxidative enzymes such as POD and PPO, and other defensive components (total phenols, H2O2, MDA, and total proteins) in relation to survival and development of the insect.
Materials and methods
Chemicals
The chemicals used in this study were of analytical grade. Tris-HCl, polyvinyl pyrolidone (PVP), EDTA, disodium hydrogen phosphate, sodium dihydrogen phosphate, Guaiacol and Thiobarbituric acid (TBA) were obtained from HiMedia Lab. Pvt. Ltd. Mumbai and 2-mercaptoethanol was procured from Loba Chemie, Mumbai. Pyrocatechol was obtained from the Central Drug House, Mumbai. Coomassie brilliant blue-G250 was obtained from Sisco Research Lab., Mumbai. Bovine serum albumin (BSA), potassium iodide (KI), and sodium carbonate (Na2CO3) were obtained from S.d. Fine Chemicals Ltd. Mumbai. Gallic acid and Folin-Ciocalteau reagent were obtained from Merck, Mumbai. Trichloroacetic acid (TCA) was obtained from Qualigens Fine Chemicals, Mumbai.
Groundnut plants (Arachis hypogaea L.)
Seeds of three groundnut genotypes were obtained from the International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Patancheru, Andhra Pradesh, India. The test material included three groundnut genotypes: ICGV 86699-resistant, NCAC 343 resistant, and TMV- 2 susceptible (Sharma et al. Citation2003). Groundnut plants were sown in plastic pots (30 cm diameter, 30 cm deep). The pots were filled with a potting mixture of soil, sand, and vermicompost (2:1:1). The plants were watered as needed. Utmost care was taken to prevent the plants from insect attack other than the experimental insect by enclosing them in cages. Twenty day old groundnut plants were used for the study. The experiment was repeated thrice. During the experimental periods, the average temperature, relative humidity, and light intensity were 39±2°C, 64.8±10%, and 24.2±4 mjoules per m2, respectively.
Helicoverpa armigera infestation
Newly emerged first instars (neonates; 1 day old) larvae of H. armigera were obtained from the stock culture maintained on artificial diet under laboratory conditions (26±1°C; 11±0.5 h photoperiod and 75±5% relative humidity) from the insectary of the Entomology Research Institute, Loyola College, Chennai, Tamil Nadu, India. Larvae were pre-starved for 4 h and 10 larvae were gently placed on each 20-day-old plant by using a camel hair brush.
Enzyme assays
Leaves were collected from the infested and uninfested plants at different time intervals. Fresh leaves (0.5 g) were frozen in liquid nitrogen and ground in 3 ml of ice cold 0.1M Tris-HCl buffer (pH 7.5) containing 5 mM 2-mercaptoethanol, 1% polyvinylpyrolidone (PVP), and 0.5 mM EDTA. The homogenate was centrifuged at 16,000×g for 25 min, and the supernatant was used as enzyme source. All spectrophotometric analyses were carried out on HITACHI UV-2010 spectrophotometer.
Peroxidase assay
The POD activity was estimated as per the method of Shannon et al. (Citation1966) with slight modifications. The reaction was started by addition of 0.1 ml enzyme extract to 2.9 ml of reaction mixture containing 0.1 M sodium phosphate buffer (pH 6.5), 0.8 mM H2O2, and 5 mM Guaiacol. Absorbance was read at 470 nm for 2 min at 15 sec interval. Enzyme activity was expressed as nano katal mg–1protein (nkat mg–1protein).
Polyphenol oxidase assay
The PPO activity was estimated as per the method of Mayer and Harel (Citation1979) with some modifications. The reaction mixture contained 2.9 ml of 0.1M sodium phosphate buffer (pH 6.8), 0.1 ml of enzyme source and 0.1 ml of substrate (0.05 M pyrocatechol). Absorbance was read at 420 nm for 3 min at 30 sec interval. Enzyme activity was expressed as nano katal mg–1protein (nkat mg–1protein).
Phenolic content
Phenolic content of treated leaves was estimated as per Zieslin and Ben-Zaken's (Citation1993) method with some modifications. Fresh leaves (0.5 g) were homogenized with 3 ml of 80% methanol and agitated for 15 min at 70°C. Methanol extract (0.1 ml) was added to 2 ml of 2% sodium carbonate (Na2CO3). After incubation for 5 min, 0.1 ml of Folin-Ciocalteau reagent was added and the solution was incubated for 10 min at room temperature. The absorbance of the blue color was measured using a spectrophotometer at 760 nm. Phenolic concentration was determined from the standard curve prepared with Gallic acid and was expressed as µg Gallic acid equivalents g–1 FW (µg GAE g–1 FW).
Hydrogen peroxide content
Hydrogen peroxide content was estimated by the method of Noreen and Ashraf (Citation2009). Fresh leaf tissue (0.1 g) was homogenized with 2 ml of 0.1% (w/v) trichloroacetic acid (TCA) in a pre-chilled pestle and mortar and the homogenate was centrifuged at 12,000×g for 15 min. To 0.5 ml of supernatant, 0.5 ml of phosphate buffer (pH 7.0), and 1 ml of 1M potassium iodide (KI) were added. The absorbance was read at 390 nm. The H2O2 concentration was determined by using an extinction coefficient of 0.28 µM cm–1 and expressed as µmol g–1 FW.
