Abstract
I investigated how seed predation differed among tree species and among microhabitats across the Cross Timbers and what that variation may tell us about how this ecotone is maintained. The ecotone is located in Oklahoma, USA, between the eastern deciduous forest and tallgrass prairie where seeds of eight common tree species were placed in three microhabitats (oak forest, tallgrass prairie, and sumac shrub/small-tree/grass mix). After nine days in the field, percent seeds remaining were scored for each of the 120 (8 species×3 microhabitats×5 replicates) dishes. I found for both wind-dispersed tree species, (ash, elm) there was significantly more predation in the prairie microsite, with similar small predation levels in the shrub and forest. For two of the three bird-dispersed species (dogwood, hackberry), there was significantly more predation in the prairie and shrub microsites compared to the forest. Red cedar, however, was not taken by predators very much anywhere. Finally, all three mammal-dispersed tree species (two oaks, pecan) showed significantly more predation in the shrub and forest microsites compared to the prairie. Whereas wind- and bird-dispersed species suffered less predation as microsites became more woody and dark, the dominant oaks showed the opposite trend. Consequently, seed predators are not preventing oaks from advancing across this ecotone, but yearly fluctuations in predator population density, especially in the shrub transitional zone, could be helping to maintain it.
Introduction
Terrestrial vegetation can be organized into large-scale biomes containing species which have common adaptations to conditions within each biome (Walter Citation1973). Between these biomes are highly dynamic, overlapping boundary areas called ecotones (Clements Citation1905). Whereas these ecotones often have species from both bordering biomes, they may also contain their own unique species. In addition, ecotones may be different in physiognomy and patch structure compared to the biomes on either side. Although studied for decades, ecotones have recently received increased attention from scientists because (1) many of them are changing at a rate not seen for a long time (e.g. woody plants have been increasing invading grasslands worldwide: Scholes & Archer Citation1997), (2) they may respond to global warming before the bordering biomes (Allen & Breshears Citation1998), and (3) they may play an important evolutionary role by generating much of the biodiversity both for themselves and for their adjacent biomes (Smith et al. Citation1997).
Many of the most common and most studied ecotones are those that involve transitions between forest and grasslands (Myster Citation2012b), for example, those between forest and prairies (Petranka & McPherson Citation1979; Knapp et al. Citation1998), between forest and savannas (Schwartz et al. Citation1996; Hoffmann et al. Citation2004), and between forest and alpine grasslands (Cierjacks et al. Citation2007; Dauby & Hik Citation2007). Here in the USA, such an ecotone is the broad transitional boundary between the eastern deciduous forest and the grasslands of the southern Great Plains which includes an area called the Cross Timbers (Collins & Klahr Citation1991; Engle et al. Citation1991). This ecotone is characterized by patches of oak (Quercus sp.) dominated closed-canopy forest, patches of tallgrass prairie, and patches of shrub/small tree/grass mix. Forest patches become bigger and more common as you move east or into lower-lying, wetter areas, but alternatively tallprairie patches become bigger and more common as you move west or into higher, dryer areas (Myster, personal observation). Shrub patches, where shrubs (e.g. Rhus copallina) encroach into the grassland asexually and may shade out the resident grasses, fill in intermediate areas not claimed by forest or prairie and may be important for facilitating tree invasion (Petranka & McPherson Citation1979), as they do in old fields of the eastern deciduous forest (Myster Citation1993; Myster & Pickett Citation1993).
Past studies in the Cross Timbers and other forest-grassland ecotones have focused on the seedling and sapling stage of the tree life cycle and investigated controlling mechanisms such as water availability in the form of rainfall, below-ground competition, burning regime, patch-type, and wind-disturbance (Petranka & McPherson Citation1979; Hoffmann et al. Citation2004; Myster Citation2009a, Citation2009b; Myster & Malahy Citation2010). The seed stage, however, is also a critical part of the regeneration niche (Grubb Citation1977) in the Cross Timbers and needs investigation (see California ecotone studies by Kennedy Citation2005; Kennedy & Diaz Citation2005) for a complete understanding of the mechanisms maintaining this ecotone. Among those seed mechanisms, predation is one of the major causes of species composition of eastern deciduous forests and fields (Myster Citation1993). On the basis of studies in similar forest/grass areas, sources of variation in the working of seed predation that warrant investigation include: (1) increased predation by litter sheltering predators (Reader Citation1990; Aguilera & Lauenroth Citation1995; Bullock et al. Citation1995), (2) different predators having different seed preferences (Myster & Pickett Citation1993), (3) different seed densities leading to different predator responses (Myster & Pickett Citation1993), (4) different seasons of the year and different years eliciting different predator responses (Lindroth & Batzli Citation1984), and (5) predation levels differing among microsites (Collins & Adams Citation1983; Collins & Uno Citation1985; Knapp et al. Citation1998).
