Abstract
Plants have developed many mechanisms to protect themselves against most potential microbial pathogens and diseases. Pathogenesis-related proteins are produced as a part of the active defenses to prevent attack. In this study, the induction of PR proteins in Eruca sativa in response to fungal pathogen Alternaria brassicicola was investigated in 10 days and one-month-old plants. Induction of pathogen resulted in a much marked increase in the activities of β-1,3-glucanase and chitinase in resistant cultivar (RTM-2002) as compared to susceptible (T-27) one. The enzyme activity gradually increased throughout the experimental period of 168 h compare to control. However, the activation of β-1,3-glucanase and chitinase was more rapid and to a greater extent in plants of RTM-2002 than in T-27. western blot analysis revealed the presence of 33 and 32 kDa β-1,3-glucanase and chitinase in induced arugula plants, respectively. The biochemical approach described in this article with E. sativa provide the basis for further efforts concentrating on the isolation and characterization of elements involved in perception and in the early steps of intracellular signal transduction.
1. Introduction
The plant pathogens have developed various independent and well-elaborated mechanisms of penetrating and accessing plant cell contents. Stopping the penetration of pathogens during plant infection is generally dependent on the accurate time-course of the pathogen perception by the plant host cells and the activation of biochemical systems resulting in induction of secondary metabolites, reactive oxygen species (ROS), and pathogenesis-related proteins (PRs), working often in combination to mount an adequate defense mechanism against the pathogen infection (Bolwell et al. Citation2001). Antoniw et al. (Citation1980) coined the term ‘pathogenesis-related proteins’ (PRs), which have been defined as ‘proteins encoded by the host plant but induced only in pathological or related situations.’ They are low-molecular weight proteins (6–43 kDa), selectively extractable and stable at low pH 3 and thermostable (Van Loon Citation1999). Two well-known examples of PRPS are β-1,3-glucanase (EC 3.2.1.39) and chitinase (EC 3.2.1.14). The β-1,3-glucanase known as laminarinases enhance fungal resistance in crop plants (Kirubakaran and Sakthivel Citation2007). These endoglucanase catalyze the hydrolytic cleavage of the (1,3)-β-D-glucosidic linkages in (1,3)-β-glucans and act primarily on glucans present in the fungal cell wall. The main substrate of chitinase is chitin a natural homopolymer of β-1,4-linked N-acetyl glucosamine residues (Kasprzewska Citation2003).
Eruca sativa Miller (arugula, rocket salad) belonging to the Brassicaceae family is a native plant of Southern Europe, Central Asia, and India. It is an oil crop and used for the preparation of some traditional medicines. The leaves and sprouts of the plant are widely used in salad for their hot pungent taste. Leaves are stimulant, stomachic, diuretic, and antiscorbutic. Alternaria species is the main fungus, which causes black spot disease on virtually every important oilseed species (Westman et al. Citation1999). It is a devastating disease resulting in 20–50% yield reductions in crop such as canola or rape (Rotem Citation1994).
In the present study, induction of PR proteins in response to fungal pathogen A. brassicicola was investigated in 10 days and one-month-old plants of E. sativa after different time intervals.
2. Materials and methods
2.1. Plant material and growth conditions
Two different cultivars of E. sativa (arugula, Rocket salad) viz., var. RTM-2002 (resistant) and T-27 (susceptible) were procured from SKN College of Agriculture, Jobner (Rajasthan, India). Seeds were surface sterilized in 0.1% HgCl2 and grown in pots containing sterilized garden soil in plant growth chamber with 60% relative humidity and temperature (26±2°C) under control conditions. Two age groups of plants, that is 10 days and one month were taken for experiments. For each group 10 pots were taken and there were 8–10 plants per pot. The fungal strain of Alternaria brassicicola (MTCC NO. 2102) was procured from IMTECH, Chandigarh.
2.1.1. Activation and maintenance of fungal culture
The lyophilized fungal strain was activated on potato carrot broth (PCB) under proper aseptic conditions in the laminar flow. The flasks were incubated in incubator shaker (28±2°C) for 120 h at 120 rpm. Activated fungal strain was then streaked on potato carrot agar (PCA) slants.
