Abstract
To determine to what extent wild species related to crops might serve as refuges for insect pests and their natural enemies, we compared the performance of the aphid Myzus persicae and its endoparasitoid Diaeretiella rapae on one cultivar of Brassica napus and Brassica oleracea, two wild species Brassica nigra and Sinapis arvensis, and one cultivar of Solanum lycopersicum. These species differ in traits associated with plant defences that may have an impact on the herbivore and its parasitoid. In contrast to our initial hypothesis, aphid population growth rate was significantly smaller on B. napus than on the other Brassicaceae species. Similarly, the performance of the parasitoid was affected by the host plant on which the aphid was feeding. However, aphid and parasitoid performance was not correlated. Thus, in temporally changing landscapes, pests and natural enemies may utilize crops and wild-related host species with contrasting impacts on their fitness.
Introduction
In agroecosystems, landscape composition and structure may influence the diversity of insect communities (Tscharntke and Brandl Citation2004). In particular, the spatial and temporal distribution of cultivated and non-cultivated areas may have an impact on the dynamics of insect populations, depending on their capacity to disperse, their adaptation to use ephemeral habitats, and their degree of host specialization (Kruess and Tscharntke Citation1994; Vialatte et al. Citation2005). For example, at the landscape level, insects may use non-crop areas as overwintering or diapause sites, reproduction zones, or alternative sources of food and hosts when crops have been harvested (see Bianchi et al. Citation2006 for review). Non-cultivated areas with annual and perennial vegetation may thus provide resources for herbivores and their natural enemies.
Movements of herbivores and parasitoids between crops and non-cultivated plants imply that insects can colonize and develop on hosts with potentially contrasting characteristics. Compared with their wild relatives, cultivated species have increased nutritional content, enlarged plant structures of economical importance such as roots, leaves and fruits, and reduced physical and chemical defences (Evans Citation1993). As a consequence, differences between cultivated and non-cultivated plant species may be large enough to affect plant–herbivore interactions. For example, larvae of the pod-borer Helicoverpa armigera preferred to feed on pods of a cultivated Cajanus species than on those of a wild pigeonpea. Pods of the cultivated species lacked trichomes and presented compounds that stimulated feeding (Green et al. Citation2002). Thus, plant characteristics of cultivated species may favour insect pests. On the other hand, differences in plant characteristics between cultivated and wild relatives can also have an impact on parasitoid development (Benrey et al. Citation1998). For example, survival and progeny size of the parasitoid Cotesia flavipes were higher when its host was feeding on cultivated Poaceae than on wild species (Sétamou et al. Citation2005). Differences in levels of secondary compounds, physical defences, and nutrient contents were advocated to explain such differences, but mechanisms were not clearly identified. Parasitoid fitness is indeed influenced by many factors derived from the plant and from the herbivore (Harvey Citation2005).
In agroecosystems, Brassicaceae crops have many wild relatives growing in non-cultivated areas or as weeds in cultivated fields. Cultivated and wild Brassicaceae differ in a number of traits associated with plant morphology and chemical defences (Slansky and Feeny Citation1977; Cole Citation1997; Harvey et al. Citation2003; Gols et al. Citation2008). In particular, most Brassicaceae crops have leaves without trichomes and present low concentrations of glucosinolates (GLS), a well-characterized group of plant secondary compounds. After damage of plant tissue by herbivores, GLS are broken down into a number of compounds potentially toxic to many insects (see Hopkins et al. Citation2009 for review). Consequently, cultivated plants are considered to be less well-defended than their wild relatives and more susceptible to attack by generalist insect herbivores: time from egg to adult was significantly shorter for Spodoptera exigua (Sznajder and Harvey Citation2003), and adult body mass of Mamestra brassicae was larger on different cabbage cultivars than on wild relatives (Gols et al. Citation2008). Moreover, specialist and generalist parasitoids may be affected positively when their hosts feed on cultivated species: development time was shorter (Benrey et al. Citation1998; Sznajder and Harvey Citation2003; Harvey and Wagenaar Citation2006; Gols et al. Citation2008), survival was higher (Benrey et al. Citation1998; Harvey et al. Citation2003; Gols et al. Citation2008; Kahuthia-Gathu et al. Citation2008), and the emerging parasitoids were larger (Benrey et al. Citation1998; Harvey et al. Citation2003; Sznajder and Harvey Citation2003; Gols et al. Citation2008) on cultivated than on wild Brassicaceae. However, all these observations have been conducted on caterpillars and their parasitoids. Little is known about the effect of plant characteristics on the performance of aphids and their natural enemies. Moreover, the impact of Brassicaceae plant characteristics on aphid physiology still raises a number of questions as aphids are phloem-feeders that cause little tissue damage to their host plants (de Vos et al. Citation2007). Finally, a recent review conducted by Gols and Harvey (Citation2009) shows that on different Brassicaceae species, performance of herbivores and their parasitoids were positively correlated in most cases. Surprisingly, there are no data on aphids and their parasitoids.
