Abstract
Exogenous application of 0.1 mM methyl jasmonate (MeJA) throughout seed soaking or fumigation of seedlings could induce resistance against the necrotrophic fungus Alternaria porri f. sp. solani in tomato. MeJA applied at 0.01, 0.1, and 1 mM was found to reduce spore germination and mycelial growth in vitro. This compound at 0.01 and 0.1 mM did not cause toxic responses in the tomato plants; however, ethylene production by seedlings was observed to increase after using of all concentrations. A significant increase in the levels of defense markers such as total phenolics, anthocyanins, and phenylalanine ammonia-lyase activity, in response to exogenous MeJA, was observed. Pretreatment of tomato by soaking the seeds in MeJA or treating them with gaseous MeJA efficiently reduced the development of disease caused by Alternaria only when MeJA was applied at 0.1 mM concentration. Seed priming is an easier method of resistance induction than exposure to gaseous MeJA.
Introduction
Diseases caused by Alternaria species are common among many plants throughout the world. The fungus affects many solanaceous plants, including tomato (Agrios Citation2005). Alternaria porri f. sp. solani causes one of the most damaging tomato diseases, resulting in heavy economic losses by reducing plant growth in field and in greenhouses. The fungus overwinters as mycelium or spores in infected plants debris and in or on seeds. When the fungus is carried by seeds, it dampens off the seedlings or causes stem lesions and collar rot after emergence; usually, however, spores are blown in from the infected debris or infected cultivated plants and weeds, especially during dew and rain, and germinate to form mycelium in leaves, stem, and fruits. Generally, Alternaria diseases are more prevalent on poorly growing plants, already weakened by different stressors.
Most attempts to control fungal pathogens involve the use of fungicides, but application of traditional chemical agents requires complicated planning and careful timing, as many of these compounds are toxic or have toxic residues in fruits and their use is therefore often restricted to certain pre-harvest time windows. Alternative methods for fungicide use are those increasing resistance of plants to their pathogens. For decades, plants have been known to have a wide variety of structural and biochemical defenses against pathogen attack (Agrios Citation2005; Van Loon et al. Citation2006). One of the biochemical defenses produced by plants is induced resistance, whereby a plant – pretreated with various biological and chemical agents – develops a rather non-specific and long-lasting whole-system resistance against many pathogens such as fungi, bacteria, and other agents. The phenomenon is known as the induced systemic resistance (ISR). Such resistance can be induced by, e.g. β-aminobutyric acid (Jackab et al. Citation2001; Ton and Mauch-Mani Citation2004) and salicylic acid (Thulke and Conrath Citation1998). Induction of resistance against fungal pathogens by a treatment involving the plant hormone methyl jasmonate (MeJA) or jasmonic acid (JA) has been shown to be effective in Arabidopsis (Thomma et al. Citation1998, Citation2000) and other species such as potato, tomato, and grapefruit (see review in Pozo et al. Citation2005; El-Khallal Citation2007). Studies involving external application of resistance elicitors might suffer strongly from physiologically unrealistic concentrations and spatial distribution of the elicitors. For agricultural pathogen management, research integrating physiological, biochemical, and molecular aspects of resistance is required to realize the full potential of inducible resistance strategies. In our opinion, when studying plant defense against fungi, it is also important to know the fungus response to MeJA, particularly the effect of the compound on spore germination in water, since the step of fungal diseases involves contact of spores with water.
Consequently, the present study was carried out to determine whether MeJA (1) is active against A. porri f. sp. solani when tested in vitro, (2) as the ISR elicitor is safe for the tomato plants treated, (3) enhances tomato defense responses, including phenolic compounds, anthocyanins, and the related enzyme phenylalanine ammonia-lyase (PAL), directly involved in the biosynthetic pathway of phenolic compounds, and (4) whether MeJA pretreatment of tomato plant provides sufficient protection against A. porri f. sp. solani.
Materials and methods
Plant and fungal materials
Seeds of tomato (Lycopersicon esculentum. cv. Beta) harvested in 2008 (germination > 80%) were obtained from the TORSEED Seed Company (Poland) and stored dry at −23°C until used.
Alternaria porri f. sp. solani (synonyms: Alternaria alternata f. sp. lycopersici, A. solani, A. arborescens) isolates were recovered from tomato seeds. The fungus was isolated by placing the seeds – after 2 min surface disinfection with 1% sodium hypochloride – on 2% potato dextrose agar (PDA) (Difco, Detroit, MI, USA) and incubating them at 25°C for 7 days. Morphological identification of A. porri f. sp. solani isolates was based on characteristics of the conidia and colony growth traits. The forma specialis of the pathogen was identified using pathogenicity tests, following Koch's postulates. The isolates obtained were kept on PDA slopes or as 15% glycerol stocks at −80°C.
