Abstract
The presence of Fe-efficient pink-pigmented methylobacterium (Fe-PPM) has been recently certified in the leaves of Celosia plant by priming of a specific Fe-siderophore receptor gene in our laboratory (GenBank accession number: FM955594). Considering Celosia as a pink-colored plant, a new proposal was made with regard to the possible contribution of Fe-PPM in the pigmentation process of this plant. To examine this, the quantitative expression level of methylobacterial-type Fe siderophore receptor gene was analyzed in differentially scored colored leaves of Celosia cristata. The normalized gene expression data analysis using 2−ΔΔCt method revealed that there is a positive correlation between the expression level of target gene and the intensity of the test leaf color as well as the total flavonoid content, total carotenoid content, and total antioxidative capacity as representatives of color intensity. The overall results lead to questioning'Do Fe-PPM reliably contributes the pigmentation process in pink-colored plants?’ However, this research will open a new gate to study the putative communicative roles of these bacteria in plants.
Abbreviations
| FRAP | = |
ferric reducing antioxidant power |
| RT-PCR | = |
reverse transcriptase-polymerase chain reaction |
| TPTZ | = |
tripyridyl triazine |
| SD | = |
standard deviations |
Introduction
Bacteria of the genus Methylobacterium are classified as α-proteobacyteria and referred to pink-pigmented facultative methylotrophs (PPFM) because of their pink-pigmentation and their ability to grow on one-carbon compounds such as methanol, methylamine as well as on a variety of multicarbon compounds (Loper and Buyer Citation1991, www.Homd.org/download/NCBI_bacteria_genome/Methylobacterium; www.micro-genomes.mpg.de, Braun Citation1995; Green Citation2006). These bacteria are commonly found in soils, on leaves, and in other parts of plants as a natural flora (Listrom and Chistoserdova Citation2002). They are extremely important because they play a significant role in global carbon cycling by utilizing the biogenic atmospheric methane/methanol/methylated amines. It is suggested that methylotrophy might be useful from ecological viewpoints in facilitating attempts to reduce methane emission in the future (Eller and Frenzel Citation2001).
The detailed relationship of methylobacteria to their growth habitats has not been clear, but they are highly studied organism. Also, it is not clear whether they are commensal bacteria or they do communicate with plants in an intimate manner? They have been previously shown that they stimulate seed germination and plant developmental processes (possibly by the production of phytohormones) as well as contribute to plant flavor (Corpe and Basile Citation1982; Holland and Polacco Citation1992, Citation1994; Zabetakis Citation1997). In addition, the production of cis and trans-type cytikinins and indole acetic acid has been reported from some strains of methylobacteria suggesting their effective roles in plant system (Ivanova et al. Citation2000, Citation2001; Koenig et al. Citation2002). One non-pigmented strain has been shown to form a root-nodulating nitrogen fixing symbiosis with a legume (Sy et al. Citation2001). All these together indicate that PPFMs interact with plants, but still the bio-molecular details of their relationships to plant system have remained unclear and elusive. It has been newly reported that the sites and the species of plants are important determinants of methylobacterium community composition (Knief et al. Citation2010). This new finding may be reflecting that methylobacteria can exhibit differential roles in different sites and in different plant species.
Celosia species are ornamental plants belongs to amaranthaceous. They are generally pink-colored plants and contain high iron content in their leaves (Gupta et al. Citation2005). Among these plants, Celosia cristata is well-characterized one.
Recently, the presence of an endosymbiotic Fe-efficient pink-pigmented methylobacterium was certified in Celosia cristata plant leaves (Gholizadeh and BaghbanKohnehrouz Citation2010). This was suggesting the existence of biochemical and molecular possible interactions between the iron-rich pink-colored plants and the iron-efficient pink-pigmented bacteria. As Celosia cristata is a pink-pigmented plant, a prediction was made with regard to the possible contribution of Fe-PPM to pigmentation process in this plant.
