Abstract
Helicoverpa armigera is a polyphagous pest damaging vast numbers of different crops leading to decrease in total production. Use of Bt transgenic to control H. armigera has worked well but has increased resistance against Bt in H. armigera and controversies about the Bt transgenic making it imperative to find another strategy to control attack. Soybean is a nonhost plant for H. armigera; reason could be laid in the defense system of the soybean. Proteinase Inhibitor (PIs) have been extensively studied for development of resistance against insect pest. Two cultivars developed by our university were investigated for the presence of proteinase inhibitors namely, MAUS-158 and MAUS-61. Partially purified inhibitors were showed inhibition of total protease activity of gut extract by 91.34±1.49 and 89.95±0.96% by MAUS-158 and MAUS-61, respectively. While inhibition of trypsin like proteases were found between 65 and 71% and inhibition of chymotrypsin like proteases ranges between 40 and 42%. The partial purification study shows stability of PIs up to 60°C. Soybean PIs are also showing more prominent inhibition pattern against trypsin than chymotrypsin.
Introduction
Every organism has evolved some effective counter measures to their enemies. Plants have also evolved to cope with predatory insects. Exploiting these peculiarities will definitely make some difference in the present status of the insect pest resistance management. Plants produce different insect control proteins (ICPs) to protect themselves from their enemies. There are number of examples of the ICPs like serine protease inhibitors, thiol protease inhibitors, amylase inhibitors, lectins, enzymes, etc. (Hilder and Boulter Citation1999). Plant-derived ICPs, attack different targets to synthetic chemicals and may be used in combination with them. Many worthwhile Integrated Pest Management (IPM) practices, such as the use of short-season varieties, partial resistant varieties and conservation of predators, are aimed at preventing the buildup of pest populations to catastrophic levels rather than the total elimination of the pest (Hilder and Boulter Citation1999).
Present study was conducted to evaluate the in vitro analysis of the soybean protease inhibitors against the Helicoverpa gut proteases (HGPs) from local soybean cultivars. Disruption of a pest's essential amino-acid metabolism by inhibition of protein digestion has been a key target, reviewed by Hilder et al. (Citation1992). Many insects particularly those belonging to the order Lepidoptera depend on serine proteases (trypsin, chymotrypsin, and elastase) as a primary protein digestive enzyme. Transgenic expressing these proteinase inhibitors have shown promising results against different economically important pests. Transgenic expressing Cowpea Trypsin Inhibitor gene have shown promising effect against tobacco budworm (Heliothis virescens) leads to increased mortality, reduced growth, and reduced plant damage (Hilder et al. Citation1987). Similar results were obtained against corn earworm (Helicoverpa zea) in US (Hoffmann et al. Citation1992). Transgenic study of soybean Kunitz trypsin inhibitors (TIs) in tobacco have show promising results against H. virescence (Lepidoptera) (Gatehouse et al. Citation1993). Transgenic study of PIs from other plants has also done, for example, (1) potato PI-2 expressed in tobacco (McManus et al. Citation1994; Jongsma et al. Citation1995) and in rice (Duan et al. Citation1996) which were investigated for deleterious effects on lepidopterans Manduca sexta (Johnson et al. Citation1989), Spodoptera litura (McManus et al. Citation1994), Spodoptera exigua (Jongsma et al. Citation1995), and Sesamia inferens (Duan et al. Citation1996); (2) oryzacystatin I from rice expressed in tomato (Urwin et al. Citation1995), poplar (Leple et al. Citation1995), potato (Cloutier et al. Citation1999, Citation2000), and oilseed rape (Girard et al. Citation1998; Bonade-Bottino et al. Citation1999) for effect against Coleopteran pests.
Helicoverpa armigera is a polyphagous pest damaging vast numbers of different crops leading to decrease in total production. Use of Bt transgenic to control H. armigera has worked well but has increased resistance against Bt in H. armigera (Barton et al. Citation1987; reviewed by Brzin and Kidric Citation1995; Brousseau et al. Citation1999; Frutos et al. Citation1999; Kranthiet al. Citation2000) and controversies about the Bt transgenic making it imperative to find another strategy to control attack. Chemical pesticides used to control H. armigera infestation have become increasingly less feasible mainly because of development of pesticide resistance in the insect population and also due to their serious threat to normal ecosystems (Armes et al. Citation1996).
Extensive cultivation of Bt-cotton can impose a continuous and intense selection pressure on bollworms leading to the development of resistance to the toxin (Kumar Citation2004). A study was carried out during 2001–2007 to monitor the variability in susceptibility of cotton bollworm, to CrylAc toxin in populations collected from 53 cotton growing districts of India (Mayee Citation2009). The study indicating a decrease in the proportion of susceptible populations warrants judicious implementation of insect resistance management strategies such as refugia, gene stacking, high toxin dosage, and integrated pest management.