To determine to what extent wild plants (including weeds) may serve as refuges for pests and natural enemies, we investigated the suitability of cultivated and non-cultivated species for the green peach aphid Myzus persicae (Sulzer) (Aphididae: Aphidinae) and for one of its parasitoid Diaeretiella rapae (M'Intosh) (Hymenoptera: Aphidiidae). We selected one cultivar of Brassica napus L. and Brassica oleracea L., and two wild species, Brassica nigra L. and Sinapis arvensis L. As M. persicae naturally colonizes plants of many families, we selected another potential cultivated host plant species Solanum lycopersicum (Solanaceae). We addressed the following questions: (1) Is the performance (measured as population growth rate) of M. persicae higher on cultivated species? and (2) Is the performance (estimated as parasitism rate, larval survival and development time, adult longevity and size) of D. rapae higher when its host feeds on cultivated species?
Of the two cultivated species, B. napus has become a major crop in Western Europe in the past 30 years. B. napus is sown in October and harvested in July. The other cultivated species B. oleracea is present at low density all yearlong. It has been cultivated for over 100 years in the Loire valley as a source of proteins for cattle. The two wild species B. nigra and S. arvensis are encountered along roadsides and field margins throughout the year. Both species are ruderals with locally abundant populations. Seeds germinate in the fall and plants start flowering the following spring. All species are annual but they differ in the nature and levels of physical and chemical defences (Le Guigo et al. Citation2011). B. nigra and S. arvensis are well-defended wild species. Their leaves are covered with trichomes and these species are rich in GLS. Concentrations of GLS in B. nigra (approximately 50 µmoles g−1 dry mass) are three times higher than in S. arvensis and 10 times higher than in the two cultivated Brassicaceae. Concentrations in B. oleracea and B. napus are very similar and relatively low (approximately 5 µmoles g−1 dry mass). These two cultivars present tougher leaves than the wild species (Le Guigo et al. 2011). Leaves of S. lycopersicum are also covered with trichomes, and this species is rich in alkaloids known to be toxic for many insects (Kennedy Citation2003).
M. persicae, commonly known as the green peach aphid or the peach-potato aphid, is highly polyphagous. This aphid is heteroecious and alternates between its primary host Prunus persica L. (Rosaceae) and many herbaceous secondary hosts including plants in the Brassicaceae and Solanaceae families. It colonizes Brassicaceae thanks to the excretion of GLS and secondary plant metabolites in the honeydew (Francis et al. Citation2005). M. persicae is attacked by a variety of natural enemies, including the endoparasitoid D. rapae (Bukovinszky et al. Citation2008). This parasitoid, recorded from more than 60 different aphid host species worldwide, is considered to attack mostly aphids feeding on Brassicaceae (Pike et al. Citation1999; Blande et al. Citation2004). Thus, we expected B. napus and B. oleracea to be more suitable for the development of M. persicae and D. rapae, compared with B. nigra and S. arvensis. We also expected S. lycopersicum to be suitable for the development of M. persicae but not of D. rapae.
Materials and methods
Plant and insect cultures
Seeds of B. nigra and S. arvensis were collected from feral populations around the city of Angers (47°28’ N, 1°27’ E-France) in 2007. Seeds of three common cultivars, B. oleracea variety acephala cv. Bonanza, B. napus cv. ‘NK Bravour’, and S. lycopersicum cv. ‘Saint Pierre’ were provided, respectively, by Baumaux Seeds in Nancy, the technical center for oilseed crops (CETIOM) in Rennes, and Jardiland in Angers (France).