Malondialdehyde content
The level of lipid peroxidation was determined in terms of Thiobarbituric acid-reactive substances (TBARS) concentration as described by Carmak and Horst (Citation1991) with minor modifications. Fresh leaf tissue (0.2 g) was homogenized in 3 ml 0.1% (w/v) trichloroacetic acid (TCA) at 4°C. The homogenate was centrifuged at 20,000×g for 15 min. The 0.5 ml of supernatant was added to 3 ml 0.5% (v/v) Thiobarbituric acid (TBA) in 20% TCA. The mixture was incubated at 95°C in a shaking water bath for 50 min, and the reaction was stopped by cooling the tubes in an ice water bath. Samples were then centrifuged at 10,000×g for 10 min, and absorbance of the supernatant was read at 532 nm. The value for nonspecific absorption at 600 nm was subtracted. The concentration of TBARS was calculated using the absorption coefficient of 155 mM–1 cm–1 and expressed as µmol g–1FW.
Protein content
Protein concentration was determined according to the method of Bradford (Citation1976) with minor modifications, using bovine serum albumin as a standard.
Damage rating, larval survival, and larval weight
After 96 h of infestation, plants were assessed for insect damage by rating them to a scale 1–8, with 1 showing no or slight damage (<10%) and 8 shows >90% damage. Larvae were recovered from the plants and counted. The larvae were then starved for 4 h, after which their weights were recorded using a digital balance (Mettler Toledo, AB304-S).
Statistical analysis
The replication data were pooled together and mean and standard error were calculated. All the data were analyzed by two-way analysis of variance (ANOVA) using SPSS (Version 15.1). When the treatment effects were statistically significant (P ≤ 0.05), Tukey's test was used to separate the means.
Results
POD activity
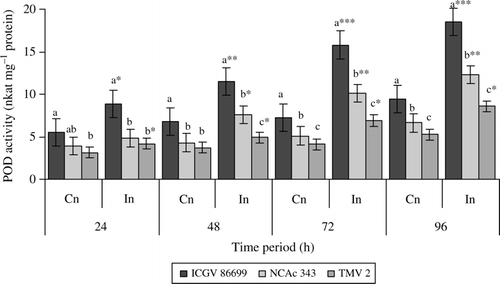
Helicoverpa armigera infestation induced POD activity in the tested groundnut genotypes (). Significant differences were observed in POD activity between control and infested plants of all the three genotypes at all the time intervals. Infested plants of ICGV 86699 exhibited nearly twofold increase in POD activity at all the time periods than that of uninfested control plants. NCAc 343 also showed higher induction at later stages. Across the genotypes significantly higher POD activity was observed both in control and infested plants of ICGV 86699 throughout the test period than that of NCAc 343 and TMV 2.
Figure 1. Peroxidase activity (nkat mg–1protein) of three groundnut genotypes after H. armigera infestation. Note: *Bars indicate the levels of statistical significance between control and infested plants within a germplasm at each time interval. *, **, ***=significance at P≤0.05, P≤0.01, and P = 0.001, respectively, by students t-test. Bars with the same letter (s) in a treatment within a time interval are not significantly different at P≤0.05. FW, fresh weight of leaf tissue; Cn, control plants; In, plants infested with H. armigera; n, 10 for each genotype.
PPO activity
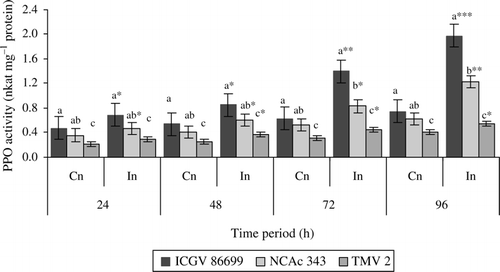
Infestation with H. armigera resulted in higher PPO activity (). Significant differences were observed between control and infested plants of all the three genotypes throughout the test period. The induced PPO activity was significantly greater in ICGV 86699 at 72 h (2.2-fold) and 96 h (2.6-fold) as compared to the uninfested control plants. NCAc 343 also showed significantly higher PPO activity in infested plants at 96 h (1.9-fold) than the uninfested control plants. At 96 h, least induction in PPO activity was observed in infested plants of TMV 2 (1.2-fold) than the respective control plants. Among the tested genotypes, no significant difference was observed in control plants of ICGV 86699 and NCAc 343 throughout the test period. However, at 72 and 96 h infested plants of ICGV 86699 showed greater PPO activity than both NCAc 343 and TMV 2.
Figure 2. Polyphenol oxidase activity (nkat mg–1protein) of three groundnut genotypes after H. armigera infestation. Note: *On bars indicates the levels of statistical significance between control and infested plants within a germplasm at each time interval. *, **, ***=Significance at P≤0.05, P≤0.01, and P≤0.001, respectively, by students t-test. Bars with the same letter (s) in a treatment within a time interval are not significantly different at P≤0.05. FW, fresh weight of leaf tissue; Cn, control plants; In, plants infested with H. armigera; n, 10 for each genotype.