Chickpea, the host plant for H. armigera, produces seven isoforms of TIs, but HGPs degraded these TIs (Giri et al. Citation1998). Several cultivars along with wild relatives of chickpea showed only 35% inhibition of HGP (Patankar et al. Citation1999). It suggests that the source of host plant PIs are ineffective against HGP.
Soybean is a nonhost plant for H. armigera; reason could be laid in the defense system of the soybean. PIs have been extensively studied for development of resistance against insect pest (Jouanin et al. Citation1998; Schuler et al. Citation1998).
Materials and methods
Two local soybean cultivars developed by Marathwada Agriculture University, Parbhani, India, were selected for the study namely, MAUS-61 and MAUS-158.
Chemicals used in study were supplied by Himedia®, Sisco Research Laboratory and N-a-benzoyl-dl-arginine-p-nitroanilide (BApNA) and succinylalanyl-alanyl-prolyl-phenylalanyl-p-nitroanilide (SAALpNA) from Fisher Scientific®, and protein molecular weight marker were procured from Genei® and Azocasein from Sigma®.
Extraction of soybean PIs
Seeds were crushed in blender and seed powder was defatted and depigmented by several washes with hexane–acetone. Defatted, dried seed powder was mixed with 10 volume of extraction buffer (0.01 M Tris–Cl, 2.6 mM EDTA, 1% PVP, pH 6.8) and incubated overnight. The suspension was centrifuged at 10,000 rpm for 35 min. The supernatant was collected and stored at 4°C. The protein concentration of the soybean seed extracts was estimated by Bradford's method, where bovine serum albumin was used as a standard (Bradford Citation1976). Saturated ammonium sulfate solution was added to the supernatant (crude extract) to obtain a precipitate formed at 0–60 and 60–90% saturation with respect to this salt. The pellet was collected from all fractions [F1 (0–60%) and F2 (60–90%)]. The pellet was dissolved in minimal volume of extraction buffer and dialyzed overnight with the same extraction buffer (Oliveira et al. Citation2002). At each concentration, the proteinase inhibitory activity and protein content were estimated. The F1 fraction, which corresponds to a 0–60% saturation range, showed a high level of inhibitory activity against trypsin. All the steps were performed at 4°C.
Extraction of HGPs
Third instar larvae of H. armigera were collected from the Division of Crop Protection, Central Institute of Cotton Research, Nagpur. Larvae were dissected to isolate gut tissue, which was immediately stored at –20°C. The midgut tissue was homogenized and mixed with extraction buffer (0.1 M Glycine, 0.1 M NaOH, pH 10.0). The mixture was stored for 30 min. The gut luminal content was removed by centrifugation at 10,000 rpm for 10 min. The resulting supernatant was analyzed for protease activity in assays and resolved on acrylamide gel and stored at –20°C. (Harsulkar et al. Citation1998).
Proteinase and PI assay
TI, HGP, and Helicoverpa gut protease inhibitor (HGPI) activity were measured using azocaseinolytic assays (Brock et al. Citation1982) as reported earlier (Tamhne et al. Citation2005). For azocaseinolytic assay, 60 ml of diluted enzyme was added to 200 ml of 1% azocasein (in 0.2 M glycine–NaOH, pH 10.0) and incubated at 37°C for 30 min. The reaction was terminated by the addition of 300 ml of 5% trichloroacetic acid. After centrifuging at 12,000 rpm for 10 min, an equal volume of 1 M NaOH was added to the supernatant, and absorbance was measured at 450 nm. One proteinase unit was defined as the amount of enzyme that increases absorbance by 1 OD under the given assay conditions. For the inhibitor assays, a suitable volume of seed extract was added to the gut proteinase extract and incubated at room temperature (27°C) for 15 min. The residual proteinase activity was then estimated for every assay, suitable controls like sample containing only proteinase inhibitor sample with substrate to estimate the activity of protease in sample and sample containing only HGP and substrate to estimate total gut protease activity.
Determination of LC50 value
The LC50 was determined by the probit analysis in the program ED50plus v1.0.
Visualization of isoforms of PIs and HGPs
A discontinuous buffer system of sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), using a 5% stacking gel and a 10% resolving gel, was done by the method described by Laemmli (Citation1970). Bromophenol blue was used as tracking dye.
Samples for SDS–PAGE were prepared in non denaturing condition. Electrophoresis was carried out at constant voltage of 150 V at 25°C. On completion of run, gel was removed and the SDS was thoroughly washed in 2.5% Triton X-100 dissolved in 0.2 M glycine–NaOH buffer (pH 10.0) or 200 mM Tris–Cl (pH 7.8). Gel was shaken gently at 50 rpm in 0.2 M glycine–NaOH buffer (pH 10.0) or 200 mM Tris–Cl (pH 7.8). To detect trypsin inhibitor isoforms, gel was incubated in 0.1% trypsin and HGP of equal activity, respectively, for 30 min. The gel was washed to remove excess trypsin or HGP and placed on exposed mammographic film (one side gelatin coated) and incubated at 37°C for 15–30 min. The gel was removed and washed gently to remove hydrolyzed gelatin. TI and HGPI activity bands were visible as unhydrolyzed gelatin. The film was then developed and contact printed. For zymography, gel was prepared with 1% gelatin, and normal procedure was carried out as above. The gel was stained instead of incubating with mammographic film.