In order to test this research proposal, an attempt was presently been made to analyze the possible correlation between the expression level of TonB-dependant Fe siderophore receptor gene (as target gene and a specific molecular marker for the detection of methylobacterial existence) and the intensity of leaf color in test plant. As the representatives of color intensity, the total flavonoid and total carotenoid contents as well as the total antioxidative capacity of the test samples were also determined and discussed.
Materials and methods
Plant materials and chemicals
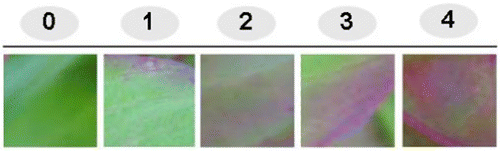
Celosia criststa seeds were taken from our laboratory stock and were grown under laboratory conditions. Five differentially colored leaf tissues were considered as experimental materials. The scores of leaf pink-color intensities were obviously rated using indexes as shown in . Leaf samples were only taken on the basis of leaf color intensity and were collected randomly from every part of growing plants.
Figure 1. Indexing of pink color intensity in Celosia leaves. The scores of the pink color intensity were rated by indexing from 0 to 4. 0: represents the lowest pink-colored leaf sample and 4: represents the highest pink-colored leaf sample.
Trizol reagent used for total RNA extraction was purchased from Gibco BRL, USA (Cat. No. 15596-013). The mRNA purification kit was from QIAGEN, USA (Cat. No.70022). Chemicals used for the synthesis cDNAs was provided from Promega (USA) cDNA synthesis kit (Cat. No. C4360). All other chemicals used in this research work were of analytical grade.
cDNA synthesis
Total cellular RNA was isolated from the thoroughly washed leaf samples using Trizol reagent. For this, about 0.2 g of leaf material was fine powdered using liquid N2 and 2 ml of Trizol reagent was added to homogenize it at room temperature (RT). Two hundred micro liter of chloroform was added to the mixture, mixed for 15 second, incubated on ice for 5 min and centrifuged at 13000×g for 15 min. The upper phase was transferred to another tube and the RNA was precipitated with equal volume of isopropanol. The pellet was washed in 1 ml of 75% ethanol, dried at RT and dissolved in 30 µl RNase- free water. The integrity of the RNA was tested on 1% non-denaturing agarose gel using TBE running buffer. Poly (A+) RNA was purified from total RNA using mRNA purification kit protocol (Cat. No.70022) and double-stranded cDNA was synthesized according to the protocol of Promega cDNA synthesis kit (Cat. No. C4360).
Real time RT-PCR assay
Primers specific for the amplification of methylobacterial-type Fe siderophore receptor transcript were designed using Primer3 software (http://www.primer3plus.com) as for previously reported sequence (Gholizadeh and BaghbanKohnehrouz Citation2010). The nucleotide sequences of the used primers were as follows: Fw: 5′ CAATCGATCACCGTCGTG 3′ and Rv: 5′ CGCTTGCTGATCTGGTTG 3′. The amplification and analysis of the gene expression levels were performed by real time reverse transcription PCR on Miniopticon system (Bio Rad, USA) by using iQ SYBR Green supermix. The PCR was programed to 25 cycles as described in . All the expression data were normalized by adjusting the expression level of actin gene as reference gene in test samples and the relative fold expression was calculated using the 2−ΔΔCt value (Livak and Schmittgen 2011).
Table 1. Real time PCR amplification steps.
Total flavonoid assessment
Total flavonoid contents of the test samples were calorimetrically measured by using aluminum chloride assay (Zhishen et al. Citation1999). To one ml of the leaf extract, 4 ml double distilled water, 0.3 ml NaNO2 (5%), 0.3 ml AlCl3 after 5 min, 2 ml NaOH after 6 min were added and diluted to 10 ml. The absorbance of the samples was measured at 510 nm. Data were adjusted using the standard solutions of catechin and presented as milligram per gram fresh weight of leaf tissues.