In vitro antifungal activity assay of MeJA against Alternaria porri f. sp. solani
To harvest spores for the germination test, 5-day-old cultures were brushed gently to loosen the spores from the mycelial surface and then rinsed with a 0.01% Tween 20 solution. The resulting spore suspension was filtered through a fine nylon mesh to remove larger mycelial parts, quantified using a hemocytometer, and generally adjusted to 106 spores ml−1. Droplets (0.05 ml) of the spore suspension were placed on glass slides in Petri dishes (10 cm) lined with filter paper moistened with distilled water. The spores were mixed with 0.05 ml of water (control) or with MeJA solutions to obtain the final 0.01, 0.1, and 1 mM concentrations. The germinated spores were counted under a light microscope where the minimum number of spores used was >300 in each experiment. For each treatment, six droplets were used. To determine the MeJA effect on A. porri f. sp. solani mycelium growth, a 5-mm diameter mycelium disc was transferred to PDA containing MeJA at concentration ranging from 0 to 1 mM. The plates were incubated in the dark at 24°C or up to 5 days. The fungus growth was determined by measuring the colony diameter. To determine mycelial dry weight, the PDA medium with mycelia was heated and filtered through the filter paper in Büchner funnel under pressure, rinsed with warm water, and dried at 75°C for 48 hours.
Tomato plant treatment with MeJA and its activity during different physiological and biochemical processes
Two methods of treating the tomato plants were used: soaking of seeds in MeJA solutions and pretreatment of 14-day-old seedlings with gaseous MeJA.
Seed germination and seedling emergence
To determine the effect of MeJA on germination rate, the seeds (50) were soaked in aqueous MeJA solutions (0.01, 0.1, 1 mM concentrations) or in water (control) for 60 min and then transferred to 5-cm diameter Petri dishes in which they were placed on filter paper moistened with 1.5 ml of water. Germination was assessed every 24 h for 11 days. To test the effect of MeJA on seedling emergence, the seeds, after they had been soaked in the MeJA solutions or in water for 60 min, were sown in sterile soil in plastic pots; the pots were placed in a growth chamber (Versatile Environmental Test Chamber MLR-350, Sanyo, Osaka) at 16/8 day/night photoperiod, 25±1°C, and 70% relative humidity. The pots were watered daily and fertilized once a week with a standard nutrient solution. The seedlings emerged were counted 7 days after sowing.
The gaseous MeJA pretreatment (fumigating) was applied to 14-day-old tomato seedlings with fully expanded leaves were used. The seedlings were placed in 2.7 l glass jars. An appropriate concentration of gaseous MeJA in the atmosphere was obtained by adding, with a hypodermic syringe through a septum, liquid 1 M MeJA in µl quantities onto a piece of filter paper placed under the cover of a jar. After 24 h exposure, the seedlings were removed from the jars and used in biochemical analyses and inoculation experiments.
Biochemical analyses
Leaves of 15-day-old seedlings raised from the MeJA primed seeds or from the MeJA-gassed 14-day-old seedlings were subjected to the following assays: chlorophyll a and b (as in Lichthentaler and Wellburn Citation1985; reducing sugars (Lever Citation1972); total phenolics (Singleton and Rossi Citation1965); antocyanins (Mancinelli Citation1984); and PAL activity (Edwards and Kessman Citation1992). To measure ethylene production, the 14-day-old seedlings were kept for 24 h in 2.7 l jars closed with covers containing rubber stoppers. Ethylene production by 15-day-old seedlings obtained from the MeJA-soaked seeds or from the seedlings treated with airborne MeJA 24 h before ethylene measurement was analyzed by removing 1 ml of gas from the head space, with a syringe and injecting into a Hewlett-Packard 5890 gas chromatograph equipped with a Poropak Q-filled aluminum column and flame-ionization detector.
Disease development in inoculated MeJA-pretreated plants
The 15-day-old plants obtained from the seeds soaked in different MeJA concentrations for 60 min or pretreated with gaseous MeJA for 24 h before inoculation were sprayed with spore suspension until run-off. To ensure good conditions for spore germination, during the initial 24 h the plants were placed in controlled-climate chambers with the relative humidity being increased to 95%. To assess disease severity, the percentage of seedlings with necrotic spots and chlorosis was determined four weeks after inoculation. The disease index involved a 0–5 scale: 0 = no symptoms; 1 = disease symptoms shown by more than 10% of the seedlings; 2 = disease symptoms shown by more than 25% of the seedlings; 3 = disease symptoms shown by more than 35% of the seedlings; 4 = disease symptoms shown by more than 45% of the seedlings; 5 = disease symptoms shown by more than 50% of the seedlings. The plants were rated resistant if scored 0–1, intermediate if scored 2–3, and susceptible if scored 4–5. Twelve seedlings from each combination were examined. The mean for each combination of all plants examined is reported as the average severity index (ASI):
where n is the number of plants per scale score and N is the total number of plants examined.