Detection of tryptic activity in the gel was performed using an overlay technique according to Vinokurov et al. (Citation2005). After electrophoresis, the gel was soaked for 15 min in buffer (50 mM Tris–HCl, pH 8.0). Then gel was covered by a nitrocellulose membrane that had presoaked for 40 min in the substrate (BApNA or SAALpNA). The gel and membrane were incubated at 37°C. The membrane was then removed and the librated p-nitroaniline was diazotized by subsequent incubations of 5 min each in 0.1% sodium nitrite, 0.5% ammonium sulfamate, and 0.05% N-(1-naphthyl) ethylenediamine. After pink bands of tryptic and chymotryptic activity formed, membranes were scanned.
Results
Inhibitor isolation and partial purification
Crude soluble protein extract obtained from the mature Soybean (Glycine max) seeds was initially precipitated at 60 and 90% saturation with ammonium sulfate and two protein fractions (F1 and F2) were obtained. The fractions were then subjected to desalting with dialysis. The F1 fraction protein showed strong inhibitory activity against trypsin, while the F2 fraction exhibited low trypsin inhibitory activity. Both fractions were subjected to SDS–PAGE to visualize the trypsin inhibitory activity ( and ).
Electrophoresis analysis
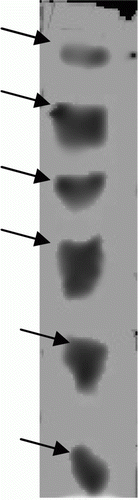
Total seven isoforms were detected in crude HGP extract (). Electrophoretic analysis of ammonium sulfate fractions on 10% SDS–PAGE resolved into protein bands ranging from ±86 kDa to more than ±120 kDa. There were four isoforms inhibiting trypsin activity () and two isoforms inhibiting chymotrypsin activity (). In case of HGP inhibition also, there were four isoforms detected ().
Protease activity of HGP
Trypsin-like activity of the crude gut extract was estimated with the trypsin-specific synthetic substrate. The HGP extract shows the specific activity of 2.1 U/mg of proteins present in the HGP extract. The presence of proteases is also checked with the reverse zymography.
Effects of soybean PIs on serine proteinases and total proteolytic activity
To assess potential effects of soybean (Glycine max), proteinase inhibitors on the digestive proteinases of H. armigera larvae, the midguts of third instar larvae was dissected. By using synthetic substrates (BApNA, SAALpNA, and azocasein), the presence of serine proteinases (trypsin) were detected in midgut extracts of H. armigera. Trypsin was assayed with specific substrate, BApNA. This substrate is specific for the determination of trypsin activity (Ahmad et al. Citation1980; Christeller et al. Citation1992; Johnston et al. Citation1995). The evidence for chymotrypsin-like activity in the alimentary tracts of Lepidoptera is less clear than that for trypsin-like activity. However, chymotrypsin-like activity was found in H. armigera by using SAALpNA as the substrate in this study. Inhibitory assays using soybean PIs showed high inhibitory activity toward trypsin followed by total gut proteolytic enzymes and chymotrypsin activity ().
High inhibitory activity toward standard trypsin than standard chymotrypsin was found (). It shows the soybean contains PIs that are more effective against trypsin than chymotrypsin. The inhibition of trypsin was almost two fold as compared to the chymotrypsin (). Partially purified F1 fractions of soybean PIs shows highest inhibition than any other fraction and was carried further to analyze the inhibitory activity against HGP. The response of the MAUS 158 toward the inhibition of trypsin and chymotrypsin was slightly higher than MAUS 61 in almost all the fractions studied.
Table 1. Percent inhibition of trypsin and chymotrypsin activity.
HGPI activity of soybean cultivar
HGPI activity of soybean PIs showed especially high inhibitory activity toward total gut proteolytic enzymes followed by trypsin and chymotrypsin (). This could be explained as the inhibitor being able to inhibit proteinases, other than those, which possess trypsin-like activity (Babu and Subrahmanyam Citation2010). This higher inhibitory activity toward general proteolysis would give more pronounced effects on larval growth and physiology (Srinivasan et al. Citation2005). In case of HGP inhibition, the varietal performance of the MAUS 158 is slightly more than MAUS 61.
Table 2. Percent inhibition of HGP by crude and F1 fraction.
Specific activity and the yield performance of soybean PIs were shown in . During each step of the partial purification the specific activity of PIs increased with the decrease in its total yield. The heat inactivated sample of F1 fraction shows highest inhibitory activity. Partial purification of the soybean PIs from both the cultivars yielded average 50% of partially purified PIs showing highest specific activity in the range of 0.59–0.82. The total PI units recovered from the sample were also higher in the MAS 158 as compared to the MAUS 61.