Plants were grown from seeds in a greenhouse compartment at Agrocampus Ouest (Angers) maintained at 22±8 °C and 40–80% r.h. under natural light. Plants were kept in 14-cm-diameter pots containing a substrate consisting of peat (80%), clay (10%), and Perlite (10%). Plants were watered daily. All plants had four to five fully expanded true leaves (approximately after 30–35 days) at the beginning of the experiments.
M. persicae was collected in 2009 near Angers on B. napus, B. oleracea, B. nigra, and S. arvensis. Stock colonies were maintained for at least 10 generations on each host plant before the beginning of the experiments, in a climate chamber kept at 18±1 °C, 70±10% r.h., and with a 16 h L:8 h D photoperiod. The parasitoid D. rapae was originally collected in 2005 from cabbage fields around Angers. It has been reared on B. oleracea infested with B. brassicae in a growth chamber in the same environmental conditions as the aphids. Adults were provided ad libitum with a 5% honey solution and a mixture of pollen, honey and yeast. Field collected parasitoids were added to the stock colonies every year to maintain genetic diversity.
Development of M. persicae on different host plant species
In this experiment, we were interested in measuring the performance of aphids on plant species on which they did not have the possibility to adapt, but on which they might move when crops have been harvested. Therefore, the aphids originated from the populations maintained on B. oleracea. Thirty plants per species were transferred from the greenhouse to the laboratory and randomized within a climate chamber at 18±1 °C, 70% r.h., and 16 h light. Each plant was enclosed in a perforated cellulosic bag. After a 2-day acclimation period, one fourth instar nymph of M. persicae reared on B. oleracea was placed on the fourth true leaf of each plant. After it had produced its first nymph, it was left untouched for 24 hours. The adult was then removed. The developmental stage of the nymphs was recorded daily to estimate mortality and the length of the pre-reproductive period. When all nymphs had developed into adults, their morph (winged or wingless) was recorded and their biomass was estimated with a high precision microbalance (Precisa 92SM-202A, Servilab, Le Mans, France, 10−5 g). The last nymph to become an adult was kept on the host plant until its death. These aphids were used to determine longevity, total fecundity, and the population growth rate r m as an estimate of fitness (Birch Citation1948). The variance of r m was estimated with the DEMP program (Giordanengo Citation2009).
Development of D. rapae on different host plants
In this experiment, we were interested in the impact of the host plant on parasitoid development and to prevent any cue given by the host plant, the parasitoid had no direct contact with the plant. To measure the performance of D. rapae on aphids that had potentially adapted to their host plants, the aphids originated from the populations maintained on B. napus, B. oleracea, B. nigra or S. arvensis. Thirty plants per species were transferred from the greenhouse to the same growth chamber as described earlier. Mated, 1-day-old, fed and naive female parasitoids were exposed to 12 second instar aphids, one at a time, in a 6-cm-diameter plastic Petri dish. Each aphid was removed after it had been stung once by the parasitoid, and placed back on its respective host plant. To compare parasitism rate of M. persicae feeding on each host plant, half of the aphids (six or less if some had died) were dissected after five days. The number of aphids containing a parasitoid larva was recorded. The other half (if possible six aphids) was kept on the host plant until mummies were formed. After approximately 10 days, the number of mummies and aphids were counted on each host plant. To estimate parasitoid development time, survival to adulthood, longevity and size of the resulting adults, mummies were then removed from the plants and kept in small vials until adult eclosion. The adult parasitoids were sexed and fed with a honey solution until their death. Mortality of the parasitoids was recorded daily. The size (as a proxy of fitness) was estimated by measuring hind tibia length using a 5× magnification lens and the Metaview© program.
Statistical analysis
To compare aphid performance on the different host plant species, Generalized Linear Models were used to analyze non-nested data calculated for each aphid (number of nymphs, aphid longevity and total fecundity). To compare the other life-history traits, data were nested per plant. Measurements from all aphids feeding on one plant or all offspring from one female parasitoid were analyzed with Generalized Estimating Equations. All computations were done with R version 2.7.1 (R Development Core Team Citation2008).