2.1.2. Preparation of spore suspension and mode of infection
Fungal spore suspension was prepared in sterilized water at a concentration of 105 spores ml–1 under aseptic conditions and kept in the incubator shaker (28±2°C) at 120 rpm for 1 h to obtain a uniformly distributed spore suspension. For the plant infection the leaf and stem surfaces of the plants were injured mildly with an abrasive to facilitate entry of spores on spraying with fungal spores using a thin-layer liquid chromatography (TLC) sprayer. The plants sprayed with autoclaved distilled water without fungal spores were served as control.
2.2. Determination of pathogenesis-related (PR) proteins
The activities of two PR proteins viz. β-1,3-glucanase and chitinase were determined using the method of Abeles et al. (Citation1970).
2.2.1. Extraction of β-1,3-glucanase
The control and inoculated tissue, that is entire leaf and stem portion of both cultivars of arugula plants viz. RTM-2002 and T-27 were taken after different time intervals (0, 2, 4, 24, 48, 72, 96, 120, 144, and 168 h) of fungal spore inoculation and homogenized in a pre-chilled mortar pestle in 4 ml of 0.05 M of potassium acetate buffer (pH 5). The homogenate was filtered through pre-moistened two-layered cheese cloth and filtrate was centrifuged at 10,000 g in a cooling centrifuge for 10 min at 4°C. The supernatant was collected and used for the estimation.
2.2.2. Assay of β-1,3-glucanase
The extract from both, control and inoculated plants was taken for the assay. The reaction mixture (1 ml) consisted of 50 µl sample, 450 µl buffer (0.05 M potassium acetate, pH 5), and 500 µl of 2% laminarin as substrate. This reaction mixture was incubated for 1 h at 40°C. After incubation the released glucose was further assayed using the method of Nelson (Citation1944) and Somogyi (Citation1952). The β-1,3-glucanase activity was calculated using the standard curve of glucose.
2.2.3. Extraction of chitinase
One gram of control and inoculated plant tissue of both varieties RTM-2002 and T-27 was taken after different time intervals (0, 2, 4, 24, 48, 72, 96, 120, 144, and 168 h) of fungal spore inoculation. The tissue was homogenized in a pre-chilled mortar pestle in 4 ml of 0.1 M sodium citrate buffer, pH 5. The homogenate was filtered through two layers of cheese cloth pre-moistened in 0.1 M sodium citrate buffer, pH 5. The filtrate was centrifuged at 10,000 g in a cooling centrifuge for 20 min and the supernatant was collected and measured.
2.2.4. Assay of chitinase
Chitinase activity was measured by the release of N-acetyl-D-glucosamine (NAG) using colloidal chitin as substrate according to the method of Reissig et al. (Citation1955). Colloidal chitin was prepared according to Berger and Reynolds (Citation1958). The chitinase activity was calculated using the standard curve of NAG.
2.3. Determination of protein
The protein content of β-1,3-glucanase and chitinase was determined using the method of Lowry et al. (Citation1951).
2.4. Western-blot
For western blot samples of 0, 4, 48, and 168 h of 10-day-old plants were taken for experiment after pathogen inoculation. Protein extract was prepared by grinding 1 g leaves with 4 ml Tris-HCl buffer (pH 8.0). Protein extract was separated on 10% SDS-PAGE (Laemmli Citation1970). After electrophoresis the proteins were electroblotted onto 0.45 µm nitrocellulose membrane (Whatman). The electrophoretic transfer of proteins was carried out from gel to membrane in a Blueflash-L Serva semi-dry transblot apparatus (96 mA, 60 min) using Tris-glycine buffer containing methanol (4 8mM Tris-HCl, pH 9.4, 39 mM glycine, 0.037% (w/v) SDS and 20% (v/v) methanol). The nitrocellulose membrane was taken out and incubated for 1 h with continuous shaking at room temperature in 5% TBST (Tris buffer saline with Tween 20; 10mM tris (pH 7.5), 150 mM NaCl) milk to block the non-specific binding sites. The membrane was then incubated overnight in primary antibody in the concentration of 1:1000 (anti-rabbit class I β-1,3 glucanase and anti-tobacco class I chitinase antibody) stock in TBST. After incubating with primary antibody, membrane was washed with TBST two times for 10 min each time and incubated in 1:8000 dilution of secondary antibody (alkaline phosphatase-conjugate-goat-anti-rabbit (IgG-ALP) in TBST buffer for 2 h at room temperature. After washing, membrane was incubated with alkaline phosphatase color development reagent containing 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium (sigma). Immediately after color development the membrane was washed in distilled water and air-dried.