Antifungal activity of leave extracts from MeJA pretreated seedlings
Fresh leaves (1 g) of 15-day-old seedlings pretreated with MeJA with either of the two priming methods described earlier were ground in a mortar with 1 ml of methanol. The methanol extracts were evaporated to dry in a rotary vacuum evaporator at 40°C. The extract residues were brought to 1 ml in sterile water. To determine effects of leave extracts on A. porri f. sp. solani spore germination, the spores were germinated in Erlenmayer flasks (50 ml) in Czapek's medium (25 ml) enriched with 5% (v/v) extracts. The spore suspensions were incubated on a rotary shaker at 200 rpm. After incubation, the spore suspensions were transferred onto glass slides for spore germination assessment.
Statistical analysis
Each treatment was run in three or five (ethylene production) replications, and all tests were repeated three or more times. All the data were subjected to statistical analysis involving Duncan's multiple range test.
Results and discussion
In vitro antifungal activity of MeJA against Alternaria porri f. sp. solani
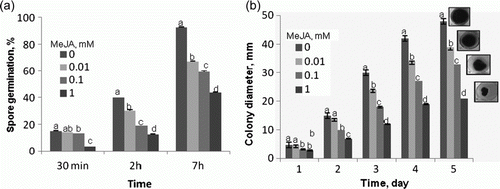
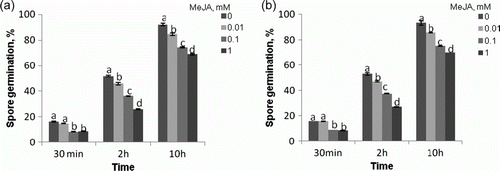
Methyl jasmonate at concentrations of 0.01, 0.1, and 1 mM was found to inhibit germination of A. porri f. sp. solani spores, a 20–50% inhibition of germination being observed (a). When applied at identical concentrations, the compound was reported to inhibit, nearly at an identical level, in vitro germination of A. alternata (Fr.) Keissl. isolated from stored apples (Kępczyńska and Kępczyński Citation2005). The inhibitory effect of MeJA was also evident in the mycelium growth (b). After one day, the mycelial growth was found to be reduced in the PDA medium containing MeJA at a concentration 0.1 and at 1 mM. However, extension of the time of incubation in the presence of MeJA at all the concentration used produced an inhibitory effect on the process: after 5 days, 25, 30, and 60% inhibition was recorded at 0.01, 0.1, and 1 mM concentrations, respectively (b). The spores and the mycelium seem to be almost equally sensitive to the chemical tested, since an identical inhibition rate was observed in spore germination and mycelium growth. The MeJA effect was also visible with respect to mycelial dry weight (data not shown). These results might suggest that MeJA can directly affect fungal growth in the plant host.
Figure 1. The effect of MeJA on A. porri f. sp. solani spore germination (a) and mycelial growth on PDA medium (b). Spores and plugs were obtained from five-day-old cultures. Vertical bars indicate±SD. Means with common letters are not significantly different at p<0.05 according to Duncan's multiple range test.
Influence of MeJA treatments on various physiological and biochemical characteristics of tomato plant
Seed germination and seedling emergence
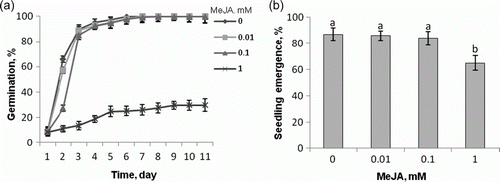
Since jasmonates are well-known inhibitors of seed germination and root growth and are senescence stimulators (Creelman and Mullet Citation1995; Wasternack Citation2007), it was deemed necessary to examine the influence of MeJA, applied in our experiment to prime the seeds or to gas the seedlings, on tomato seed germination and seedling emergence. As shown in , only at its highest concentration (1 mM) did MeJA inhibit tomato seed germination: it was reduced by 80% reduction, seedling emergence being reduced by about 30% (b). When applied at the same concentration, MeJA was reported to inhibit germination of seeds of Amaranthus caudatus (Kępczyński and Bialłecka Citation1994) and Xanthium pennsylvanicum (Nojavan-Asghari and Ishizawa Citation1998), the 5 mM concentration producing an inhibitory effects in seeds of Helianthus annuus (Corbineau et al. Citation1988). Somatic embryos, devoid of the seed coat, proved more sensitive to MeJA than seeds: the hormone, when applied to Medicago sativa, showed inhibitory effects with respect to somatic embryo germination and conversion to seedlings already at 0.5 mM (Ruduś et al. Citation2006). Thus, it is clearly evident that plants differ in their sensitivity to MeJA treatment.
Figure 2. Dynamics of seed germination (a) and seedling emergence (b) of Lycopersicon esculentum Mill. cv. Beta after pretreatment of seeds by 60 min soaking in water or MeJA solutions. The seedling emergence was determined after 7 days after sewing. Vertical bars indicate±SD. Means with common letters are not significantly different at p<0.05 according to Duncan's multiple range test.