Phenolic content
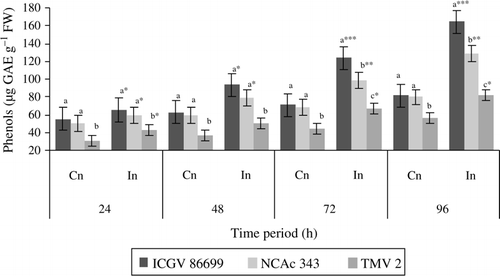
Significant differences were found between control and infested plants of all the three genotypes throughout the test period (). However, ICGV 86699 showed much increase in phenolic content in infested plants at 72 (1.7-fold) and 96 h (2.2-fold). At 72 h, both NCAc 343 and TMV2 showed 1.4-fold increases in phenolic content than their respective control plants. While at 96 h, NCAc 343 showed 1.6-fold and TMV 2 showed 1.4-fold increases in phenolic content than the respective control plants. Among the tested genotypes, ICGV 86699 and NCAc-343 exhibited higher phenolic content in control plants throughout the test period than that of TMV 2. However, infested plants of ICGV 86699 revealed higher phenolic content at 72 and 96 h than the infested plants of NCAc 343 and TMV 2.
Figure 3. Phenolic content (µg GAE g–1 FW) of groundnut genotypes after H. armigera infestation. Note: *Bars indicate the levels of statistical significance between control and infested plants within a germplasm at each time interval. *, **, ***=significance at P≤0.05, P≤0.01, and P≤0.001, respectively, by students t-test. Bars with the same letter (s) in a treatment within a time interval are not significantly different at P≤0.05. Values (Mean±SEM), GAE, Gallic acid equivalents; FW, fresh weight of leaf tissue; Cn, control plants; In, plants infested with H. armigera; n, 10 for each genotype.
Hydrogen peroxide content
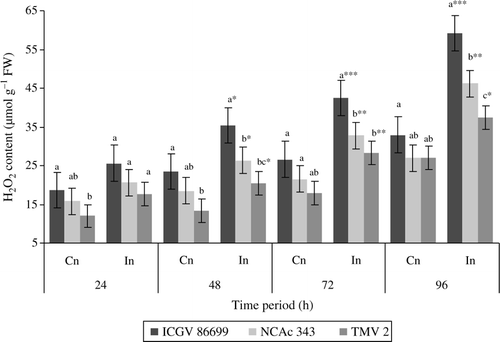
Plants infested with H. armigera had higher H2O2 content than the uninfested control plants (). All the three genotypes exhibited a significant increase in H2O2 throughout the test period. Higher induction of H2O2 content was observed at later stages. At 96 h after infestation, ICGV86699, NCAc 343, and TMV 2 showed an increase of 1.8-, 1.7-, and 1.3-fold than the respective uninfested control plants. Among the tested genotypes, control plants of ICGV 8699 and NCAc 343 showed higher H2O2 content at all the time intervals than that of TMV 2. While as infested plants of ICGV 86699 exhibited higher H2O2 at 48, 72, and 96 h after infestation as compared to the infested plants of NCAc 343 and TMV 2.
Figure 4. H2O2 content (µmol g–1 FW) of groundnut genotypes after H. armigera infestation. Note: *Bars indicate the levels of statistical significance between control and infested plants within a germplasm at each time interval. *, **, ***=significance at P≤0.05, P≤0.01, and P≤0.001, respectively, by students t-test. Bars with the same letter (s) in a treatment within a time interval are not significantly different at P≤0.05. Values (Mean±SEM), FW, fresh weight of leaf tissue; Cn, control plants; In, plants infested with H. armigera; n, 10 for each genotype.
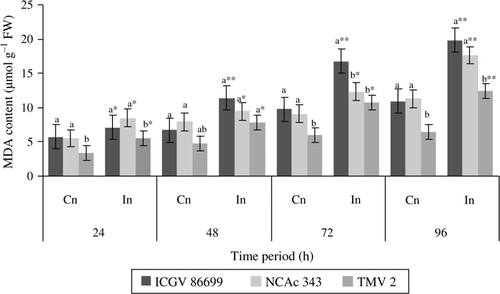
Malondialdehyde content
Herbivore infestation induced significantly higher MDA in all the three genotypes at all the time intervals (). ICGV 86699 and TMV 2 infested plants showed much increase in MDA content throughout the test period. The ICGV showed 1.6-, 1.7-, and 1.8-fold increases in MDA content than the respective uninfested control plants at 48, 72, and 96 h, respectively. TMV 2 showed 1.6-, 1.7-, and 1.8-fold increases in MDA at 48, 72, and 96 h, respectively, than the respective control plants. Among the genotypes tested, ICGV 86699 and NCAc 343 showed higher MDA content both in control and infested plants at 24 and 96 h. Control plants of ICGV 86699 and NCAc 343 showed higher MDA content at 48 and 72 h than the control plants of TMV 2. No significant differences were observed among the infested plants of the three genotypes at 48 h, however at 72 h, infested plants of ICGV 86699 exhibited higher MDA content than that of NCAc 343 and TMV 2. At 96 h control and infested plants of ICGV 86699 and NCAc 343 exhibited higher MDA content than the control and infested plants of TMV 2.
Figure 5. MDA content (µmol g–1 FW) of groundnut genotypes after H. armigera infestation. Note: *Bars indicate the levels of statistical significance between control and infested plants within a germplasm at each time interval. *, **, ***=significance at P≤0.05, P≤0.01, and P≤0.001, respectively, by students t-test. Bars with the same letter (s) in a treatment within a time interval are not significantly different at P≤0.05. Values (Mean±SEM), FW, fresh weight of leaf tissue; Cn, control plants; In, plants infested with H. armigera; n, 10 for each genotype.