2.5. Statistical analysis
The data were analyzed statistically following the method of analysis of variance (Gomez and Gomez Citation1984). The data were analyzed statistically with SPSS-17 statistical software (SPSS Inc.). Mean values were statistically compared by Duncan's Multiple Range test (DMRT). It was significant at ≤0.05% level. The data reported in graphs are means of four replications and all the treatments were repeated three times.
3. Results
In the present study on PRPs of E. sativa, the results show that β-1,3-glucanase activity increased rapidly by 48 h after pathogen infection in 10-day-old plants of RTM-2002, reaching a level of 2.4-fold higher than the corresponding control. In T-27, β-1,3-glucanase activity increased significantly by 72 h reaching a level of 1.8-folds higher than the corresponding control (A). However, the enzyme activity was higher in var. RTM-2002 when compared to var. T-27. A 1.5-fold increase in β-1,3-glucanase activity was recorded at 24 h after pathogen infection in one month cultivars of RTM-2002 when compared to control. In T-27, 1.4-fold increases were recorded at 48 h after pathogen infection in one-month-old plants (B). Significant increase in chitinase activity in 10-day-old plants was detected in both resistant (RTM-2002) and susceptible (T-27) cultivars of E. sativa, 24 h after pathogen induction. A 2.3- and 1.6-fold increase in chitinase activity was observed in plants of resistant and susceptible cultivars respectively at 24 h after pathogen infection in 10-day-old plants (A). The enzyme activity gradually increased throughout the experimental period of 168 h. However, the activation of chitinase was more rapid and to a greater extent in plants of RTM-2002 than in T-27. A 2.5- and 2-fold increase in chitinase activity was recorded after 48 and 72 h in pathogen infected RTM-2002 and T-27, respectively, when compared to control in one-month-old plants (B). The maximum enzyme activity was recorded at 48 and 72 h after the induction and thereafter a gradual decline in enzyme activity was noticed in one month plants. The levels of chitinase activity were higher in resistant cultivar than in the susceptible cultivar. Chitinases, belonging to PR-3 group of PR proteins catalyze the hydrolysis of β-1,4 linkages of the NAG polymer, called chitin, which is a major component of the cell walls of many fungi (Collinge et al. Citation1993). It has been demonstrated that constitutive overexpression of chitinase in plants can enhance disease resistance (Datta et al. Citation2001).
Figure 1. β-1,3-glucanase activity in control and pathogen (Alternaria brassicicola) inoculated 10 days (A) and one month (B) old arugula (Eruca sativa) plants viz. T-27 and RTM-2002. – • – T-27 control, – ○ – T-27 inoculated, –▾– RTM-2002 control, – ▿ – RTM-2002 inoculated. Each value represents the mean of four replicates with SE determined. Mean differences were significant at ≤0.05% level.
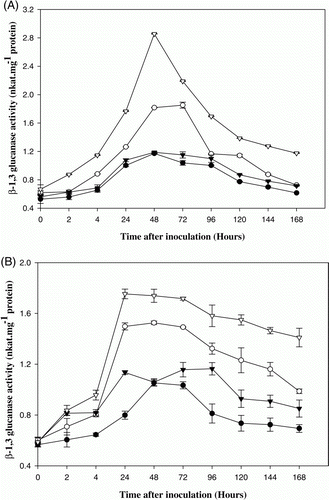
Figure 2. Chitinase activity in control and pathogen (Alternaria brassicicola) inoculated 10 days (A) and one month (B) old arugula (Eruca sativa) plants viz. T-27 and RTM-2002. – • – T-27 control, – ○ – T-27 inoculated, – ▾ – RTM-2002 control, – ▿ – RTM-2002 inoculated. Each value represents the mean of four replicates with SE determined. Mean differences were significant at ≤0.05% level.