Contents of chlorophyll and reducing sugars
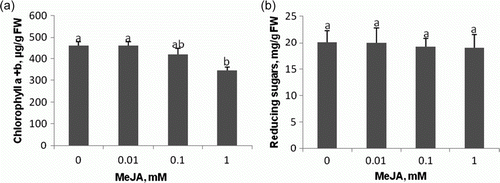
Senescence stimulation by jasmonates proceeds via, i.e., inhibition of photosynthesis (Creelman and Mullet Citation1995). The chlorophyll content in leaves of 15-day-old seedling, raised from seeds soaked for 60 min in 0.1 mM MeJA, was only slightly affected (a). However, a significant chlorophyll loss was observed in leaves of the treated with MeJA applied at 1 mM. Weidhase et al. (Citation1987) and Reinbothe et al. (Citation1994) showed that during MeJA induced defense in barley, proteolytic degradation of photosynthetic proteins, e.g. chlorophyll a/b-binding chloroplast proteins, leads to chlorophyll loss at MeJA concentrations exceeding 0.05 mM. Similarly, the Cucurbita pepo cotyledon treatments with 0.045 mM resulted in a decreased chlorophyll level, compared to the control (Ananiev et al. Citation2004). The genes involved in photosynthesis, such as ribulose coding bisphosphate carboxylase/oxygenase, chlorophyll a/b-binding protein and light-harvesting complex II were down regulated by a MeJA treatment (Cheong and Choi Citation2003). To check if the loss of chlorophylls is connected with changes in sugar levels, the contents of reduced sugars were determined (b). No change in the total reduced sugar content was observed. Similarly, JA had no effect on the levels of glucose and fructose in potato cells (Takahashi et al. Citation1995).
Figure 3. Chlorophyll (a) and reducing sugars (b) contents in leaves of 15-day-old seedling raised from seeds pretreated by 60 min soaking in water (control), or MeJA solutions. Vertical bars indicate±SD. Means with common letters are not significantly different at p<0.05 according to Duncan's multiple range test.
Ethylene production
Methyl jasmonate is known to stimulate ethylene production in many plants, e.g. tomato and Arabidopsis (reviewed by Zhao et al. Citation2005). Since our study involved two kinds of MeJA treatment, it was purposeful to find out if the hormone regulated ethylene production in our systems. The ethylene production ability of 15-day-old seedlings raised from seeds soaked for 60 min in MeJA solutions and the seedlings treated with gaseous MeJA 24 h before ethylene measurement was found to increase, compared to the control seedlings, depending on the elicitor concentration used (a). A rapid increase in ethylene emission was observed also after JA treatment of tomato leaves (O'Donnell et al. Citation1996). A treatment involving about 3 min immersion of tomato fruits in 0.1 mM MeJA solution resulted in increased ethylene production (Yu et al. Citation2009). However, MeJA at 0.05 mM concentration in the medium during callus growth, proliferating cell suspension, and development of somatic embryos during in vitro somatic embryogenesis of M. sativa had no effect on ethylene production (Kępczyńska et al. Citation2009). On the other hand, the hormone applied to germinating A. caudatus seeds at 0.1 and 1 mM concentrations inhibited their ethylene production markedly or almost completely, respectively (Kępczyński et al. Citation1999). Thus, the influence of exogenous jasmonates on ethylene production should be analyzed separately for each experiment.
MeJA effects on defense markers
Since many authors reported that production of phenol compounds, sometimes called phenolics, and PAL activity may serve as markers of induced plant resistance to diseases (Hahlbrock and Scheel Citation1989; Klarzyński et al. Citation2000), we addressed the question whether MeJA affects the content of phenolic compounds and activity of PAL, the main enzyme responsible for phenolics accumulation.
Total phenolics and anthocyanin content
Results in b show that there were significant differences in the total phenolic content between the control and the leaves both from seedlings grown from seeds soaked for 60 min in the MeJA solutions used and from the gaseous MeJA-treated seedlings. Soaking the seeds in MeJA at concentrations of 0.1 and 1 mM produced a stimulatory effect, although not too high, on the synthesis of phenolic compounds in leaves of 15-day-old seedlings; the two concentrations produced increases of about 12 and 20%, respectively. The same level of increase was observed after the treatment involving gaseous MeJA. Similar effects of the hormone on the level of phenolics were observed after postharvest exposure of plums to 250 ppm (1.5 mM) of MeJA for 4 days (Hereida and Cisneros-Zevallos Citation2009).
Figure 4. Ethylene production (a), total phenolics (b), anthocyanins (c) contents and PAL activity (d) in 15-day-old tomato seedlings raised from seeds pretreated by 60 min soaking in water (control), or MeJA solutions (▪) or 24 h gasified 14-day-old seedlings (□) Vertical bars indicate ±SD. Means with common letters are not significantly different at p<0.05 according to Duncan's multiple range test. Statistical analysis was carried out separately for soaking and fumigating.