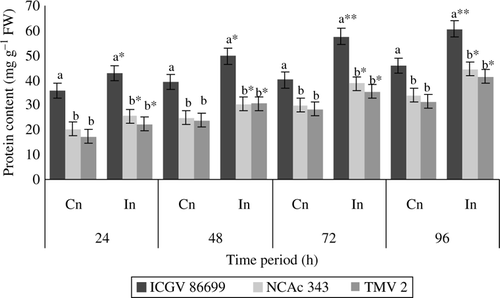
Protein content
Significant difference was recorded in protein content between control and infested plants of all the genotypes throughout the test period (). Across the genotypes, higher protein content was recorded both in control and infested plants of ICGV 86699 at all the time periods than the respective treatments of NCAc 343 and TMV 2.
Figure 6. Protein content (mg g–1 FW) of groundnut genotypes after H. armigera infestation. Note: *Bars indicate the levels of statistical significance between control and infested plants within a germplasm at each time interval. *, **, ***=significance at P≤0.05, P≤0.01, and P≤0.001, respectively, by students t-test. Bars with the same letter (s) in a treatment within a time interval are not significantly different at P≤0.05. Values (Mean±SEM), FW, fresh weight of leaf tissue; Cn, control plants; In, plants infested with H. armigera; n, 10 for each genotype.
Leaf damage, larval survival, and weight
Leaf damage was higher in TMV 2 plants (7.5) than NCAc 343 (3.6) and ICGV 86699 (2.5) (). After 96 h of infestation, larval survival was significantly lower in ICGV 86699 (35.5%) and NCAc 343 (39.4%) as compared to TMV 2 (75.9%). Likewise, weights (mg per larva) of the larvae recovered after 96 h were lower in ICGV 86699 (13.8) and NCAc 343 (17.3) than in TMV 2 (45.8).
Table 1. Leaf damage, survival and weight of H. armigera larvae after 96 h of infestation on three groundnut genotypes.
Discussion
Induced resistance is an important component of plant defense that allows plants to be phenotypically plastic in order to face different stresses and is economical, environment friendly, and effective. Moreover, understanding of plant response to arthropod herbivores will provide new insights into basic mechanisms of chemical communication and plant–animal co-evolution and may also facilitate new approaches to crop protection and improvement. In this study we examined the defensive biochemical response of the three groundnut genotypes to H. armigera feeding.
Increased levels of defense-related proteins are a common phenomenon occurring in plants on account of biotic and abiotic stress (Broze et al. Citation2010). Present studies showed increased POD activity in H. armigera infested plants than the control ones. Both constitutive and induced levels of POD showed a progressive increase throughout the test period in all the tested genotypes. ICGV 86699 showed higher induction of POD activity at later stages than that shown by NCAc 343 and TMV 2. The overall induction was more in ICGV 86699 both among the treatments and across the genotypes. Similar results were observed for PPO activity. Constitutive levels of PPO activity were almost the same in ICGV 86699 and NCAc 343 throughout the test, but at later stages, the induced levels were higher in ICGV 86699. Enzyme activities were activated by H. armigera infestation in all the three genotypes, however, the expression rhythm of activities varied among the three genotypes. The POD and PPO are the important defensive enzymes in plants against a number of biotic and abiotic stresses (Zhao et al. Citation2009; Gulsen et al. Citation2010; Usha Rani and Jyothsna 2010). Induction of these enzymes in response to insect herbivory is a common phenomenon (Heng-Moss et al. Citation2004; Han et al. Citation2009; Gulsen et al. Citation2010). Observed patterns of different POD and PPO activities in different genotypes could be due to the differences in levels of resistance against insect pests. Similarly, infestation by insect pests significantly increased POD and PPO activities in the leaves of three chrysanthemum varieties, with activity levels being higher in more resistant genotypes (He et al. Citation2011). Induction in POD activity has been implicated as an immediate response of plants in response to biotic stresses including insect attack (Moloi and van der Westhuizen Citation2006). A ninefold increase in POD activity was observed in Russian wheat aphid infestation-resistant wheat cultivar, but only a threefold increase in the susceptible cultivar after infestation (Ni et al. Citation2001). Increase in POD activity on account of insect infestation in plants detoxifies the peroxides, thus reducing plant tissue damage (Gulsen et al. Citation2010). Moreover, the role of POD in cell wall toughening and toxic secondary metabolite production and its simultaneous oxidant and antioxidant properties enables it to play an important role in integrated defense response of plants to a variety of stresses (Moore et al. Citation2003; Allison and Schultz Citation2004; Han et al. Citation2009). Reduction of nutrient quality of plant tissues by PPO and therefore reducing the consumption by herbivores makes it an important component of plant defense (Thipyapong et al. 2006; Bhonwong et al. Citation2009). Furthermore, highly reactive quinones formed on account of phenolic oxidation by PPO, alkylate the amino acids of proteins reducing their digestibility (Bhonwong et al. Citation2009). An overall similarity was observed in the patterns of expression of antioxidant enzymes in the resistant groundnut genotypes along with a susceptible genotype. Our results are in accordance with many previous studies where herbivore infestation has been reported to induce POD (Heng-Moss et al. Citation2004; Huang et al. Citation2007; Zhang et al. Citation2008; Chen et al. Citation2009; Han et al. Citation2009; Gulsen et al. Citation2010; Usha Rani and Jyothsna 2010; He et al. Citation2011), and PPO activities (Ramiro et al. Citation2006; Thipyapong et al. 2006; Bhonwong et al. Citation2009; Zhao et al. Citation2009; He et al. Citation2011). The ability of the resistant genotypes to increase POD and PPO activities suggest that genotypes with a higher level of resistance would either have a higher up-regulation capacity for defensive enzymes or have a more sensitive up-regulation response or both (Gulsen et al. Citation2010).