Table 3. Specific activity and yield of proteinase inhibitors after each step of partial purification.
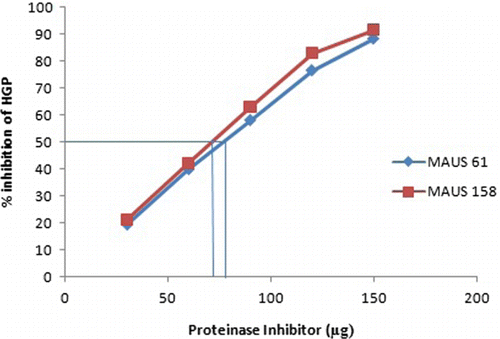
Both the varieties were checked for the HGPI activity. The comparative inhibition pattern was same for both varieties. Calculated IC50 value for MAUS 158 is 66.28 µg and for MAUS 61 it is 69.06 µg ().
Electrophoretic profile of HGP and TI isoforms
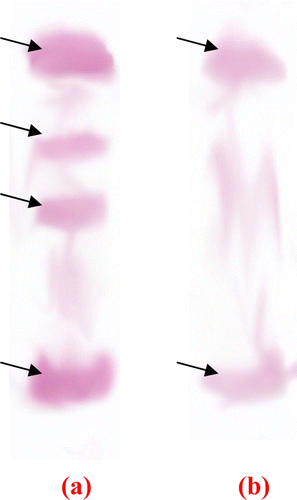
shows the isoforms of HGP. Total seven isoforms were found in the HGP extract. Presence of specific trypsin like and chymotrypsin like proteases were detected by overlay technique. a and b shows presence of specific trypsin and chymotrypsin protease respectively. There are total four isoforms of trypsin and two isoforms of chymotrypsin were detected.
Figure 3. Isoforms of specific trypsin and chymotrypsin proteases using overlay technique. (a) Trypsin like proteases (using BApNA substrate) and (b) chymotrypsin like proteases (using SAALpNA substrate).
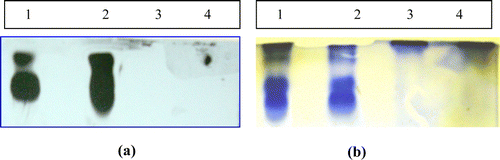
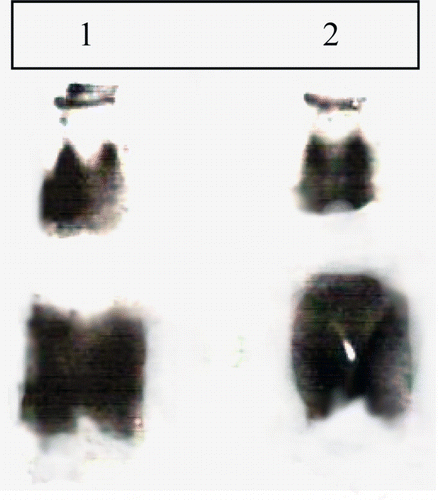
When incubated with partially purified soybean PI, it has been found that two of seven isoforms were not inhibited. This explains why 100% inhibition was not achieved in PI-HGP reaction. a and b depicts the electrophoretic patterns of TIs in different ammonium sulfate precipitated fractions. F1 fraction shows two high potential isoforms of TI (a). Also in case of F1 fraction, some low molecular weight bands were not consistent in case of gel–X-ray film contact technique (a). But those bands were clearly seen in zymography analysis of the same samples (b). Equal quantity of protein (30 µg) was loaded in each lane. There were maximum four isoforms found in the F1 fraction of PI from both the cultivars. F2 fraction shows single band at the top showing high molecular weight PI, not clearly seen in a.
Figure 4. (a) Gel–X-ray film contact technique and (b) zymography. Lanes 1 and 3 corresponds to the F1 and F2 saturated fraction of soybean cultivar MAUS-158. Lanes 2 and 4 corresponds to the F1 and F2 saturated fraction of soybean cultivar MAUS-61.
Zymography analysis of the chymotrypsin PIs shows less number of isoforms as compared to the trypsin inhibiting isoforms () clearly shows the partial purification of PIs with increase in the band intensity in the F1 fraction than the crude sample. Highest chymotrypsin inhibitory activity was found in F1 fraction showing two isoforms.
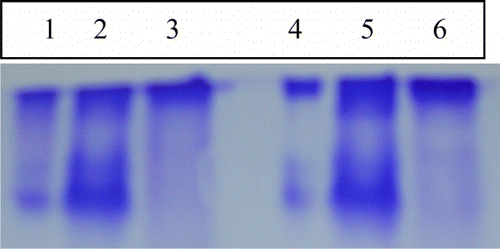
Electrophoretic visualization of HGPI isoforms
The gel–X-ray contact analyzed samples of the MAUS-158 and MAUS-61 shows average six isoforms of PI acting against HGP. The band of HGPI appeared almost at the same position as in the TI isoforms. No band appeared in the F2 fraction in any of the cultivar. There was very less HGP inhibitory activity in the F2 fraction. shows the isoforms inhibiting HGP activity.