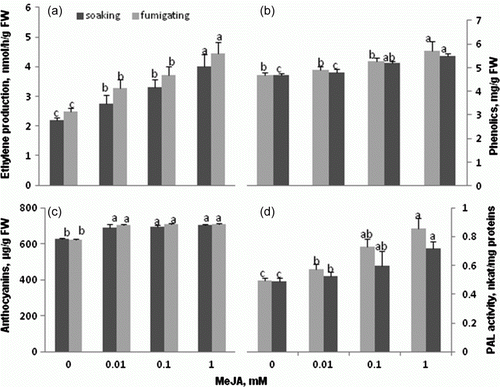
Likewise, the content of anthocyanins, polyphenol flavonoids, was found to increase in leaves of the 15-day-old tomato seedlings raised from seeds soaked for 60 min in MeJA solutions or gassed, regardless of MeJA concentration used (c). The observed increase of the tomato seedling anthocyanin content agrees with the results reported by Franceschi and Grimes (Citation1991) and Saniewski et al. (Citation2006) who noted induction of anthocyanin accumulation in light-grown soybean seedlings and in shoots of Crassula multicava treated with MeJA.
PAL activity
The observed increase in the total phenolic and anthocyanins contents, induced by MeJA treatments, corresponds to increased PAL activity (d). Activity of the enzyme, the major agent of the phenylpropanoid pathway, was enhanced both in leaves of the seedlings raised from seeds in soaked MeJA solutions and in leaves of the seedlings gassed for 24 h although the latter treatment was slightly more efficient in enhancing the enzyme activity. PAL activity has been previously shown to increase in MeJA-treated lettuce (Campos-Vargas and Saltveit Citation2002; Hereida and Cisneros-Zevallos 2009).
Disease development on inoculated plants pretreated with MeJA
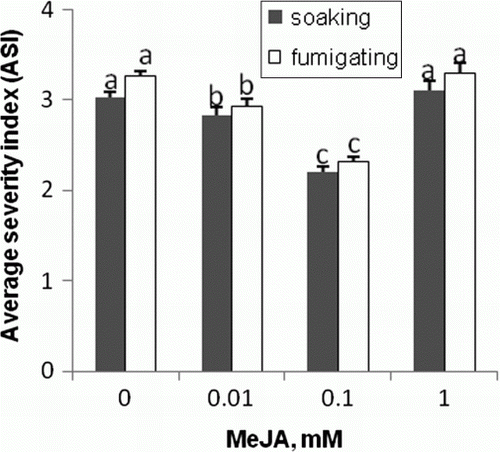
Having observed reactions of (1) the fungus to MeJA treatment in in vitro conditions and (2) tomato seedlings pretreated with the hormone, we followed the in vivo response of the fungus to MeJA (). MeJA pretreatment of seeds and seedlings resulted in efficient reduction of fungus development on the 15-day-old tomato seedlings inoculated with A. porri f. sp. solani applied as a spore suspension, the reduction was visible at MeJA concentration of 0.01 and 0.1 mM (). The highest concentration was not efficient in protecting the seedlings against the fungus. In Arabidopsis, pretreatment of 4-week-old plants with gaseous MeJA applied for 48 h resulted in efficient reduction of development of diseases caused by the necrotrophic fungi Alternaria brassicicola, Plectospharella cucumerina, and Botrytis cinerea, 150 nM being the most efficient concentration (Thomma et al. Citation2000). Seed treatment with 0.045 mM MeJA clearly protected melon seedlings against soil-borne Didymella bryoniae (Buzi et al. Citation2004). Differences in the efficiency between the MeJA doses are probably related to the plant species, duration of exposure, and plant age. In Arabidopsis, protection of plants effected by gaseous MeJA showed a dose-dependent increase up to 150 nM (Thomma et al. Citation2000), 0.1 mM being the concentration efficient in our study involving with tomato plants. The lack of the protective effect at a high concentration (1 mM) () confirmed the earlier findings of Thomma et al. (Citation2000) who used MeJA doses higher than 150 nM (0.003 and 0.006 mM). The reason for this reduced efficacy at higher MeJA doses is unclear. However, it may be related to the negative effect of high MeJA doses on physiological processes; seed germination, seedling emergence (), and chlorophyll content (a) decreased after treatment with 1 mM MeJA. Alternaria species are known to be particularly aggressive against weak plants (Agrios Citation2005). The observed lack of protection efficacy in the tomato plants primed with the highest MeJA dose (1 mM), with the accompanying increase in the levels of defense markers, might have been caused by increased ethylene production induced by the MeJA treatment. According to our earlier observations, ethylene was involved in MeJA-related reversal of the in vitro development inhibition of A. alternata (Kępczyńska and Kępczyński Citation2005); the hormone is an endogenous regulator of B. cinerea and A. alternata spore germination and mycelial growth (Kępczyńska Citation1989, Citation1993, Citation1994). Probably, priming the seeds with 1 mM MeJA enhances rather than inhibits the fungus development on the resultant seedlings.