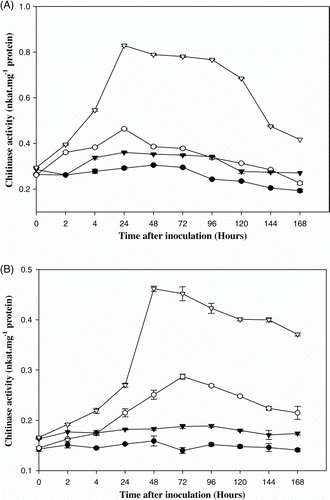
Western blot analysis of arugula plant samples of resistant and susceptible variety for β-1,3-glucanase and chitinase revealed induction of these proteins with molecular mass of 33 and 32 kDa after pathogen inoculation, that was constitutively present at a lower amount in control plants but strongly induced after pathogen induction. The intensity of β-1,3-glucanase protein increased with time and highly intense band was seen at 48 h after inoculation in both var. RTM-2002 and T-27, respectively (). In the case of chitinase activity, induction of a 32 kDa chitinase protein was recorded and its intensity was also higher at 48 h in var. RTM-2002 as compare to control, But in var. T-27 it was approximately same in intensity. It is assumed that this may be due to the pathogen inoculation. Control plants of both resistant and susceptible varieties showed these PR proteins in low amount as compared to pathogen in inoculated (). The results show that in the resistant variety the protein was seen with higher intensity during early stages of pathogenesis, whereas the same was induced during later stages of pathogenesis in the susceptible variety. The results indicate that the induction of PR proteins in E. sativa varieties varying in dark spot resistance is specifically induced in response to pathogen infection.
Figure 3. Western blot of PR proteins showing the detection of β-1,3-glucanase activity from Eruca sativa after pathogen (A. brassicicola) infection in var. RTM-2002 (A) and T-27 (B) after different time intervals; lane 1: Marker, lane 2: 0 h control, lane 3: 4 h control, lane 4: 4 h inoculated, lane 5: 48 h control, lane 6: 48 h inoculated, lane 7: 168 h control, lane 8: 168 h inoculated.
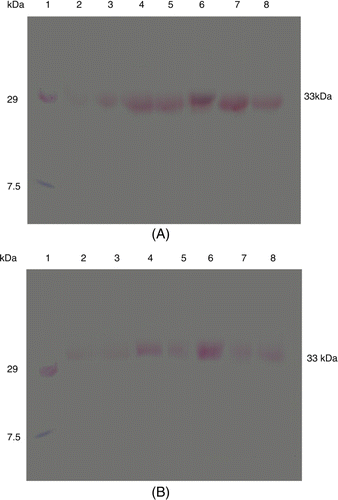
Figure 4. Western blot of PR proteins showing the detection of chitinase activity from Eruca sativa after pathogen (A. brassicicola) infection in var. RTM-2002 (A) and T-27 (B) after different time intervals; lane 1: Marker, lane 2: 0 h control, lane 3: 4 h control, lane 4: 4 h inoculated, lane 5: 48 h control, lane 6: 48 h inoculated, lane 7: 168 h control, lane 8: 168 h inoculated.
4. Discussion
Pathogenesis-related proteins are a class of proteins associated with a pathological state or other biotical stresses. They are coordinately induced, accumulate systemically and locally, and are linked to the development of systemic acquired response (SAR) (Ferreira et al. Citation2007). Plants produce enzymes such as β-1,3-glucanase and chitinase (Santos et al. Citation2004) that can break down the cell wall components of pathogens. These enzymes are important determinants of the resistance of plants to fungal diseases (Funnell et al. Citation2004). It has been demonstrated that genetically engineered over-expression of PR proteins can increase resistance in plants (Velazhahan and Muthukrishnan Citation2004). The activation of defense mechanisms in plants is considered to be consequent upon an initial recognition event in which the host plant detects molecular components of the pathogen, known as elicitors (Van't Slot and Knogge Citation2002). These elicitor molecules bind to a receptor(s) on the plasma membrane of plant cells and activate the signaling events required for the onset of the defense responses (Umemoto et al. Citation1997). Some PR proteins are constitutively expressed in plants at low levels, but the expression of most of the PR proteins is turned on in response to a pathogen attack. Induction of PR proteins is a consequence of the activation of plant defensive pathways, which limit the entry, or the further spread of the pathogen.