Discussion
Improvement in genetic makeup of the crop plant is a dynamic process, naturally as well as artificially. It demands the periodic checking of the cultivars to access its inherent strength (Pandey and Khush Citation1995; Lewis et al. Citation1997). Present study is an attempt to look potential of soybean PI found in the local cultivar.
Trypsin and serine proteases are key enzymes in the digestive system of the lepidopterian insects showing about 95% of the total digestive activity (Srinivasan et al. Citation2006; Liu et al. Citation2009). Inhibiting the insect digestive proteases will lead to the strong physiological stress on the insect for the availability of the essential amino acids. This will lead to the considerable weight loss and reduction in reproductive strength of the insect. H. armigera is a polyphagous pest with after 180 reported hosts causing significant economical loss of agricultural products (Manjunath et al. Citation1989).
Action of the proteinase inhibitors has been well studied. Previous study on the response of soybean PIs against HGP shows moderate response of about 70% inhibition (Parde et al. Citation2010). Host PIs are ineffective against insect pest and does not show faint response against insect protease (Broadway Citation1995, Citation1996, Citation1997; Jongsma et al. Citation1995). Even insect proteinases can inactivate the host PIs (Michaud Citation1997; Girard et al. Citation1998; Giri et al. Citation1998). Several nonhost plants of H. armigera were found to have good source of PIs (Harsulkar et al. Citation1999; Parde et al. Citation2010) as new sources of potent PIs.
In present study, soybean cultivars namely, MAUS 158 and MAUS 61 were checked for its strength against the HGP. Both the cultivars showed percent inhibition of HGP up to 90% in different cases. The total activity of HGP is inhibited more than activity of trypsin and chymotrypsin. It shows there are different protease inhibitors present in soybean contributing the inhibition. Inhibitors are also prone to action of certain proteases. Insects can digest PIs by altering their genetic makeup by the process of adaptation. They can secrete the proteases that can digest the PI molecules (Michaud Citation1997; Girard et al. Citation1998; Giri and Kachole Citation1998; Giri et al. Citation1998). This situation demands high tolerant PI or the effective approach to compensate the mechanism of acquired resistance (Harsulkar et al. Citation1999). In further study of transgenic, use of combination of different PI genes rather using single PI gene for insect control will definitely reduce the chances of acquired resistance in insects. Highest inhibition of trypsin-like enzymes in HGP extracts shows predominance of trypsin in the digestive mechanism of the H. armigera.
Plant PIs are involved in the plants defense system (Haq et al. Citation2004). PIs may also function as storage proteins (Mosolov et al. Citation2001; Birk Citation2003; Shewry Citation2003). This assures the enough production ability of PI. PIs are also much stable than any other protein in the plant system. They are stable even at 60°C. The activity of the PI increased during each step of the partial purification. Highest activity of PI found in F1 fraction subjected to heat treatment. It could be explained as the inactivation or degradation of proteins/enzymes present in the sample interfering with the activity of the PI. As the specific activity increased, the yield and total protein content of the sample decreased showing the efficiency of the purification step. This property of PI promises the efficiency in today's climate-changing days. Secretion of the PIs increases as plants rich maturity. Study shows that the TI activity was highest at 60 days after flowering (DAF) in verities of chickpea (Harsulkaret al. 1997). Present technology for insect control (Bt) promises protection of plant for near about 120 days after sowing. Then the expression of the Bt decreases with time. Fusion technology of PI with Bt may promises the protection of plant throughout its life.
Plants secrete number of PIs in response to the attack of enemies. Each PI has different specificity. Difference in the PIs can be visualized with techniques like polyacrylamide gel electrophoresis (PAGE) (Laemmli Citation1970). Performing PAGE with slight modification like addition of gelatin or casein will allow visualizing the PIs isoforms easily (Liota and Stetler-stevenson Citation1990). In present study, there are maximum four and minimum two isoforms inhibiting trypsin action were detected with zymography as well as Gel-X-ray film contact technique, respectively. (Pichare and Kachole Citation1994; Mulimani et al. Citation2002). Both the techniques were found more or less equally effective to study the PI. To study detail diversity of PI 2D gel electrophoresis is must which will help to reveal specificity of PI in-depth.
Detection of such inhibitory activity from different plant species may lead to the discovery of effective inhibitory compound against pests. That will help us to control the insect attack on the crop and reduces the economical loss without disturbing ecological balance.
Acknowledgements
The authors are thankful to Dr K.R. Kranthi, Director, C.I.C.R., Nagpur and Dr Sandhya Kranthi, HOD, Crop Protection Division, C.I.C.R., Nagpur for the providing with H. armigera culture.