Analyses were performed with different distributions: number of nymphs and aphid fecundity were analyzed assuming a Poisson distribution and a log link function for count data; nymph mortality, parasitism rate, survival, and sex ratio with a Binomial distribution and a logit link function for binary data; pre-reproductive period, development time, and longevity with a Gamma distribution and an inverse link function for data expressed in days. Finally, aphid biomass, the intrinsic rate of increase r m , and parasitoid size were analyzed assuming a Gaussian distribution with an identity link for data with a normal distribution. Over-dispersed data were corrected with a quasi-function distribution.
Results
Development of M. persicae on different host plant species
Most larval and adult life-history traits of M. persicae did not differ significantly between B. napus, B. oleracea, B. nigra, and S. arvensis (N=117 host plants). Host plant species did not have an effect on the total number of nymphs laid in 24 hours, nymph mortality and total adult fecundity (). All adults were apterous. However, plant species had a significant effect on the length of the pre-reproductive period, adult biomass, and adult longevity (). Adults of M. persicae were significantly smaller and took more time to reproduce on B. napus compared with the other three Brassicaceae species. These differences in life-history traits translated into differences in population growth rates with r m significantly smaller on B. napus ().
Table 1. Larval and adult traits (means±S.E.) of Myzus persicae feeding on Solanum lycopersicum (N=29 plants except for the measurements on aphid biomass), Brassica napus (N = 29), Brassica oleracea (N = 29), Brassica nigra (N = 30), or Sinapis arvensis (N = 29). Data on nymph mortality, the length of the pre-reproductive period and biomass were nested per plant. All differences between S. lycopersicum and the four Brassicaceae species were significant (p<0.001). Analyses were performed with different distributions: Poisson (P) with a log link function for count data, Binomial (B) with a logit link function for binary data, Gamma (G) with an inverse link function for data expressed in days, and Gaussian (N) with an identity link function for data with a normal distribution. Different superscript letters indicate significant differences among means for the Brassicaceae only.
M. persicae performed poorly on S. lycopersicum: even though the total number of nymphs laid in 24 hours did not differ significantly among host plant species (χ 2 4=7.87, p = 0.198; N=146 host plants), nymph mortality was on average four times higher on S. lycopersicum than on the Brassicaceae (χ 2 4=76.1, p < 0.001, ). Compared with aphid development on the Brassicaceae host plant species, the pre-reproductive period (ANOVA, F 4=216, p < 0.001) was longer, while adult biomass (F 4=152, p < 0. 001), longevity (F 4=12.4, p < 0.001), and total fecundity (F 4=107, p < 0.001; ) were significantly lower on S. lycopersicum. All these differences in life-history traits translated into a significantly lower population growth rate on Solanum compared with the Brassicaceae plant species (F 4=107, p < 0.001; ).
Development of D. rapae on aphids feeding on different host plants
We could not measure performance of D. rapae on S. lycopersicum because aphid survival was too low. Most life-history traits did not differ significantly among D. rapae that parasitized or emerged from aphids feeding on B. napus, B. oleracea, B. nigra, or S. arvensis. Parasitism rate estimated as % parasitized aphids after 5 days or at mummy stage, survival rate from egg to adult, sex ratio expressed as % females, and longevity of the resulting adults did not differ between plant species (N=112 plants; ). Unexpectedly, development time from egg to adult was significantly shorter on B. napus than on B. oleracea (). The resulting female parasitoids were larger when the aphids fed on B. napus (F 3=8.87, p = 0.03), while male parasitoids were larger on S. arvensis (F 3=9.76, p = 0.02; ).
Figure 1. Hind tibia length (µm) measured as a proxy of Diaeretiella rapae body size when its host Myzus persicae was feeding on Brassica napus (N = 27 plants), B. oleracea (N = 27), B. nigra (N = 29), or Sinapis arvensis (N = 29). Female parasitoids were larger when the aphids fed on B. napus (F=8.87, p = 0.03), while male parasitoids were larger on S. arvensis (F=9.76, p = 0.02). Males were always smaller than females on all plant species (F=11.17, p < 0.001). Significant differences among host plants are indicated by capital letters for the female parasitoids and lowercase letters for the males.