Total carotenoid assay
Total carotenoid contents of the samples were measured by a spectrophotometric method using acetone and petroleum ether as extracting solvents and measuring the absorbance at 450 nm (Ranganna Citation1997). Data were adjusted by standard curve and presented as milligram per /100 gram fresh weight of test samples.
Total antioxidation ability assay
The total antioxidant capacity of test leaf materials was determined by using FRAP assay (Benzie and Strain Citation1996). To 1 ml of plant extract in 0.1 M phosphate buffer (pH 7.0), 3 ml of FRAP reagent (10 mM TPTZ, 20 mM FeCl3. 6H2O, and 300 mM sodium acetate buffer (pH 3.6) in the ratio of 1:1:10) were added and the reaction mixture was incubated at 37°C for 4 min. The assessment was carried out spectrophotometerically at A593. Antioxidant potential was determined against the standard curve of ferrous sulphate (Fe, 100–1000 µM). FRAP values were calculated as follows:
FRAP values of all test samples were presented as (µmol FeII per 100 mg leaf fresh weight).
Statistics
All of the experimental samples including all assays were analyzed in duplicate and the data were presented as mean values + SD.
Results and discussion
It has been previously understood that methylobacteria have got passive interactive roles in a wide variety of plants, including their contributions in seed germination, flavoring, growth and developmental processes, and nitrogen fixation (Corpe and Basile Citation1982; Holland and Polacco Citation1992, Citation1994; Zabetakis Citation1997). In the previous research work, we certified the presence of an endosymbiotic Fe-efficient methylobacteria in the leaves of Celosia cristata plant (Gholizadeh and BaghbanKohnehrouz Citation2010). We also suggested that iron efficient bacteria might be existed on high iron content plants such as Celosia (Gholizadeh and BaghbanKohnehrouz Citation2010).
As a different set of studies, we conducted our experiments to examine whether endosymbiotic methylobacteria contribute the pink-pigmentation process and color development in Celosia leaves? Considering methylobacteria as pink-pigmented bacteria (Nemecek-Marshall et al. Citation1995; Listrom and Chistoserdova Citation2002) and Celosia as well pink-colored plant (Gupta et al. Citation2005), a communicative role of pink-pigmented methylobacteria in pink-pigmentation process of Celosia leaves was predicted. To examine this, the scores of color intensities were obviously rated by indexing from 0 to 4, as described in Materials and methods section, so the comparison between the indexes of leaves showed significant differences in their colors (). As the first experiment using real time RT-PCR, the presence and the level of the expression of methylobacterial-type TonB dependent Fe siderophore receptor gene were examined as determinants of the existence and the rate of methylobacteria in differentially indexed leaf samples. Data related to the relative fold changes (all normalized by actin gene and related to S0 using 2−ΔΔCt values) in the expression level of target gene is shown in .
Figure 2. Real-time RT-PCR detection of methylobacterial type Fe-siderophore receptor transcript. Expression level of siderophore receptor gene was analyzed in five differentially indexed leaf samples using iQ SYBR Green dye (provided by Bio Rad, USA) on Miniopticon Real Time PCR Detection system (Bio Rad, USA). S: represents the test sample; 0–4: represents the indexing number of leaf samples. Data presented as the mean values of two replicates±SD.
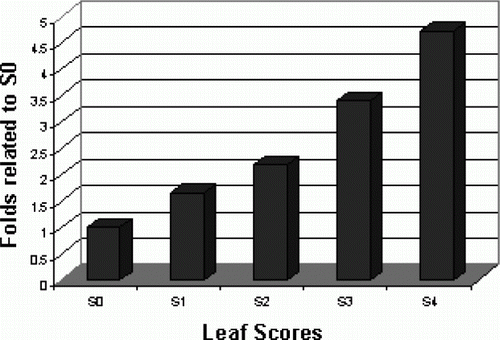
As the results revealed, the expression level of the target gene is considerably varied in different samples. An enhanced pattern of expression is detectable as the rating of the color index is increased in the leaf samples. In comparison with the control sample S0, there is about 1.65 to 4.75 folds increase from S1 to S4 in the expression level of the target gene (). On the other hand, data revealed that there is a positive correlation between the expression level of the methylobacterium-specific gene and the intensity of leaf color in test samples.