Figure 5. Disease incidence noted on tomato plants raised from seeds pretreated by 60 min soaking in water and different concentrations of MeJA (▪) or 24 h gasified 14-day-old seedlings (□) four weeks after inoculation by A. porri f. sp. solani spore suspension. Twelve plants were evaluated for each replicate. Vertical bars indicate ±SD. Means with common letters are not significantly different at p<0.05 according to Duncan's multiple range test. Statistical analysis was carried out separately for soaking and fumigating.
It is interesting that the use of two different modes of application and timing of MeJA treatment produced similar defense-related biochemical (accumulation of total phenols and anthocyanins, PAL activity) and physiological (plant disease reduction) responses. It could be hypothesized that the lack of differences between treating the seed or seedlings with identical dosages of MeJA might be associated with saturation of all the available receptors of this inducer, which resulted in identical levels of defense markers in 15-day seedlings, responsible for a similar tomato seedling resistance against Alternaria. The results presented are in agreement with those reported by Thaler (Citation1999) who observed that spraying of tomato plants with JA or MeJA produced an increase in the level and activity of polyphenol oxidase (POP) three days after the treatment, and that the elevated activity was maintained for at least three weeks. Likewise, Shailasree et al. (Citation2001) showed the resistance induced in seeds of pearl millet with β-aminobutiric acid (BABA) to have remained operative during the vegetative and reproductive growth phases of the plants.
Fungitoxicity of leave extracts from MeJA-pretreated tomato seeds or seedlings
Since the highest MeJA concentration (1 mM) had a high inhibitory effect on the in vitro development of A. porri f. sp. solani (), but did not affect the process in vivo (), it was interesting to examine the effect of extracts from leaves of the seedlings after either of the MeJA treatments on the in vitro spore germination of the pathogen (). Depending on the MeJA concentrations used both for seed soaking or seedling gassing, inhibition of spore germination in the presence of extracts from 15-day-old seedling leaves was observed. Although the inhibitory effect of MeJA present in water on spore germination was stronger (a) than that observed in the presence of leave extracts (), these observation might suggest that MeJA – probably present in the leaves alone or with other compounds induced by the MeJA pretreatment – is responsible not only for inhibition of the in vitro spore germination, but probably also for inhibition of the in vivo fungus development. These results might also indicate that MeJA was capable of being transported from the place of its application, i.e., seeds, to the developing seedling leaves. According to data published by Thorpe et al. (Citation2007), the exogenously applied MeJA is transported through phloem and xylem.
Figure 6. The influence of extracts obtained from 15-day-old tomato seedlings raised from seeds pretreated by 60 min soaking in water and different concentrations of MeJA (a) or 24 h gasified 14-day-old seedlings (b) on A. porri f. sp. solani conidia germination. Vertical bars indicate±SD. Means with common letters are not significantly different at p<0.05 according to Duncan's multiple range test.
The presented results indicate that MeJA at 0.1 mM may be used as an activator of ISR against A. porri f. sp. solani, a soil-borne pathogen of tomato and possibly in combination with existing crop protection methods may serve for control above fungus. Although the contents of defense markers were highest after pretreatment with the highest concentration (1 mM), protection against the fungus was observed to lack in efficacy. It was probably a result of the plants being weakened by using the high MeJA dose, 1 mM, as evidenced by reduction in seed germination and seedling emergence rates, loss of chlorophyll, increase in ‘stress’ ethylene, since Alternaria diseases are known to be more prevalent on plants growing poorly because of some kind of stress.
Conclusion
On the basis of the results obtained we conclude that optimizing the MeJA dose and pretreatment timing is very important. Moreover, since the level of plant protection against the fungus after 4 weeks is, regardless of the MeJA treatment (seed soaking or seedling gassing), almost the same, seed soaking in MeJA solutions in a more effective and easier method than using MeJA as a gas.
The results of the present study may be useful for both the basic research on physiology, ecology and evolution of disease defense and the applied research addressing the development of tools for agricultural plant protection.
Acknowledgements
This work was supported by a grant from the Ministry of Science and Higher Education No. NN310107034. We are indebted to Dr Teresa Radziejewska for linguistic assistance.
References
- Agrios , G . 2005 . Plant pathology , 5th ed , 452 – 456 . San Diego , (CA) : Academic Press .
- Ananiev , ED , Ananieva , D and Todorov , I . 2004 . Effect of methyl ester of jasmonic acid, abscisic acid and benzyladenine on chlorophyll synthesis in excised cotyledons of Cucurbita pepo (zucchini) . Bulg J Plant Physiol. , 30 ( 1–2 ) : 51 – 63 .