References
- Ahmad , Z , Saleemuddin , M and Siddi , M . 1980 . Purification and characterization of three alkaline proteases from the gut of the larva of army worm, Spodoptera litura . Insect Biochem. , 10 : 667 – 673 .
- Armes , NJ , Jadhav , DR and DeSouza , KR . 1996 . A survey of insecticide resistance in Helicoverpa armigera in the Indian subcontinent . Bull Entomol Res. , 86 : 499 – 514 .
- Babu , SR and Subrahmanyam , B . 2010 . Bio-potency of serine proteinase inhibitors from Acacia senegal seeds on digestive proteinases, larval growth and development of Helicoverpa armigera (Hübner) . Pest Biochem Physiol. , 98 : 349 – 358 .
- Barton , K , Whitely , H and Yang , NS . 1987 . Bacillus thuringiensis δ-endotoxin in transgenic Nicotiana tabacum provides resistance to lepidopteran insects . Plant Physiol. , 85 : 1103 – 1109 .
- Birk , Y . 2003 . Plant protease inhibitors: significance in nutrition, plant protection, cancer prevention and genetic engineering , 170 Berlin : Springer .
- Bonade-Bottino , M , Lerin , J , Zaccomer , B and Jouanin , L . 1999 . Physiological adaptation explains the insensitivity of Baris coerulescens to transgenic oilseed rape expressing oryzacystatin I . Insect Biochem Mol Biol. , 29 : 131 – 138 .
- Bradford , MM . 1976 . A rapid and sensitive method for the quantitation of microgram of quantities of protein utilizing the principal of protein-dye binding . Anal Biochem. , 72 : 248 – 254 .
- Broadway , RM . 1995 . Are insects resistant to plant proteinase inhibitors? . J Insect Physiol. , 41 : 107 – 116 .
- Broadway , RM . 1996 . Dietary proteinase inhibitors alter complement of midgut proteases . Arch Insect Biochem Physiol. , 32 : 39 – 53 .
- Broadway , RM . 1997 . Dietary regulation of serine proteinases that are resistant to serine proteinase inhibitors . J Insect Physiol. , 43 : 855 – 874 .
- Brock , RM , Forsberg , CW and Buchanan-Smith , JG . 1982 . Proteolytic activity of rumen microorganisms and effect of proteinase inhibitors . Appl Environ Microbiol. , 44 : 561 – 569 .
- Brousseau R , Masson L , Hegedus D. 1999 . Insecticidal transgenic plants: are they irresistible? Review. AgBiotechNet July, ABN022 CAB International , UK , 22 .
- Brzin , J and Kidric , M . 1995 . Proteinases and their inhibitors in plants: role in normal growth and in response to various stress conditions . Biotechnol Genet Eng Rev. , 13 : 421 – 467 .
- Christeller , JT , Laing , WA , Markwick , NP and Burgess , EP . 1992 . Midgut protease activities in 12 phytophagous lepidopteran larvae: dietary and protease inhibitor interactions . Insect Biochem Mol Biol. , 22 ( 7 ) : 735 – 746 .
- Cloutier , C , Fournier , M , Jean , C , Yelle , S and Michaud , D . 1999 . Growth compensation and faster development of Colorado potato beetle (Coleoptera: Chrysomelidae) feeding on potato foliage expressing oryzacystatin I . Arch Insect Biochem Physiol. , 40 : 69 – 79 .
- Cloutier , C , Jean , C , Fournier , M , Yelle , S and Michaud , D . 2000 . Adult Colorado potato beetles, Leptinotarsa decemlineata compensate for nutritional stress on oryzacystatin I-transgenic potato plants by hypertrophic behaviour and overproduction of insensitive proteases . Arch Insect Biochem Physiol. , 44 : 69 – 81 .
- Duan , X , Li , X , Xue , Q , Abo-el-saad , M , Xu , D and Wu , R . 1996 . Transgenic rice plants harbouring an introduced potato inhibitor II gene are insect resistant . Nat Biotechnol. , 14 : 494 – 498 .
- Frutos , R , Rang , C and Royer , M . 1999 . Managing insect resistance to plants producing Bacillus thuringiensis toxins . Crit Rev Biotechnol. , 19 : 227 – 276 .
- Gatehouse , AMR , Shi , Y , Powell , KS , Brough , C , Hilder , VA , Hamilton , WDO , Newell , CA , Merryweather , A , Boulter , D and Gatehouse , JA . 1993 . Approaches to insect resistance using transgenic plants . Phil Trans R Soc Lond. , B342 : 279 – 286 .
- Girard , C , Le Metayer , M , Bonade-Bottino , M , Pham-Delegue , MH and Jouanin , L . 1998 . High level of resistance to proteinase inhibitors may be conferred by proteolytic cleavage in beetle larvae . Insect Biochem Mol Biol. , 28 : 229 – 237 .