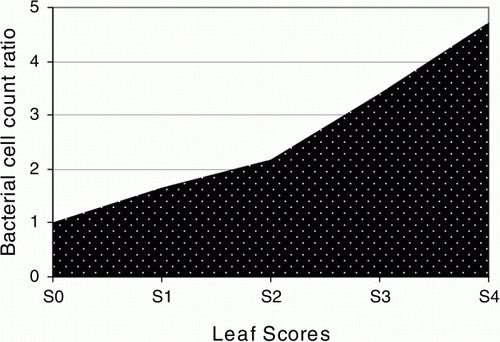
Based on the results obtained, different levels of Fe efficient-pink-pigmented methylobacterium (Fe-PPM) are predicted in differentially indexed pink-colored leaves of Celosia plant. Mainly, TonB-dependent receptors are found on the outer membranes of Gram-negative bacteria such as methylobacteria to compete for scarce recourses to survive (Lim Citation2010). Among them, TonB-dependent Fe siderophore receptors are responsible for the active import of Fe 3 + -siderophore complexes into bacterial cells. Our bioinformatics analysis data revealed that the homolog of these receptor genes is not present in plants. Based to our knowledge, the presence and the levels of the expressed methylobacterial-type TonB-dependent Fe siderophore receptor sequence could be considered as representatives of methylobacterial attendance and rates in the leaves of the Celosia plant. Therefore, methylobacterial cell count ratios (related to S0) in S1, S2, S3, and S4 samples were predicted to be 1.65, 2.18, 3.40, and 4.67, respectively (). However, these predicted values are needed to be determined and approved by microbiologists.
Figure 3. Prediction of methylobacterial cell count ratio. Methylobacterial attendance and rates in the leaves of the Celosia plant were predicted to be consistent to the levels of the specific target gene expression in the test leaf samples.
Priming of a methylobacterial mRNA by using oligo dT primer in Celosia leaves is so interested. This is a very rare phenomenon. Bacterial mRNA with short poly(A+) tails is known to exist. These prokaryotic poly(A+) tracts found to be ranging from 15 to 60 adenylate residues and associated with only 2–60% of the molecules of a given mRNA species (Sarkar Citation1997). In some cases like those of Mycobacterium and Pseudomonas, oligo (dT) can prime reverse transcription of several mycobacterial mRNAs and convert them to cDNAs (Adilakshmi et al. Citation2000; Saravanamuthu et al. Citation2004).
Our results potentially lead to question'Do the Fe-PPM reliably contribute the pigmentation process in pink-colored plants?′ We think this potential could be predicable in Celosia plant. However, it needs to be more discussed and unraveled in the future investigations.
Considering plant pigments as indicators of leaf color, the experiments were proceeded to determine the pigment contents and to find out their relations to methylobacterial target gene expression in differentially indexed samples.
Flavonoids and carotenoids are mostly responsible compounds in pink-pigmentation process in various pink-colored plants. They, respectively, belong to phenolic and isopropanoid metabolic pathways, known as ubiquitous secondary metabolites in plant system (Harborne and Turner Citation1984). Using colorimetric methods we assessed the total favonoid and total carotenoid contents in five differentially indexed pink-colored leaf samples of Celosia plant. The results showed that the total falavonoid content is increased from 2.1 to 8.9 mg/g between S0 and S4 test samples (). Similarly, the total carotenoid content is increased from 0.26 to 0.88 mg/g between lowest (S0) and highest (S4) indexed leaf samples ().