It is difficult to assign a reasonable role of PRPs in plant resistance to pathogens because the numerous data on PRPs as disease resistance factor are mostly correlative, such as, stronger accumulation of PRPs in inoculated resistant as compared to susceptible plants, constitutive expression of PRPs in plants with high level of natural disease resistance. PRPs are induced in resistance or SAR-expressing plants, as well as PRs from transgenic resistant plants exhibit high microbial activity (Anand et al. Citation2004) suggesting their direct role in disease resistance. Chitinases are a group of enzymes that hydrolyze chitin, the major component of many fungal cell walls. They play key roles in active defense response in plants, alone or in combination with chitinases (Mauch et al. Citation1988). In resistant, varieties immediately after pathogen penetration, hydrolytic enzymes act on fungal germlings and weaken them leading to no disease development, whereas in susceptible host before induction of PR proteins to a required level the pathogen may penetrate and colonize the tissue. The mechanisms of direct break-down or damage of pathogens, PRs can operate in a distinct pathway involving the hydrolytic release of chitin and glucan fragments from fungal cell walls. These oligosaccharides are endowed with elicitor activity and can induce a chain of defense reactions in the host plant (Kombrink et al. Citation2001).
From the results it is clear that chitinase activity in plants sample was generally higher in inoculated plants as compared to control. It has been demonstrated that enhanced chitinase activity in plants can indeed reduce the damage caused by pathogens (Broglie et al. Citation1991). The β-1,3-glucanase and chitinase activity was determined in two age groups of plants, that is 10 days and one month. The enzyme activities were found to be higher in 10-day-old plants as compared to one-month-old plants.This may be due to the higher metabolic rate and growing cells of young plants as compared to the mature plants. They also showed strong evidence of systemic resistance for airborne communication, young plants were more effective emitters of cues as well as more responsive receivers of volatile cues. The induction of chitinase and other hydrolytic enzymes is perceived to be one of the coordinated, often complex and multifaceted defense mechanisms, which are triggered in response to pathogen attack. Chitinases are synergistically induced during attack by fungal pathogens. Their induction is generally considered to be part of a non-specific defense response initiated in plants after pathogen attack. PRs accumulate rapidly at the intra-or extra-cellular level under various biotic and abiotic stimuli, including fungal, elicitor, and physical or chemical treatments (Graham et al. Citation2003). The proteomic studies carried throughout this study indicated that, in both resistant and susceptible Eruca cultivars, plants inoculated with A. brassicicola showed a significantly higher amount of PRPs than the healthy ones.
The consistent and rapid increase in activity in the resistant cultivar after pathogen infection suggests a possible role for β-1,3-glucanase and chitinase in the defense mechanism of E. sativa against A. brassicicola. A direct role for β-1,3-glucanases in the defense of plants against pathogens has been proposed, because the substrate for the enzyme, β-1,3-glucan is a major component of the cell walls of many fungi (Wessels and Sietsma Citation1981). Furthermore, β-1,3-glucanases are known to release oligosaccharides from the walls of fungi, which in turn, act as signals in the elicitation of host defense responses (Ham et al. Citation1991). In Fusarium-infected musk-melon, there was evidence of higher β-1,3-glucanase activity in resistant plants than in susceptible plants (Ward et al. Citation1991). In this study, we presented evidence that E. sativa has chitinase and β-1,3-glucanase PR proteins. In the plants inoculated with A. brassicicola, the activities of chitinase and β-1,3-glucanase were increased significantly in the resistant genotype in comparison with the susceptible genotype. Most plants respond to pathogen attacks by synthesizing an assortment of PR proteins.
Western blot analysis revealed a 33 and 32 kDa β-1,3-glucanase and chitinase proteins to be induced in pathogen-induced plants of arugula, respectively. An important understanding from this study is that the application of fungal pathogen induces signaling process that begins upstream activation of PR proteins. Expression of pathogen inducible PR proteins has been well correlated with disease resistance (Vidhyasekaran Citation1997). Further, it has been concluded that plants with elevated levels of chitinase and β-1,3-glucanase expression are more resistant to fungal pathogens (Datta et al. Citation2001). The results, therefore, suggest a possible role of PR proteins in defense mechanism of E. sativa against the dark spot disease.