An increase in phenolic content was observed both in control and infested plants throughout the test period. Initially, both ICGV 86699 and NCAc 343 had higher constitutive and induced levels of phenols than that of TMV 2. At later stages, ICGV 86699 alone exhibited significantly greater phenolic content in infested plants than the infested plants of NCAc 343 and TMV 2. Accumulation of total phenolics in plants in response to herbivory is a general phenomenon (Sharma et al. Citation2009; Usha Rani and Jyothsna 2010), and has been correlated with negative effects on insect larval growth and development (Green et al. Citation2003). Quinones and ROS such as superoxide anion, hydroxide radicals, H2O2, and singlet oxygen produced by the oxidation of phenols leads to the activation of defensive enzymes in plants (Johnson and Felton Citation2001). Similar results have been reported earlier in various plants in response to insect attack (Green et al. Citation2003; Sharma et al. Citation2009; Usha Rani and Jyothsna 2010).
The ROS production is one of the important and abrupt plant responses to various stresses including herbivory (Maffei et al. Citation2007), which play an important part in signaling insect–plant interactions. Hydrogen peroxide content increased in H. armigera infested plants. Significant accumulation of H2O2 was observed in infested plants of all the tested genotypes. Across the three tested genotypes, ICGV 86699 and NCAc 343 had higher H2O2 content in uninfested control plants throughout the test period than that of TMV 2. However, infested plants of ICGV 86699 showed higher levels of H2O2 than that of NCAc 343 and TMV 2. The H2O2 has been found to play an important role in plant defense against oxidative changes due to biotic and abiotic stresses (Maffei et al. Citation2006, Citation2007; Noreen and Ashraf Citation2009; Shivaji et al. Citation2010). Accumulation of H2O2 in response to herbivory has been studied in many plants where it triggers physiological and molecular plant responses that result in induction of defensive enzymes and other defensive compounds to prevent or minimize the insect attack and/or pathogen invasion (Walling Citation2000; Torres et al. Citation2006; Maffei et al. Citation2007; Howe and Jander Citation2008). Furthermore, H2O2 could also function as a messenger for the induction of defense systems that can lead to the increased production of toxic secondary metabolites (Berglund and Ohlsson Citation1995) and oxidative enzymes (Gechev et al. Citation2002).
One of the important lipid peroxidation product involved in singling the plant defense against many stresses is MDA (Gechev et al. Citation2002; Huang et al. Citation2007). The MDA content in groundnut genotypes increased due to H. armigera attack indicating the stress levels. All the three tested genotypes showed higher MDA contents in infested plants than their respective controls. ICGV 86699 and NCAc 343 had higher MDA levels than TMV 2. MDA is commonly used to determine lipid peroxidation and is involved in defense-related signaling processes in plants (Huang et al. Citation2007). Higher H2O2 levels, under biotic stress may lead to the lipid peroxidation, which in turn could function as a signal for the induction of defense systems and enhance the production of secondary metabolites (Berglund and Ohlsson Citation1995; Zhang et al. Citation2008). Moreover, lipid peroxidation has been reported to stimulate the emission of plant volatiles in response to herbivory that attract the natural enemies of herbivores (Arimura et al. Citation2009). Induced levels of MDA in response to insect infestation may result in synthesis of complex defense compounds as reported in alfalfa varieties after aphid infestation by Huang et al. (Citation2007). Cucumber seedlings showed accumulation of MDA on account of the Bemisia tabaci attack (Zhang et al. Citation2008).
A protein-based defense is one of the important and widely studied defenses in plants against biotic and abiotic stresses (Chen et al. Citation2009). Protein concentration was greater in H. armigera infested plants. Among the three genotypes, ICGV 86699 exhibited higher protein content both in control and infested plants than the respective treatments of NCAc 343 and TMV 2. To combat with the biotic and abiotic stresses, plants produce a number of defense-related enzymes and other protein-based defensive compounds (Chen et al. Citation2009). Increase in protein concentration following H. armigera damage might be due to the production of more defense-related enzymes and other protein-based defensive compounds, many of which are detrimental to herbivore fitness (Lawrence and Koundal Citation2002; Zavala et al. Citation2004; Chen et al. Citation2009).
Minimum damage was observed in resistant genotypes than the susceptible one. Also the larval survival and weights were lower in insects fed on resistant genotypes than those fed on the susceptible one. Effect of a particular genotype on larval growth and development is an important aspect of plant resistance. Higher induction of secondary metabolites and other defensive compounds in the insect resistant genotypes on account of insect damage might result in reduced damage, decreased survival, and lower larval weights (Sharma et al. Citation2005; Bhonwong et al. Citation2009; Chen et al. Citation2009). Moreover, higher activities of POD and PPO have been linked with reduced insect growth and development in many plants (Stout et al. Citation1999; Chaman et al. Citation2001; Bhonwong et al. Citation2009; Sethi et al. Citation2009). Sharma et al. (Citation2005) observed that resistant genotypes of host plants showed minimum weight gain in H. armigera larvae compared to susceptible genotypes.