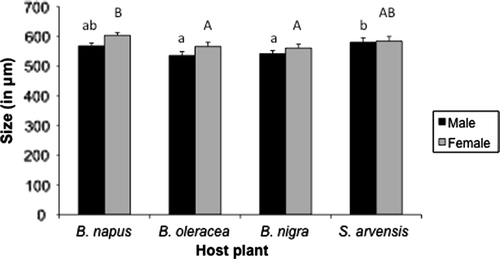
Table 2. Parasitism rate, larval survival and development time, adult sex ratio and longevity for Diaeretiella rapae reared on Myzus persicae feeding on Brassica napus (N = 27 plants), Brassica oleracea (N = 27), Brassica nigra (N = 29), or Sinapis arvensis (N = 29). All data are nested per female parasitoid. Means±S.E. are given for each host plant species. Analyses were performed with different distributions: Binomial (B) with a logit link function for binary data, or Gamma (G) with an inverse link function for data expressed in days. Different superscript letters indicate significant differences among means.
Discussion
In contrast to our initial hypothesis, M. persicae did not perform better on the cultivated species. Differences in life-history traits translated into differences in population growth rates with r m significantly smaller on B. napus compared with the other three Brassicaceae species (). Moreover, even though M. persicae is a generalist aphid feeding on a large number of host plants, its development seemed hampered on S. lycopersicum (). Similarly, performance of the parasitoid D. rapae was affected by the plant on which its host was feeding. Development time from egg to adult was shorter () and female parasitoids were larger on B. napus, while males were larger on S. arvensis (). Thus, in opposite with the results observed for the aphid, B. napus seemed a good host plant species for D. rapae even though the impacts of the plant on the parasitoid fitness were not as clear as for the herbivore.
Our results contrast with previous studies that have shown that M. persicae develops better on cultivated species like B. napus and B. oleracea compared with the wild species, B. nigra, B. fruticulosa, and B. spinescens (Cole Citation1997). Cole's results indicate a significant negative correlation between the intrinsic growth rate of M. persicae and the concentrations of some GLS in Brassica species or cultivars. Other authors have also observed a negative impact of plant secondary compounds, in particular GLS or their breakdown products, on population growth rate of M. persicae. Insect performance was negatively related to GLS concentration in B. oleracea (van Emden 1972 cited in Feeny Citation1977), Arabidopsis thaliana (Mewis et al. Citation2005; Kim et al. Citation2008), and B. oleracea, B. napus, B. nigra and S. alba (Hodge et al. Citation2006). In our study, aphid performance did not seem related to GLS concentration as M. persicae population growth rate was significantly lower on B. napus, the species characterized by the lowest GLS concentration. However, differences in GLS profiles (Kuśnierczyk et al. Citation2007; Poelman et al. Citation2009) and other plant characteristics might also be important. M. persicae has been shown to respond to phloem nitrogen levels (van Emden and Bashford Citation1969; Karley et al. Citation2008), which are expected to be lower in cultivated than in wild species (Slansky and Feeny Citation1977). Yet, our results indicate that the population growth rate of the aphid was not significantly different between one cultivar of B. oleracea and the two wild species B. nigra and S. arvensis.
An alternative hypothesis to explain the differences observed in host plant suitability is a specialization of M. persicae on the Brassicaceae. The poor performance of the aphid on S. lycopersicum might have been the result of artificial selection of clones that have specialized on Brassicaceae in the field or in the laboratory as aphids were reared on B. oleracea for at least 10 generations. In previous studies (Vorburger Citation2006; Kasprowicz et al. Citation2008), from aphids collected in the field, authors identified a small number of clones and at least one clone that exhibited marked preference for Brassica crops. Thus, host plant adaptation in this aphid seems to occur, even under strong temporal and spatial variation in the availability of various vegetable and oilseed Brassica throughout the year. Moreover, this species is able to excrete or detoxify GLS (Francis et al. Citation2005; Ramsey et al. Citation2010). All these studies support the fact that M. persicae might be able to specialize on Brassicaceae. Host plant specialization could explain why, in our study, aphid performance was high on wild species characterized by high concentrations of GLS, and low on non-Brassicaceae species like S. lycopersicum. However, it does not explain why M. persicae performed poorly on B. napus, and why it did not perform better on B. oleracea as the aphid could have adapted to this host plant.