5. Conclusion
Both these PRPs (β-1,3-glucanase and chitinase) were induced in E. sativa plants after inoculation of fungal pathogen A. brassicicola. The results show that in the resistant variety the protein was seen with higher intensity during early stages of pathogenesis, whereas the same was induced during later stages of pathogenesis in the susceptible variety. In the varietal difference of both these cultivars var. RTM-2002 showed higher accumulation of these PRPs when compared to the T-27 cultivar. The present results on the role of PRs in defense response represent a basis for insight into PRs importance in plants. The PR proteins are relevant to important plant performances, such as development, disease resistance, and general adaptation to stressful environment.
References
- Abeles , FB , Bosshart , RP , Forrence , LE and Habig , WE . 1970 . Preparation and purification of glucanase and chitinase from bean leaves . Plant Physiol. , 47 : 129 – 134 .
- Anand , A , Lei , ZT , Summer , LW , Mysore , KS , Arakane , Y , Backus , WW and Muthukrishnan , S . 2004 . Apoplastic extracts from a transgenic wheat line exhibiting lesion-mimic phenotype have multiple pathogenesis-related proteins that are antifungal . Plant-Microbe Interact. , 17 : 1306 – 1317 .
- Antoniw , JF , Ritter , CE , Pierpoint , WS and Van Loon , LC . 1980 . Comparison of three pathogenesis-related proteins from plants of two cultivars of tobacco infected with TMV . J Gen Virol. , 47 : 79 – 87 .
- Berger , LR and Reynolds , DM . 1958 . The chitinase system of a strain of Streptomyces griseus . Biochem Biophys Acta. , 29 : 522 – 534 .
- Bolwell , PP , Page , A , Pislewska , M and Wojtaszek , P . 2001 . Pathogenic infection and the oxidative defences in plants apoplast . Protoplasma. , 217 : 20 – 32 .
- Broglie , K , Chet , I , Holliday , M , Cressman , R , Biddle , P , Knowlton , S , Maurais , CJ and Broglie , R . 1991 . Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani . Science. , 254 : 1194 – 1197 .
- Collinge , DB , McKragh , KM , Mikkelsen , JD , Nielsen , KK , Rasmussen , U and Vad , K . 1993 . Plant chitinases . Plant J. , 3 : 31 – 340 .
- Datta , K , Tu , JM , Oliva , N , Ona , I , Velazhahan , R , Mew , TW , Muthukrishnan , S and Datta , SK . 2001 . Enhanced resistance to sheath blight by constitutive expression of infection related rice chitinase in transgenic elite Indica rice cultivars . Plant Sci. , 160 : 405 – 414 .
- Ferreira , RB , Monteiro , S , Freitas , R , Santos , CN , Chen , Z , Batista , LM , Duarte , J , Borges , A and Teixeira , AR . 2007 . The role of plant defense proteins in fungal pathogenesis . Mol Plant Pathol. , 8 : 677 – 700 .
- Funnell , DL , Lawrence , CB , Pedersen , JF and Schardl , CL . 2004 . Expression of the tobacco β-1,3-glucanase gene, PR-2d, following induction of SAR with Peronospora tabacina . Physiol Mol Plant Pathol. , 65 : 285 – 296 .
- Gomez , KA and Gomez , AA . 1984 . Statistical procedures for agricultural research , New York (NY) : Wiley .
- Graham , MY , Weidner , J , Wheeler , K , Pelow , ML and Graham , TL . 2003 . Induced expression of pathogenesis-related protein genes in soybean by wounding and the Phytophthora sojae cell wall glucan elicitor . Physiol Mol Plant Pathol. , 63 : 141 – 149 .
- Ham , KS , Kauffmann , S , Albersheim , P and Darvill , AG . 1991 . Host pathogen interactions. XXXIX. A soybean pathogenesis related protein with β-1,3-glucanase activity releases phytoalexin elicitor-active heat-stable fragments from fungal walls . Mol Plant-Microbe Interact. , 4 : 545 – 552 .