- Giri , AP , Harsulkar , AM , Deshpande , VV , Sainani , MN , Gupta , VS and Ranjekar , PK . 1998 . Chickpea defensive proteinase inhibitors can be inactivated by podborer gut proteinases . Plant Physiol. , 116 : 393 – 401 .
- Giri , AP and Kachole , MS . 1998 . Amylase inhibitors of pigeonpea (Cajanus cajan L.) seeds . Phytochemistry. , 47 : 197 – 202 .
- Haq , SK , Atif , SM and Khan , RH . 2004 . Protein proteinase inhibitor genes in combat against insects, pests, and pathogens: natural and engineered phytoprotection . Arch Biochem Biophys. , 431 : 145 – 159 .
- Harsulkar , AM , Giri , AP , Gupta , VS , Sainani , MN , Deshpande , VV , Patankar , AG and Ranjekar , PK . 1998 . Characterization of Helicoverpa armigera gut proteinases and their interaction with proteinase inhibitors using gel–X-ray film contact print technique . Electrophoresis. , 19 : 1397 – 1402 .
- Harsulkar , AM , Giri , AP , Patankar , AG , Gupta , VS , Sainani , MN , Ranjekar , PK and Deshpande , VV . 1999 . Successive use of non host Plant proteinase inhibitors Required for effective inhibition of Helicovrpa armigera gut proteinases and larval growth . Plant Physiol. , 121 : 497 – 506 .
- Hilder , VA and Boulter , D . 1999 . Genetic engineering of crop plants for insect resistance – a critical review . Crop Protect. , 18 : 177 – 191 .
- Hilder , VA , Gatehouse , AMR and Boulter , D . 1992 . “ Transgenic plants conferring insect tolerance: protease inhibitor approach ” . In Transgenic plants , Edited by: Kung , S and Wu , R . 310 – 338 . New York (NY) : Academic Press .
- Hilder , VA , Gatehouse , AMR , Sheerman , SE , Barker , RF and Boulter , DA . 1987 . A novel mechanism of insect resistance engineered into tobacco . Nature. , 300 : 160 – 166 .
- Hoffmann , MP , Zalom , FG , Wilson , LT , Smilanick , JM , Malyj , LD , Kiser , J , Hilder , VA and Barnes , WM . 1992 . Field evaluation of transgenic tobacco containing genes encoding Bacillus thuringiensis δ-endotoxin or cowpea trypsin inhibitor: efficacy against Helicoverpa zea (Lepidoptera: Noctuidae) . J Econ Entomol. , 85 : 2516 – 2522 .
- Johnson , R , Narvaez , J , An , G and Ryan , C . 1989 . Expression of proteinase inhibitors I and II in transgenic tobacco plants: effects on natural defense against Manduca sexto larvae . Proceedings of the National Academy of Sciences USA , 86 : 9871 – 9875 .
- Johnston , KA , Lee , MJ , Brough , C , Hilder , VA , Gatehouse , AMR and Gatehouse , JA . 1995 . Protease activities in the larval midgut of Heliothis virescens: evidence for trypsin and chymotrypsin-like enzymes . Insect Biochem Mol Biol. , 25 ( 3 ) : 375 – 383 .
- Jongsma , MA , Bakker , PL , Peters , J , Bosch , D and Stiekema , WJ . 1995 . Adaptation of Spodoptera exigua larvae to plant proteinase inhibitors by induction of gut proteinase activity insensitive to inhibition . Proc Natl Acad Sci USA. , 92 : 8041 – 8045 .
- Jouanin , L , Bonade-Bottino , M , Girard , C , Morrot , G and Giband , M . 1998 . Transgenic plants for insect resistance . Plant Sci. , 131 : 1 – 11 .
- Kranthi , KR , Kranthi , S , Ali , S and Banerjee , SK . 2000 . Resistance to Cry1Ac δ-endotoxin of Bacillus thuringiensis in a laboratory selected strain of Helicoverpa armigera (Hübner) . Curr Sci. , 78 : 1001 – 1004 .
- Kumar PA. 2004 . Cautious use of Bt genes in transgenic crops . Curr Sci . 86 : 632 – 633 .
- Laemmli , UK . 1970 . Cleavage of structural proteins during the assembly of the head of bacteriophage T4 . Nature. , 227 : 680 – 685 .
- Leple , JC , Bonade-Bottino , M , Augustin , S , Pilate , G , Dumanois Le Tan , V , Delplanque , A , Cornu , D and Jouanin , L . 1995 . Toxicity to Chrysomela tremulae (Coleoptera: Chrysomelidae) of transgenic poplars expressing a cysteineproteinase inhibitor . Mol Breed. , 1 : 319 – 328 .
- Lewis , WJ , van Lenteren , JC , Phatak , SC and Tumlinson , III JH . 1997 . A total system approach to sustainable pest management . Proc Natl Acad Sci USA. , 94 : 12243 – 12248 .