Performance of the parasitoid D. rapae was indirectly affected by the plant on which M. persicae was feeding but, unexpectedly, was not correlated with the performance of its host. In a recent review, Gols and Harvey (Citation2009) noted that on different Brassicaceae species, performance of herbivores and their parasitoids were positively correlated in most cases. In a previous study, we also observed a positive relationship between the performance of Brevicoryne brassicae, a Brassicaceae aphid specialist that sequesters GLS, and D. rapae (Le Guigo et al. 2011). In this study, aphid adults were smaller and female parasitoids were larger on B. napus ( and ), which is in contrast with the assumption that larger host size translates into higher quality resource for the parasitoid (Harvey Citation2005). On one hand, D. rapae might have been favoured by the low concentration of GLS in B. napus. Even though M. persicae excretes GLS and secondary plant metabolites (Francis et al. Citation2005), other studies with herbivores that detoxify or excrete toxic plant secondary compounds have shown that the development of parasitoids may still be negatively affected by harmful compounds encountered in the host plant (Harvey et al. Citation2007). On the other hand, differences in parasitoid performance among host plants were small or not significant for most of the life-history traits that we measured, whereas aphid performance was clearly affected by the host plant on which it was feeding. Previous studies have also shown that parasitoids are less affected than the herbivores by host plant characteristics (Barbosa et al. Citation1986; Harvey, Citation2005; Gols and Harvey Citation2009, Schädler et al. Citation2010).
In conclusion, our study indicates that M. persicae and D. rapae are able to exploit cultivated and wild species of Brassicaceae, with contrasting impacts on their fitness. Non-cultivated areas may serve as refuges when crops are not available. To determine potential movements of insect pests and natural enemies between cultivated and non-cultivated areas, future work should address whether herbivores and/or parasitoids can utilize one host plant, a group of species or even a landscape compartment. Results may offer new perspectives in the design of cropping systems that manage crop colonization by both herbivores and their natural enemies.
Acknowledgements
We would like to thank Michèle Travers, Ferréol Braud, and Isabelle Besse for technical assistance, Yannick Outreman for statistical advice, and Frédéric Francis for comments on a previous version. The project benefited from suggestions by Yannick Outreman, Anne-Marie Cortesero, Emmanuel Corcket and Claire Campion. We acknowledge the French Ministry of Agriculture for funding. P.L.G. was supported by a graduate fellowship from the region Pays-de-la-Loire (contract # 2007-7623).
References
- Barbosa , P , Saunders , JA , Kemper , J , Trumbule , R , Olechno , J and Martinat , P . 1986 . Plant allelochemicals and insect parasitoids-Effects of nicotine on Cotesia congregata (Say) (Hymenoptera: Braconidae) and Hyposoter annulipes (Cresson) (Hymenoptera: Ichneumonidae) . J Chem Ecol. , 12 : 1319 – 1328 .
- Benrey , B , Callejas , A , Rios , L , Oyama , K and Denno , R . 1998 . The effects of domestication of Brassica and Phaseolus on the interaction between phytophagous insects and parasitoids . Biol Control. , 11 : 130 – 140 .
- Bianchi , FJJA , Booij , CJH and Tscharntke , T . 2006 . Sustainable pest regulation in agricultural landscapes: a review on landscape composition, biodiversity and natural pest control. Proc R Soc London . Ser. B. , 273 : 1715 – 1727 .
- Birch , LC . 1948 . The intrinsic rate of natural increase of an insect population . J Anim Ecol. , 17 : 15 – 26 .
- Blande , JD , Pickett , JA and Poppy , GM . 2004 . Attack rate and success of the parasitoid Diaeretiella rapae on specialist and generalist feeding aphids . J Chem Ecol. , 30 : 1781 – 1795 .
- Bukovinszky , T , van Veen , FJF , Jongema , Y and Dicke , M . 2008 . Direct and indirect effects of resource quality on food web structure . Science , 319 : 804 – 807 .