Figure 4. Detection of the total flavonoid contents in relation to target gene expression levels. (Left) total flavonoid contents were determined using aluminum chloride method by measuring the absorbance of the samples at 510 nm. (Right) target gene expression levels of the differentially indexed samples were plotted against their total flavonoid contents.
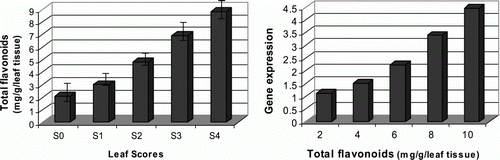
Figure 5. Detection of the total carotenoid contents in relation to target gene expression levels. (Left) total carotenoid contents were assessed using acetone and petroleum ether as extracting solvents and measuring the absorbance of the samples at 450 nm. (Right) target gene expression levels were plotted against total carotenoid contents of the differentially indexed leaf samples. All data were presented as the mean values of two replicates±SD.
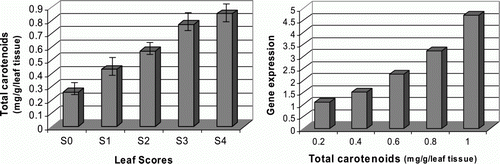
The overall results for flavonoid and carotenoid contents revealed that their contents are linearly increased with the increasing pink-color indexing number of the leaf tissues. This experiment data indicated that both flavonoid and carotenoid derived pigments contribute in pink-color development in the leaves of Celosia plant.
Evaluation of the methylobacterial specific target gene expression levels against flavonoid and carotenoid contents of the leaf samples indicated that the target gene expression level is positively correlated with the total flavonoid and carotenoid contents in the leaf tissues ( and ). On the other hand, the variation in the rates of pink-pigmented methylobacteria is more evident between the lower and higher pink-color indexed leaf samples. This can be an important result, since it may reflect the interactive role of Fe-PPM bacteria in pink-pigmentation process in Celosia leaves.
The earlier evidences have shown that pink-pigmented methylobacteria interact with plants, but the chemical and molecular details of their interactions are yet elusive. However, the results presented by our research team provide some more evidences for the presence of iron-efficient pink-pigmented methylobacteria on iron-rich pink-colored plant and their potential metabolic communications. In addition, to prove that the pink-pigmented methylobacteria contribute to the color of the leaves some data are essential such as: (1) The number of bacteria should be estimated by using microbiological approaches, (2) The extraction properties of the pink methylobacterial pigment as well as its light absorption characteristics should be investigated and compared to the Celosia flavonoids and carotenoids, and (3) The possible communicative biochemical pathway between bacteria and test plant needs to be identified with regard to pink-pigmentation process. These complementary investigations are recommended for the microbiology scientists. We hope they will help us to approve this predicted plant-microbe communication.
Bacteria commonly develop different strategies to colonize habitats to satisfy their requirements (Braun Citation1997). Iron is considered to play a crucial role in plant-microbe interactions and is known as one of the limiting factors for bacterial growth in planta (Briat Citation1992; Expert et al. Citation1996). Based on our experiments results, it seems that methylobacterial population develops Fe-siderophore receptor compounds and siderophore-mediated strategy to efficiently uptake ferric ions from high iron content Celosia leaves. In contrast, pink-pigmented bacteria contribute to pink-color development in Celosia pink-colored leaves.
Flavonoids and carotenoids have been well-characterized compounds as antioxidants due to their redox properties. They play an important role in absorbing and neutralizing free radicals, quenching oxygen or decomposing peroxides allowing them to act as reducing agents or hydrogen donors (Beckman Citation2000). To identify the antioxidative capacity of differentially indexed leaf samples we performed FRAP test that is generally known as a simple and reproducible method for the assessment of total antioxidation status from different biological samples (Benzie and Strain Citation1996). The results showed that the total antioxidative capacity is increased as the indexing number of the colored leaf samples increased (). The FRAP value is considerably varied from 0.12 to 0.47 µmol FeII per 100 mg between S0 and S4.