- Kasprzewska , A . 2003 . Plant chitinases – regulation and function . Cellul Mol Biol Lett. , 8 : 809 – 824 .
- Kirubakaran , SI and Sakthivel , N . 2007 . Cloning and over expression of antifungal barley chitinase gene in Escherichia coli . Protein Exp Purif. , 52 : 1159 – 1166 .
- Kombrink E , Ancillo G , Büchter R , Dietrich J , Hoegen E , Ponath Y , Schmelzer E , Strömberg A , Wegener S. 2001 . The role of chitinases in plant defense and plant development . 6th international workshop on PR-proteins. May 20–24, 2001, Spa, Belgium. Book of abstracts , 11 .
- Laemmli , UK . 1970 . Cleavage of structural proteins during the assembly of the head of bacteriophage T4 . Nature. , 227 : 680 – 685 .
- Lowry , OH , Roserbrough , NJ , Farr , AL and Randell , RJ . 1951 . Protein measurement with the Folin-phenol reagent . J Biol Chem. , 193 : 265 – 275 .
- Mauch , F , Mauch-Mani , B and Boller , T . 1988 . Antifungal hydrolases in pea tissue. II. Inhibition of fungal growth by combinations of chitinase and β-1,3-glucanase . Plant Physiol. , 88 : 936 – 942 .
- Nelson , N. 1944 . A photometric adaption of the Somogyi method for the determination of glucose . J Biol Chem. , 153 : 375 – 380 .
- Reissig , JL , Strominger , JL and Leloir , LF . 1955 . A modified colorimetric method for the estimation of N-acetylamino sugars . J Biol Chem. , 217 : 959 – 966 .
- Rotem , J . 1994 . The genus Alternaria. Biology, epidemiology, and pathogenecity , St. Paul : APS Press .
- Santos , IS , Machado , OLT , Da Cunha , M and Gomes , VM . 2004 . A chitinase from Adenanthera pavonina L. seeds: purification, characterization and immunolocalization . Plant Sci. , 167 : 1203 – 1210 .
- Somogyi , M . 1952 . Notes on sugar determination . J Biol Chem. , 195 : 19 – 23 .
- Umemoto , N , Kakitani , M , Iwamatsu , A , Yoshikawa , M , Yamoka , N and Ishida , I . 1997 . The structure and function of a soybean β-glucan-elicitor binding protein . Proc Nat Acad Sci USA. , 94 : 1029 – 1034 .
- Van Loon , LC . 1999 . “ Occurrence and properties of plant pathogenesis-related proteins ” . In Pathogenesis-related proteins in plants , Edited by: Datta , SK and Muthukrishnan , S . 1 – 19 . Boca Raton (FL) : CRC Press LLC .
- Van't Slot , KAE and Knogge , W . 2002 . A dual role for microbial pathogen-derived effector proteins in plant disease and resistance . Crit Rev Plant Sci. , 21 : 229 – 271 .
- Velazhahan , R and Muthukrishnan , S . 2004 . Transgenic tobacco plants constitutively overexpressing a rice thaumatin-like protein (PR-5) show enhanced resistance to Alternaria alternata . Biol Plant. , 47 : 347 – 354 .
- Vidhyasekaran , P . 1997 . Fungal pathogenesis in plants and crops , 553 New York (NY) : Marcel Dekker Inc .
- Ward , ER , Uknes , SJ , Williams , SC , Dincher , SS , Wiederhold , DL , Alexander , DC , Ahl-Goy , P , Métraux , JP and Ryals , JA . 1991 . Coordinate gene activity in response to agents that induce systemic acquired resistance . Plant Cell. , 3 : 1085 – 1094 .
- Wessels , JGH and Sietsma , JH . 1981 . “ Fungal cell walls: a survey ” . In Encyclopedia of plant physiology , Edited by: Tanner , W and Louewus , FA . 352 – 394 . New York (NY) : Springer-Verlag. New Series .
- Westman , AL , Kresovich , S and Dickson , MH . 1999 . Regional variation in Brassica nigra and other weedy crucifers for disease reaction to Alternaria brassicicola and Xanthomonas campestris pv.campestris . Euphytica. , 106 : 253 – 259 .