- Cole , RA . 1997 . The relative importance of glucosinolates and amino acids to the development of two aphid pests Brevicoryne brassicae and Myzus persicae on wild and cultivated brassica species . Entomol Exp Appl. , 85 : 121 – 133 .
- de Vos , M , Kim , JH and Jander , G . 2007 . Biochemistry and molecular biology of Arabidopsis-aphid interactions . BioEssays , 29 : 871 – 883 .
- Evans , LT . 1993 . Crop evolution, adaptation and yield , Cambridge , , UK : Cambridge University press .
- Feeny , P . 1977 . Defensive ecology of the Cruciferae . Ann Mo Bot Gard. , 64 : 221 – 234 .
- Francis , F , Vanhaelen , N and Haubruge , E . 2005 . Glutathione S-transferases in the adaptation to plant secondary metabolites in the Myzus persicae aphid . Arch Insect Biochem Physiol. , 58 : 166 – 174 .
- Giordanengo P. 2009 . DEMP 1.3, programme php pour calculer les paramètres démographiques (tables de survie) . Université de Picardie Jules Verne, Amiens (France). Available from: http://www.u-picardie.fr/PCP/UTIL/demp.php/
- Gols , R , Bukovinszky , T , van Dam , NM , Dicke , M , Bullock , JM and Harvey , JA . 2008 . Performance of generalist and specialist herbivores and their endoparasitoids differs on cultivated and wild Brassica populations . J Chem Ecol. , 34 : 132 – 143 .
- Gols , R and Harvey , JA . 2009 . Plant-mediated effects in the Brassicaceae on the performance and behaviour of parasitoids . Phytochem Rev. , 8 : 187 – 206 .
- Green , PWC , Stevenson , PC , Simmonds , MSJ and Sharma , HC . 2002 . Can larvae of the pod-borer, Helicoverpa armigera (Lepidoptera: Noctuidae), select between wild and cultivated pigeonpea Cajanus sp. (Fabaceae)? . Bull Entomol Res. , 92 : 45 – 51 .
- Harvey , JA . 2005 . Factors affecting the evolution of development strategies in parasitoid wasps: the importance of functional constraints and incorporating complexity . Entomol Exp Appl. , 117 : 1 – 13 .
- Harvey , JA , Gols , R , Wagenaar , R and Bezemer , TM . 2007 . Development in an insect herbivore and its pupal parasitoid reflect differences in direct plant defense . J Chem Ecol. , 33 : 1556 – 1569 .
- Harvey , JA , van Dam , NM and Gols , R . 2003 . Interactions over four trophic levels: foodplant quality affects development of a hyperparasitoid as mediated through a herbivore and its primary parasitoid . J Anim Ecol. , 72 : 520 – 531 .
- Harvey , JA and Wagenaar , R . 2006 . Development of the herbivore Pieris rapae and its endoparasitoid Cotesia rubecula on crucifers of field edges . J Appl Entomol. , 130 : 465 – 470 .
- Hodge , S , Pope , TW , Holaschke , M and Powell , G . 2006 . The effect of β-aminobutyric acid on the growth of herbivorous insects feeding on Brassicaceae . Ann Appl Biol. , 148 : 223 – 229 .
- Hopkins , RJ , van Dam , NM and van Loon , JJA . 2009 . Role of glucosinolates in insect-plant relationships and multitrophic interactions . Ann Rev Entomol. , 54 : 57 – 83 .
- Kahuthia-Gathu , R , Lohr , B and Poehling , HM . 2008 . Effect of common wild crucifer species of Kenya on fitness of two exotic diamondback moth parasitoids, Cotesia plutellae and Diadegma semiclausum . Crop Prot. , 27 : 1477 – 1484 .
- Karley , AJ , Hawaes , C , Ianetta , PPM and Squire , GR . 2008 . Intraspecific variation in Capsella bursa-pastoris in plant quality traits for insect herbivores . Weed Res. , 48 : 147 – 156 .
- Kasprowicz , L , Malloch , G , Pickup , J and Fenton , B . 2008 . Spatial and temporal dynamics of Myzus persicae clones in fields and suction traps . Agri Forest Entomol. , 10 : 91 – 100 .