- Buzi , A , Chilosi , G , De Sillo , D and Magro , P . 2004 . Induction of resistance in melon to Didymella bryoniae and Sclerotinia sclerotiorum by seed treatments with acibenzolar-S-methyl and methyl jasmonate but not with salicylic acid . J Plant Pathol. , 152 : 34 – 42 .
- Campos-Vargas , R and Saltveit , ME . 2002 . Involvement of putative chemical wound signals on the induction of phenolic metabolism in wounded lettuce . Physiol Plant. , 114 : 73 – 84 .
- Cheong , J.-J and Choi , Y . 2003 . Methyl jasmonate as a vital substance in plants . Trends Genet. , 19 ( 7 ) : 409 – 413 .
- Corbineau , F , Rudnicki , RM and Come , D . 1988 . The effect of methyl jasmonate on sunflower (Helianthus annuus L.) seed germination and seedling development . Plant Growth Regul. , 7 : 157 – 169 .
- Creelman , RC and Mullet , JE . 1995 . Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress . Proc Natl Acad Sci USA. , 92 : 114 – 119 .
- Edwards , R and Kessman , H . 1992 . editor. Molecular plant pathology. A practical approach , Edited by: Bowles , DJ, . 45 – 62 . Oxford : IRL Press .
- El-Khallal , SM . 2007 . Induction and modulation of resistance in tomato plants against Fusarium wilt disease by bioagent fungi (arbuscular mycorrhiza) and/or hormonal elicitors (jasmonic acid & salicylic acid): 2-changes in the antioxidant enzymes, phenolic compounds and pathogen related-proteins . Aust J Basic Appl Sci. , 1 ( 4 ) : 717 – 732 .
- Franceschi , VR and Grimes , HD . 1991 . Induction of soybean vegetative torage protein and anthocyanins by low-level atmospheric methyl jasmonate . Proc Natl Acad Sci USA. , 88 : 6745 – 6749 .
- Hahlbrock , K and Scheel , D . 1989 . Physiology and molecular biology of phenylpropanoid metabolism . Annu Rev Plant Physiol Plant Mol Biol. , 40 : 347 – 369 .
- Hereida , JB and Cisneros-Zevallos , L . 2009 . The effect of exogenous ethylene and methyl jasmoniate on the accumulation of phenolic antioxidants in selected whole and wounded fresh produce . Food Chem. , 115 : 1500 – 1508 .
- Jackab , G , Cottier , V , Toquin , V , Rigoli , G , Zimmerli , L , Metraux , JP and Mauch-Mani , B . 2001 . β-aminobutyric acid-induced resistance in plants . Eur J Plant Pathol. , 107 : 29 – 37 .
- Kępczyńska , E . 1989 . Ethylene requirement during germination of Botrytis cinerea spores . Physiol Plant , 77 : 369 – 372 .
- Kępczyńska , E . 1993 . Involvement of ethylene in the regulation and development of the fungus Botrytis cinerea Pers Ex Fr . Plant Growth Regul. , 13 : 65 – 69 .
- Kępczyńska , E . 1994 . Involvement of ethylene in spore germination and mycelial growth of Alternaria alternata . Mycol Res. , 98 : 118 – 120 .
- Kępczyński , J and Bialłecka , B . 1994 . Stimulatory effect of ethephon, ACC, gibberellin A3 and A4 + 7 on germination of methyl jasmonate inhibited Amaranthus caudatus L. seeds . Plant Growth Regul. , 14 : 211 – 216 .
- Kępczyński , J , Bialłecka , B and Kępczyńska , E . 1999 . Ethylene biosynthesis in Amaranthus caudatus seeds in response to methyl jasmonate . Plant Growth Regul. , 28 : 59 – 65 .
- Kępczyńska , E and Kępczyński , J . 2005 . Inhibitory effect of methyl jasmonate on development of phythopathogen Alternaria alternata (Fr.) Keissl and its reversal by ethephon and ACC . Acta Physiol Plant , 27 ( 4A ) : 491 – 496 .
- Kępczyńska , E , Ruduś , I and Kępczyński , J . 2009 . Abscisic acid and methyl jasmonate as regulator of ethylene biosynthesis during somatic embryogenesis of Medicago sativa L . Acta Physiol Plant. , 31 : 1263 – 1270 .
- Klarzynski , O , Plesse , B , Joubert , JM , Yvin , JC , Knopp , M , Kloareg , B and Fritig , B . 2000 . Linear β-1,3-glucans are elicitors of defense responses in tobacco . Plant Physiol , 123 : 1027 – 1037 .
- Lever , M . 1972 . A new reaction for colorimetric determination of carbohydrates . Anal Biochem , 47 : 273 – 279 .
- Lichthentaler , HK and Wellburn , AR . 1985 . Determination of total carotenoids and chlorophylls A and B of leaf in different solvents . Biol Soc Trans. , 11 : 591 – 592 .
- Mancinelli , AL . 1984 . Photoregulation of anthocyanin synthesis . Plant Physiol. , 75 : 447 – 453 .