- Liota , LA and Stetler-stevenson , WG . 1990 . Cancer Biol. , 1 : 96 – 106 .
- Liu , Y , Sui , YP , Wang , JX and Zhao , XF . 2009 . Characterization of the trypsin-like protease (Ha-TLP2) constitutively expressed in the integument of the cotton bollworm, Helicoverpa armigera . Arch Insect Biochem Physiol. , 72 : 74 – 87 .
- Manjunath TM , Bhatnagar VS , Pawar CS , Sithanantham S. 1989 . Economic importance of Heliothis spp. in India and assessment of their natural enemies and host plants . In King EG , Jackson RD , Proceedings of the Workshop on Biological Control of Heliothis: Increasing the Effectiveness of Natural Enemies. Far Eastern Regional Research Office , US Department of Agriculture , New Delhi , , India , 197 – 228 .
- Mayee CD. 2009 . Biotech cotton in India: current status and impact on textile industry . Proc Indian Natl Sci Acad . 79 : 195 – 211 .
- McManus , MT , White , DWR and McGregor , PG . 1994 . Accumulation of a chymotrypsin inhibitor in transgenic tobacco can affect the growth of insect pests . Transgenic Res. , 3 : 50 – 58 .
- Michaud , D . 1997 . Avoiding protease-mediated resistance in herbivorous pests . Trends Biotechnol. , 15 : 4 – 6 .
- Mosolov , VV , Grigor'eva , LI and Valueva , TA . 2001 . Plant proteinase inhibitors as multifunctional proteins . Appl Biochem Microbiol (Moscow) , 37 : 643 – 650 .
- Mulimani , VH , Sudheendra , K and Giri , AP . 2002 . Detection of legume protease inhibitors by Gel-X-ray film contact print technique . Biochem Mol Biol Educ. , 30 : 40 – 44 .
- Oliveira , AS , Pereira , RA , Lima , LM , Morais , AHA , Melo , FR , Franco , OL , Bloch , C Jr , Grossi-de-Sa , MF and Sales , MP . 2002 . Activity toward bruchid pest of a kunitz-type inhibitor seeds of the Algaroba tree (Prosopis juliflora D.C.) . Pest Biochem Physiol. , 72 : 122 – 132 .
- Panda , N and Khush , GS . 1995 . Host plant resistance to insects , Oxon (London) : International Irrigation Information Center .
- Parde , VD , Sharma , HC and Kachole , MS . 2010 . In vivo inhibition of Helicoverpa armigera gut pro-proteinase activation by non-host plant protease inhibitors . J Insect Physiol. , 56 : 1315 – 1324 .
- Patankar , AG , Harsulkar , AM , Giri , AP , Gupta , VS , Sainani , MN , Ranjekar , PK and Deshpande , VV . 1999 . Diversity in inhibitors of trypsin and Helicoverpa armigera gut proteinases in cheakpea (Cicer arientinum) and its wild relatives . Theor Appl Genet. , 99 : 719 – 726 .
- Pichare , MM and Kachole , MS . 1994 . Detection of electrophoretically separated protease inhibitors using X-ray film . J Biochem Biophys Methods. , 28 : 215 – 224 .
- Schuler , TH , Poppy , GM , Kerry , BR and Denholm , I . 1998 . Insect resistant transgenic plants . Trends Biotechnol. , 16 : 168 – 175 .
- Shewry , PR . 2003 . Tuber storage proteins . Ann Bot. , 91 : 755 – 769 .
- Srinivasan , A , Giri , AP and Gupta , VS . 2006 . Structural and functional diversities in lepidopteran serine proteases . Cell Mol Biol Lett. , 11 : 132 – 154 .
- Srinivasan , A , Giri , AP , Harsulkar , AM , Gatehouse , JA and Gupta , VS . 2005 . A Kunitz trypsin inhibitor from chickpea (Cicer arietinum L.) that exerts anti-metabolic effect on podborer (Helicoverpa armigera) larvae . Plant Mol Biol. , 57 ( 3 ) : 359 – 374 .
- Tamhane , VA , Chaugule , NP , Giri , AP , Dixit , AR , Sainani , MN and Gupta , VS . 2005 . In vivo and in vitro effect of Capssicum annum proteinase inhibitors on Helicoverpa armigera gut proteinases . Biochimica et Biophysica Acta. , 1722 : 156 – 167 .
- Urwin , PE , Atkinson , HJ , Waller , DA and McPherson , MJ . 1995 . Engineered oryzacystatin I expressed in transgenic hairy roots confers resistance to Globodera pallida . Plant J. , 8 : 121 – 131 .
- Vinokorov , KS , Oppert , B and Elpidina , EN . 2005 . An overlay technique for postelectrophoretic analysis of proteinase spectra in complex mixtures using p-nitroanilide substrates . Anal Biochem. , 337 : 164 – 166 .