Figure 6. Assessment of the total antioxidant capacity. The total antioxidant abilities of the differentially indexed leaf samples (S0–S4) were assessed using FRAP method by measuring the absorbance of the test samples at A593. All data were presented as the mean values of two replicates±SD.
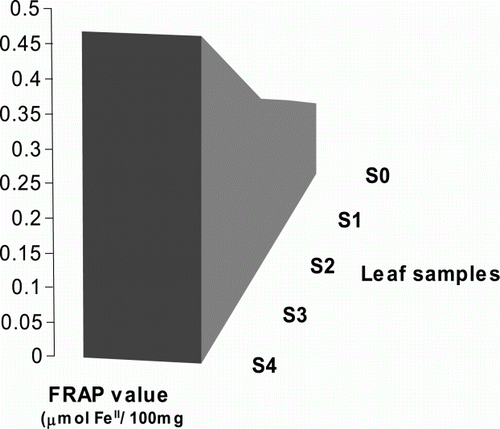
In general, plants elevate their antioxidative status as defense mechanism to combat the danger posed by the various environmental stresses. However, our present results may give more attention to the communicative role of methylobacterial population in the leaves of Celosia plant. Whether these bacteria really existed and involved in the elevation of antioxidative capacity in highly pink-colored leaves of Celosia to increase its resistance against environmental cues or not? Our study highlights the molecular variation of Fe-PPM in Celosia leaves to provide further assessment criteria for characterization of plant-bacteria interactions in details.
Despite our attempts, more clarity is needed to show how Fe-PPM bacteria and plants do communicate. Also numerous investigations are needed to shed light on the suggested communicative roles as well as on molecular interactions that may be existed between high iron content pink-pigment plants and iron efficient pink-pigment bacteria in the future.
Acknowledgements
The author of this paper is thankful to RIFS (Research Institute for Fundamental Sciences), University of Tabriz, Iran for funding of this work.
References
- Adilakshmi , T , Ayling , PD and Ratledge , C . 2000 . Polyadenylylation in mycobacteria: evidence for oligo(dT)-primed cDNA synthesis . Microbiology , 146 : 633 – 638 .
- Beckman , KB and Ames , BN . 2000 . Oxidants and agingin , Amsterdam : Elsevier .
- Benzie , FF and Strain , JJ . 1996 . The ferric reducing ability of plasma as leisure of antioxidant power . Anal Biochem. , 239 : 70 – 76 .
- Braun , V . 1995 . Energy-coupled transport and signal transduction through the gram-negative outer membrane via TonB-ExbB-ExbD dependent receptor proteins . FEMS Microbiol Rev. , 16 : 295 – 307 .
- Braun , V . 1997 . Avoidance of iron toxicity through regulation of bacterial iron transport . Biol chem. , 378 : 779 – 786 .
- Briat , JF . 1992 . Iron assimilation and storage in prokaryotes . J General Microbiol. , 138 : 2475 – 2483 .
- Corpe , WA and Basile , DV . 1982 . Methanol-utilizing bacteria associated with green plants . Develop in Indust Microbiol. , 23 : 483 – 493 .
- Eller , G and Frenzel , P . 2001 . Changes in the activity and community structure of methan-oxidizing bacteria over the growth period of rice . Appl Environ Microbiol. , 67 : 2395 – 2403 .
- Expert , D , Enard , C and Masclaux , C . 1996 . The role of iron in plant host-pathogen interactions . Trends in Microbiol. , 4 : 232 – 237 .
- Gholizadeh , A and BaghbanKohnehrouz , B . 2010 . A molecular evidence for the presence of methylobacterial-type Fe siderophore receptor in Celosia cristata . Plant Soil Environ. , 56 : 133 – 137 .
- Green , PN . 2006 . Methylobacterium , New York : Springer .