Conclusion
Helicoverpa armigera infestation induced the enzyme activities of POD, PPO, and amounts of total phenols, H2O2, MDA, and proteins. A quick response was shown by all the three genotypes to H. armigerainfestation. Reduced plant damage, decreased survival, and lower larval weights recorded in insect resistant genotypes might be due to higher enzyme activities, the amounts of total phenols, and H2O2 and MDA contents in the infested plants. These results show the differential defensive responses of the three groundnut genotypes against H. armigera and offer a perspective on plant resistance in insect plant interaction.
Acknowledgement
We are highly thankful to Dr. H.C. Sharma (Principal Scientist, Entomology, ICRISAT, India) for providing the seeds and for his valuable comments and suggestions. First author greatly acknowledges the help and financial support of the corresponding author.
References
- Agrawal , AA . 2000 . Benefits and costs of induced plant defense for Lepidium virginicum (Brassicaceae) . Ecology. , 81 : 1804 – 1813 .
- Agrawal , AA , Fishbein , M , Jetter , R , Salminen , JP , Goldstein , JB , Freitag , AE and Sparks , JP . 2009 . Phylogenetic ecology of leaf surface traits in the milkweeds (Asclepias spp.): chemistry, ecophysiology, and insect behavior . New Phytol. , 183 : 848 – 867 .
- Allison , SD and Schultz , JC . 2004 . Differential activity of peroxidase isozyme in response to wounding, gypsy moth, and plant hormones in northern red oak (Quercus rubra L.) . J Chem Ecol. , 30 ( 7 ) : 1363 – 1379 .
- Arimura , G , Matsui , K and Takabayashi , J . 2009 . Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions . Plant Cell Physiol. , 50 : 911 – 923 .
- Berglund , T and Ohlsson , AB . 1995 . Defensive and secondary metabolism in plant tissue cultures, with special reference to nicotinamide, glutathione and oxidative stress . Plant Cell Tiss Org Cult. , 43 : 137 – 145 .
- Bhonwong , A , Stout , MJ , Attajarusit , J and Tantasawat , P . 2009 . Defensive role of tomato polyphenol oxidase against cotton bollworm (Helicoverpa armigera) and beet armyworm (Spodoptera exigua) . J Chem Ecol. , 35 : 28 – 38 .
- Boka , K , Orban , N and Kristof , Z . 2007 . Dynamics and localization of H2O2 production in elicited plant cells . Protoplasma. , 230 : 89 – 97 .
- Bostock , RM . 2005 . Signal crosstalk and induced resistance: straddling the line between cost and benefit . Annu Rev Phytopathol. , 43 : 545 – 580 .
- Bradford , M . 1976 . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding . Anal Biochem. , 72 : 248 – 254 .
- Broze , AK , Broeckling , CD , De-le-Pena , C , Lewis , MR , Greene , E , Callaway , RM , Sumner , LW and Vivanco , JM . 2010 . Plant neighbor identity influences plant biochemistry and physiology related to defense . BMC Plant Biology. , 10 : 115
- Bruinsma , M and Dicke , M . 2008 . “ Herbivore-induced indirect defense: from induction mechanisms to community ecology ” . In Induced plant resistance to herbivory , Edited by: Schaller , A . 31 – 60 . Berlin , , Germany : Springer Verlag .
- Carmak , I and Horst , JH . 1991 . Effects of aluminium on lipid peroxidation, superoxide dismutase, catalase and peroxidase activities in root tips of soybean (Glycine max) . Physiol Plant. , 83 : 463 – 468 .
- Chaman , ME , Corcuera , LJ , Zuniga , GE , Cardemil , L and Argandona , VH . 2001 . Induction of soluble and cell wall bound peroxidases by aphid infestation in barley . J Agric Food Sci. , 49 : 2249 – 2253 .
- Chen , Y , Ni , X and Buntin , GD . 2009 . Physiological, nutritional and biochemical bases of corn resistance to foliage-feeding fall armyworm . J Chem Ecol. , 35 : 297 – 306 .
- Franceschi , V , Krokene , P , Christiansen , E and Krekling , T . 2005 . Anatomical and chemical defenses of conifer bark against bark beetles and other pests . New Phytol. , 167 : 353 – 376 .
- Freeman , HA , Nigam , SN , Kelley , TG , Ntare , BR , Subrahmanyam , P and Doughton , D . 1999 . The world groundnut economy, facts, trends, and outlook , 1 – 14 . Patancheru , AP (India) : ICRISAT .
- Gatehouse , JA . 2002 . Plant resistance towards herbivores: a dynamic interaction . New Phytol. , 156 : 145 – 169 .
- Gechev , T , Gadjev , I , Van Breusegem , F , Inze , D , Dukiandjiev , S , Toneva , V and Minkov , I. 2002 . Hydrogen peroxide protects tobacco from oxidative stress by inducing a set of antioxidant enzymes . Cell Mol Life Sci. , 59 : 708 – 714 .
- Green , PWC , Stevenson , PC , Simmonds , MSJ and Sharma , HC . 2003 . Phenolic compounds on the pod surface of pigeonpea, Cajanus cajan, mediate feeding behavior of larvae of Helicoverpa armigera . J Chem Ecol. , 29 : 811 – 821 .