- Nojavan-Asghari , M and Ishizawa , K . 1998 . Inhibitory effect of methyl jasmonate on the germination and ethylene production in cocklebur seeds . J Plant Growth Regul. , 17 : 13 – 18 .
- O'Donnell , PJ , Calvert , C , Atzorn , R , Wasternack , C , Leyer , HMO and Bowles , DJ . 1996 . Ethylene as a signal mediating the wound response of tomato plants . Science. , 274 : 1914 – 1917 .
- Pozo , MJ , Van Loon , LC and Pieterse , CMJ . 2005 . Jasmonates – signals in plant – microbe interactions . J Plant Growth Regul. , 23 : 211 – 222 .
- Reinbothe , S , Mollenhauer , B and Reinbothe , CH . 1994 . JIPs and RIPs: the regulation of plant gene expression by jasmonates in response to environmental cues and pathogens . Plant Cell. , 6 : 1197 – 1209 .
- Ruduś , I , Kępczyńska , E and Kępczyński , J . 2006 . Comparative efficacy of abscisic acid and methyl jasmonate for indirect somatic embryogenesis in Medicago sativa L . Plant Growth Regul. , 48 : 1 – 11 .
- Saniewski , M , Horbowicz , M and Puchalski , J . 2006 . Induction of anthocyanins accumulation by methyl jasmonate in shoots of Crassula multicava Lam . Acta Agrob. , 59 : 43 – 50 .
- Shailasree , S , Sarosh , BR , Vasanthi , NS and Shetty , HS . 2001 . Seed treatment with β-aminobutyric acid protects Pennisetum glaucum systemically from Sclerospora graminicola . Pest Manag Sci. , 57 : 721 – 728 .
- Singleton , VL and Rossi , JA . 1965 . Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents . Am J Enol Viticult. , 16 : 144 – 158 .
- Takahashi , K , Fujino , K , Kikuta , Y and Koda , Y . 1995 . Involvement of the accumulation of sucrose and the synthesis of cell wall polysaccharides in the expansion of potato cells in response to jasmonic acid . Plant Sci. , 111 : 11 – 18 .
- Thaler , JS . 1999 . “ Jasmonic acid mediated interactions between plants, herbivores, parasitoids, and pathogens: a review of field experiments in tomato ” . In Inducible plant defenses against pathogens and herbivores: biochemistry, ecology, and agriculture , Edited by: Agrawal , AA , Tuzun , S and Bent , E . 319 – 334 . St. Paul , (MN) : American Phytopathological Society Press .
- Thomma , BPHJ , Eggermont , K , Broekaert , WF and Cammue , BPA . 2000 . Disease development of several fungi on Arabidopsis can be reduced by treatment with methyl jasmonate . Plant Physiol Biochem , 38 ( 5 ) : 421 – 427 .
- Thomma , BPHJ , Eggermont , K , Penninckx , IAMA , Mauch-Mani , B , Vogelsang , R , Cammue , BPA and Broekaert , WF . 1998 . Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens . Proc Natl Acad Sci USA. , 95 : 15107 – 15111 .
- Thorpe , MR , Ferrieri , AP , Herth , MM and Ferrieri , RA . 2007 . C-imaging: methyl jasmonate moves in both phloem and xylem, promotes transport of jasmonate and photoasymilate even after proton transport is decoupled . Planta. , 226 : 541 – 551 .
- Thulke , O and Conrath , U . 1998 . Salicylic acid has a dual role in the activation of defense-related genes in parsley . Plant J. , 14 : 35 – 42 .
- Ton , J and Mauch-Mani , B . 2004 . β-aminobutyric acid induced resistance against necrotrophic pathogen based on ABA-dependent priming for callose . Plant J. , 38 : 119 – 130 .
- Van Loon , LC , Rep , M and Pieterse , CMJ . 2006 . Significance of inducible defense-related proteins in infected plants . Annu Rev Phytopath. , 44 : 135 – 162 .
- Wasternack , C . 2007 . Jasmonates: an update on biosynthesis, signal transduction and action in plant response, growth and development . Ann Bot. , 1 : 1 – 17 .
- Weidhase , RA , Kraell , HM , Lehmann , J , Liebissch , HW , Lerbs , W and Parthier , B . 1987 . Methyl jasmonate-induced changes in polypeptide pattern of senescing barley leaf segments . Plant Sci. , 51 : 177 – 186 .
- Yu , M , Shen , L , Fan , B , Zhao , D , Zheng , Y and Sheng , J . 2009 . The effect of MeJA on ethylene biosynthesis and induced disease resistance to Botrytis cinerea in tomato . Posthar Biol Technol. , 54 : 153 – 158 .
- Zhao , J , Davis , LC and Verpoorte , R . 2005 . Elicitor signal transduction leading to production of plant secondary metabolites . Biotech Adv. , 23 : 283 – 333 .