- Gupta , S , Lakshmi , AJ , Manjunath , MN and Prakash , J . 2005 . Analysis of nutrient and antinutrient content of underutilized green leafy vegetables . Food Sci Technol. , 38 : 339 – 345 .
- Harborne , JB and Turner , BL . 1984 . Plant Chemosystematics , London : Academic Press .
- Holland , MA and Polacco , JC . 1992 . Urease-null and hydrogenase-null phenotypes of a phylloplane bacterium reveal altered nickel metabolism in two soybean mutants . Plant Physiol. , 98 : 942 – 948 .
- Holland , MA and Polacco , JC . 1994 . PPFMs and other covert contaminants: is there more to plant physiology than just plant? Annual Review of Plant Physiology . Plant Mol Biol. , 45 : 197 – 209 .
- Ivanova , EG , Doronina , NV and Trotsenko , YA . 2001 . Aerobic methylobacteria are capable of synthesizing auxins . Microbiol. , 70 : 392 – 397 .
- Ivanova , EG , Doronina , NV , Shepeliakovskaia , AO , Laman , AG , Brovko , FA and Trotsenko , YA . 2000 . Facultative and obligate aerobic methylobacteria synthesize cytokinins . Mikrobiologiya , 69 : 764 – 769 .
- Knief , G , Ramette , A , Frances , L , Alonso-Blanco , C and Vorholt , JA . 2010 . Site and plant species are important determinants of the Methylobacterium community composition in the plant phyllosphere . ISME J. , 4 : 719 – 728 .
- Koenig , RL , Morris , RO and Polacco , JC . 2002 . tRNA is the source of low-level trans-zeatin production in methylobacterium spp . J Bacteriol. , 184 : 1832 – 1842 .
- Lim , BL . 2010 . TonB-dependent receptors in nitrogen fixing bacteria . Microbes Environ. , 25 : 67 – 74 .
- Listrom , ME and Chistoserdova , L . 2002 . Plants in the pink: cytokinin production by methylobacterium . J Bacteriol. , 184 : 1818
- Livak , KJ and Schmittgen , TD . 2001 . Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method . Methods , 25 : 402 – 408 .
- Loper , JE and Buyer , JS . 1991 . Siderophores in microbial interactions on plant surfaces . Mol Plant-Microbe Interact. , 4 : 5 – 13 .
- Nemecek-Marshall , M , MacDonald , RC , Franzen , JJ , Wojciechowski , CL and Fall , R . 1995 . Methanol emission from leaves: enzymatic detection of gas-phase methanol and relation of methanol fluxes to stomatal conductance and leaf development . Plant Physiol. , 108 : 1359 – 1368 .
- Ranganna , S . 1997 . Manual of analysis of fruit and vegetable products , New Delhi : Tata Mc Graw Hill .
- Sarkar , N . 1997 . Polyadenylation of mRNA in prokaryotes . Annu Rev Biochem. , 66 : 173 – 197 .
- Saravanamuthu , SS , Götz , FV , Salunkhe , P , Chozhavendan , R , Geffers , R , Buer , J , Tümmler , B and Steinmetz , I . 2004 . Evidence for Polyadenylated mRNA in Pseudomonas aeruginosa . J Bacteriol. , 186 : 7015 – 7018 .
- Sy , A , Giraud , E , Jouraud , P , Garcia , N , Willems , A , de Lajudie , P , Prin , Y , Neyra , M , Gillis , M , Boivin-Masson , C and Dreyfus , B . 2001 . Methylotrophic Methylobacterium bacteria nodulate and fix nitrogen in symbiosis with legumes . J Bacteriol. , 183 : 214 – 220 .
- Zabetakis , I . 1997 . Enhancement of flavour biosynthesis from strawberry (Fragraria ananassa) callus cultures by Methylobacterium species . Plant Cell Tissue Organ Cult. , 50 : 179 – 183 .
- Zhishen , J , Mengcheng , T and Jianming , W . 1999 . The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals . Food Chem. , 64 : 555 – 559 .