- Kennedy , GG . 2003 . Tomato, pests, parasitoids and predators . Ann Rev Entomol. , 48 : 51 – 72 .
- Kim , JH , Lee , BW , Schroeder , FC and Jander , G . 2008 . Identification of indole glucosinolate breakdown products with antifeedant effects on Myzus persicae (green peach aphid) . The Plant J. , 54 : 1015 – 1026 .
- Kruess , A and Tscharntke , T . 1994 . Habitat fragmentation, species loss and biological Control . Science , 264 : 1581 – 1484 .
- Kuśnierczyk , A , Winge , P , Midelfart , H , Armbruster , WS , Rossiter , JT and Bones , AM . 2007 . Transcriptional responses of Arabidopsis thaliana ecotypes with different glucosinolate profiles alter attack by polyphagous Myzus persicae and oligophagous Brevicoryne brassicae . J Exp Bot. , 58 : 2537 – 2552 .
- Le Guigo , P , Qu , Y and Le Corff , J . 2011 . Plant-mediated effects on a toxin-sequestering aphid and its endoparasitoid . Basic Appl Ecol. , 12 : 72 – 79 .
- Mewis , I , Appel , HM , Hom , A , Raina , R and Schultz , JC . 2005 . Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects . Plant Physiol. , 138 : 1149 – 1162 .
- Pike , KS , Starý , P , Miller , T , Allison , D , Graf , G , Boydston , L , Miller , R and Gillespie , R . 1999 . Host range and habitats of the aphid parasitoid Diaeretiella rapae (Hymenoptera: Aphidiidae) in Washington state . Environ Entomol. , 28 : 61 – 71 .
- Poelman , EH , van Dam , NM , van Loon , JJA , Vet , LEM and Dicke , M . 2009 . Chemical diversity in Brassica oleracea affects biodiversity of insect herbivores . Ecology , 90 : 1863 – 1877 .
- R Development Core Team . 2008 . R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing . Available from: http://www.R-project.org/
- Ramsey , JS , Rider , DS , Walsh , TK , de Vos , M , Gordon , KHJ , Ponnala , L , Macmil , SL , Roe , BA and Jander , G . 2010 . Comparative analysis of detoxification enzymes in Acyrthosiphon pisum and Myzus persicae . Insect Mol Biol. , 19 : 155 – 164 .
- Schädler , M , Brandl , R and Kempel , A . 2010 . Host plant genotype determines bottom-up effects in an aphid–parasitoid–predator system . Entomol Exp Appl. , 135 : 162 – 169 .
- Sétamou , M , Jiang , N and Schulthess , F . 2005 . Effect of the host plant on the survivorship of parasitized Chilo partellus Swinhoe (Lepidoptera: Crambidae) larvae and performance of its larval parasitoid Cotesia flavipes Cameron (Hymenoptera: Braconidae) . Biol Control , 32 : 183 – 190 .
- Slansky , F Jr and Feeny , P . 1977 . Stabilization of the rate of nitrogen accumulation by larvae of the cabbage butterfly on wild and cultivated food plants . Ecol Monogr. , 47 : 209 – 228 .
- Sznajder , B and Harvey , JA . 2003 . Second and third trophic level effects of differences in plant species reflect dietary specialisation of herbivores and their endoparasitoids . Entomol Exp Appl. , 109 : 73 – 82 .
- Tscharntke , T and Brandl , R . 2004 . Plant–insect interactions in fragmented landscapes . Ann Rev Entomol. , 49 : 405 – 430 .
- van Emden , HF and Bashford , MA . 1969 . A comparison of the reproduction of Brevicoryne brassicae and Myzus persicae in relation to soluble nitrogen concentration and leaf age (leaf position) in the Brussel sprout plant . Entomol Exp Appl. , 12 : 351 – 364 .
- Vialatte , A , Dedryver , CA , Simon , JC , Galman , M and Plantegenest , M . 2005 . Limited gene exchanges between populations of an insect pest living on uncultivated and related cultivated host plants. Proc R Soc London . Ser. B. , 272 : 1075 – 1082 .
- Vorburger , C . 2006 . Temporal dynamics of genotypic diversity reveal strong clonal selection in the aphid Myzus persicae . J Evol Biol. , 19 : 97 – 107 .