- Gulsen , O , Eickhoff , T , Heng-Moss , T , Shearman , R , Baxendale , F , Sarath , G and Lee , D . 2010 . Characterization of peroxidase changes in resistant and susceptible warm-season turfgrasses challenged by Blissus occiduus . Arthropod-Plant Interact. , 4 : 45 – 55 .
- Han , Y , Wang , Y , Bi , JL , Yang , XQ , Huang , Y , Zhao , X , Hu , Y and Cai , QN . 2009 . Constitutive and induced resistance in aphid-resistant and aphid-susceptible cultivars of wheat . J Chem Ecol. , 35 : 176 – 182 .
- Hanley , ME , Lamont , BB , Fairbanks , MM and Rafferty , MC . 2007 . Plant structural traits and their role in anti-herbivore defense . Perspect Plant Ecol Evol Syst. , 8 : 157 – 178 .
- He , J , Chen , F , Chen , S , Lv , G , Deng , Y , Fang , Z , Guan , Z and He , C . 2011 . Chrysanthemum leaf epidermal surface morphology and antioxidant and defense enzyme activity in response to aphid infestation . J Plant Physiol. , 168 : 687 – 693 .
- Heng-Moss , TM , Sarath , G , Baxendale , F , Novak , D , Bose , S , Ni , X and Quisenberry , S . 2004 . Characterization of oxidative enzyme changes in buffalograsses challenged by Blissus occiduus . J Econ Entomol. , 97 : 1086 – 1095 .
- Howe , GA and Jander , G . 2008 . Plant immunity to herbivores . Ann Rev Plant Biol. , 59 : 41 – 66 .
- Huang , W , Zhikuan , J and Qingfang , H . 2007 . Effects of herbivore stress by Aphis medicaginis Koch on the malondialdehyde contents and activities of protective enzymes in different alfalfa varieties . Acta Ecol Sin. , 27 ( 6 ) : 2177 – 2183 .
- Johnson , KS and Felton , GW . 2001 . Plant phenolics as dietary antioxidants for herbivorous insects: a test with genetically modified tobacco . J Chem Ecol. , 27 : 2579 – 2597 .
- Kappers , IF , Hoogerbrugge , H , Bouwmeester , HJ and Dicke , M . 2011 . Variation in herbivory-induced volatiles among cucumber (Cucumis sativus) varieties has consequences for the attraction of carnivorous natural enemies . J Chem Ecol. , 37 : 150 – 160 .
- Karban , R . 2011 . The ecology and evolution of induced resistance against herbivores . Func Ecol. , 25 : 339 – 347 .
- Kessler , A and Baldwin , IT . 2002 . Plant responses to insect herbivory: the emerging molecular analysis . Ann Rev Plant Biol. , 53 : 299 – 328 .
- Lawrence , PK and Koundal , KR . 2002 . Plant protease inhibitors in control of phytophagous insects . EJB Electron J Biotechn. , 5 : 93 – 109 .
- Maffei , ME , Mithofer , A , Arimura , GI , Uchtenhagen , H , Bossi , S , Bertea , CM , Cucuzza , LS , Novero , M , Volpe , V Quadro , S . 2006 . Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide . Plant Physiol. , 140 : 1022 – 1035 .
- Maffei , ME , Mithofer , A and Boland , W . 2007 . Insects feeding on plants: rapid signals and responses preceding the induction of phytochemical release . Phytochemistry. , 68 : 2946 – 2959 .
- Mayer , AM and Harel , E . 1979 . Polyphenoloxidases in plants . Phytochemistry. , 18 : 193 – 215 .
- Miranda , M , Ralph , SG , Mellway , R , White , R , Heath , MC , Bohlmann , J and Constabel , CP . 2007 . The transcriptional response of hybrid poplar (Populus trichocarpa×P. deltoides) to infection by Melampsora medusae leaf rust involves induction of flavonoid pathway genes leading to the accumulation of proanthocyanidins . Mol Plant Microbe Interact. , 20 : 816 – 831 .
- Moloi , MJ and van der Westhuizen , AJ . 2006 . The reactive oxygen species are involved in resistance responses of wheat to the Russian wheat aphid . J Plant Physiol. , 163 : 1118 – 1125 .
- Moore , JP , Paul , ND , Whittakar , JB and Taylor , JE . 2003 . Exogenous jasmonic acid mimics herbivore-induced systemic increase in cell wall bound peroxidase activity and reduction in leaf expansion . Func Ecol. , 17 : 549 – 554 .
- Ni , X , Quisenberry , SS , Heng-Moss , T , Markwell , J , Sarath , G , Klucas , R and Baxendale , FP . 2001 . Oxidative responses of resistant and susceptible cereal leaves to symptomatic and nonsymptomatic cereal aphid (Hemiptera: Aphididae) feeding . J Econ Entomol. , 94 : 743 – 751 .
- Noreen , Z and Ashraf , M . 2009 . Change in antioxidant enzymes and some key metabolites in some genetically diverse cultivars of radish (Raphanus sativus L.) . Environ Exp Bot. , 67 : 395 – 402 .
- Orozco-Cardenas , ML , Narvaez-Vasquez , J and Ryan , CA . 2001 . Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin and methyl jasmonates . Plant Cell. , 13 : 179 – 191 .
- Purrington , CB . 2000 . Costs of resistance . Curr Opin Plant Biol. , 3 : 305 – 308 .
- Ramiro , DA , Guerreiro-Filho , O and Mazzafera , P . 2006 . Phenol contents, oxidase activities, and the resistance of coffee to the leaf miner Leucoptera coffeella . J Chem Ecol. , 32 : 1977 – 1988 .
- Rasmann , S and Agrawal , AA . 2009 . Plant defense against herbivory: progress in identifying synergism, redundancy, and antagonism between resistance traits . Curr Opin Plant Biol. , 12 : 473 – 478 .
- Sankar , B , Jaleel , CA , Manivannan , P , Kishorkumar , A , Somasundaram , R and Panneerselvam , P . 2007 . Effect of paclobutrazol on water stress amelioration through antioxidants and free radical scavenging enzymes in Arachis hypogaea L . Colloids Surf B Biointerfaces. , 60 : 229 – 235 .
- Senthil-Nathan , S , Kalaivani , K , Choi , MY and Paik , CH . 2009 . Effects of jasmonic acid-induced resistance in rice on the plant brownhopper, Nilaparvata lugens Stal (Homoptera: Delphacidae) . Pestic Biochem Physiol. , 95 : 77 – 84 .
- Sethi , A , McAuslane , HJ , Rathinasabapathi , B , Nuessly , GS and Nagata , RT . 2009 . Enzyme induction as a possible mechanism for latex-mediated insect resistance in romaine lettuce . J Chem Ecol. , 35 : 190 – 200 .
- Shannon , LM , Kay , E and Lew , JY . 1966 . Peroxidase isozymes from horse radish roots. Isolation and physical properties . J Biol Chem. , 241 : 2166 – 2172 .
- Sharma , HC , Pampathy , G , Dhillon , MK and Ridsdill-Smith , JT . 2005 . Detached leaf assay to screen for host plant resistance to Helicoverpa armigera . J Econ Entomol. , 98 ( 2 ) : 568 – 576 .
- Sharma , HC , Pampathy , G , Dwivedi , SL and Reddy , LJ . 2003 . Mechanism and diversity of resistance to insect pests in wild relatives of groundnut . J Econ Entomol. , 96 ( 6 ) : 1886 – 1897 .
- Sharma , HC , Sujana , G and Rao , DM . 2009 . Morphological and chemical components of resistance to pod borer, Helicoverpa armigera in wild relatives of pigeonpea . Arthropod-Plant Interact. , 3 ( 3 ) : 151 – 161 .
- Shivaji , R , Camas , A , Ankala , A , Engelberth , J , Tumlinson , JH , Williams , WP , Wilkinson , JR and Luthe , DS . 2010 . Plants on constant alert: elevated levels of jasmonic acid and jasmonate-induced transcripts in caterpillar-resistant maize . J Chem Ecol. , 36 : 179 – 191 .
- Stout , MJ , Findantsef , AL , Duffey , SS and Bostock , RM . 1999 . Signal interactions in pathogen and insect attack: systemic plant-mediated interactions between pathogens and herbivores of the tomato, Lycopersicon esculentum . Physiol Mol Plant Pathol. , 54 : 97 – 114 .
- Thipyapong P Mahanil S Bhonwong A Attajarusit J Stout MJ Steffens JC 2004 . Increasing resistance of tomato to lepidopteran insects by overexpression of polyphenol oxidase , In: W.J Ashcroft Proceedings of the Ninth International Symposium on the Processing Tomato , Nov 15–18 . Melbourne , , Australia : Acta Hort 29 , 724 – 38 .
- Torres , MA , Jones , JDG and Dangl , JL . 2006 . Reactive oxygen species signaling in response to pathogens . Plant Physiol. , 141 : 373 – 378 .
- Usha Rani , P and Jyothsna , Y . 2010 . Biochemical and enzymatic changes in rice as a mechanism of defense . Acta Physiol Plant. , 32 : 695 – 701 .
- Walling , LL . 2000 . The myriad plant responses to herbivores . Plant Growth Regul. , 19 : 195 – 216 .
- Wu , J and Baldwin , IT . 2010 . New insights into plant responses to the attack from insect herbivores . Annu Rev Genet. , 44 : 1 – 24 .
- Zavala , JA , Patankar , AG , Gase , K , Hui , D and Baldwin , IT . 2004 . Manipulation of endogenous trypsin proteinase inhibitor production in Nicotiana attenuata demonstrates their function as antiherbivore defenses . Plant Physiol. , 134 : 1181 – 1190 .
- Zhang , SZ , Hau , BZ and Zhang , F . 2008 . Induction of the activities of antioxidative enzymes and the levels of malondialdehyde in cucumber seedlings as a consequence of Bemisia tabaci (Hemiptera: Aleyrodidae) infestation . Arthropod-Plant Interact. , 2 : 209 – 213 .
- Zhao , LY , Chen , JL , Cheng , DF , Sun , JR , Liu , Y and Tian , Z . 2009 . Biochemical and molecular characterizations of Sitobion avenae-induced wheat defense responses . Crop Prot. , 28 : 435 – 442 .
- Zieslin , N and Ben-Zaken , R . 1993 . Peroxidase activity and presence of phenolic substances in penduncles of rose flowers . Plant Physiol Biochem. , 31 : 333 – 339 .