Therefore to better understand Cross Timbers seed processes and what controls and maintains this ecotone, I executed field experiments to: (1) investigate variation among common tree species in seed predation, (2) investigate variation among common microhabitats across the ecotone in seed predation, and (3) test the interactive effects of species and microhabitat in seed predation.
Materials and methods
The study site was located approximately 15 km west of Stillwater Oklahoma on a Cross Timbers area surrounding Lake Carl Blackwell in Payne County (36° 07′ 13″ N, 97° 13′ 20″ W). This is an ecological research area managed by Oklahoma State University (Petranka & McPherson Citation1979). Lake Carl Blackwell is in the Central Redbed Plains composed mainly of red sandstones and shales (Johnson et al. Citation1972), similar to soils and species composition found throughout the western Cross Timbers (Francaviglia Citation2000). Annual temperature ranges between 38°C and –18°C and there is an annual precipitation of 820 mm which occurs mainly during the growing season (Hoagland et al. Citation1999). Cross Timbers soils are of generally low fertility (Therrell & Stahle Citation1998).
Cross Timbers forest is dominated by post oak (Quercus stellata Wang.) and blackjack oak (Quercus marilandica Muen. Arevalo Citation2002), but includes other oaks and trees such as eastern red bud (Cercis canadensis L.), hickory (Carya sp. L.), slippery elm (Ulmus rubra Muhl.), scotch elm (Ulmus glabra), and american elm (Ulmus americana L.; Clark & Hallgren Citation2003). The forest borders an understory tree and shrub area consisting of the trees: rough-leaved dogwood (Cornus drummondi C.A. Mey), red cedar (Juniperus virginiana L.), and mexican plum (Prunus mexicana S. Wats.) with oak (Quercus sp.) seedlings intermixed beneath sumac shrub (Rhus sp.; Petranka & McPherson 1979). This understory tree and shrub area gives way to a mowed prairie only a few inches tall dominated by the grasses: Indian grass (Sorghastrum nutans L.), switchgrass (Panicum virgatum L.), little bluestem (Schizachyrium scoparium Michx. Nash), and big bluestem (Andropogon gerardii Vitman): Hoagland et al. Citation1999) and by forbs such as milkweed (Asclepias sp.), leadplant (Amorpha sp.), and ladies tresses orchid (Spiranthes lacera Raf. var. gracilis). Oklahoma State University mows the prairie twice a year in July and November.
On 5 July 2010, three 15 m transects were established in each of the three plant communities that make up the study site: (1) closed-canopy oak forest, (2) shrub/understory tree/grass mix, and (3) tallgrass prairie. Along each transect, five study microsites were established 3 m apart. At each microsite a plastic box (0.5 m×0.5 m×0.2 m) was anchored to the ground with nails. Within each box, eight plastic Petri dishes (20 cm diameter: Hulme Citation1994) were evenly placed. The boxes protected the seeds from effects of wind and weather and had two 20 cm diameter side holes cut out of them large enough to allow insects, birds, and mammals to enter and eat the seeds. Ten fruits/seeds each of Carya illinoensis (pecan), Celtis occidentalis (hackberry), C. drummondii (rough-leaved dogwood), Fraxinus americana (white ash), J. virginiana (red cedar), Q. marilandica (blackjack oak), Quercus stellate (post oak), and U. glabra (scotch elm: ) were randomly placed in separate dishes within each box. For nomenclature, I used the Plants Database, 2005. USDA. http://plants.usda.gov. All of the tree species were dispersing seeds during the time of study and seeds were collected locally from one individual tree the same day they were put out. Seeds were hand-sorted using gloves, visually inspected for damage, and then floated to further exclude nonviable seeds (as in Haught & Myster Citation2008). The wind-dispersed species are white ash and scotch elm, the bird-dispersed species are hackberry, rough-leaved dogwood, and red cedar, and the mammal-dispersed species are pecan, blackjack oak, and post oak.
Table 1. Seed characteristics of the eight test species sorted by increasing fresh mass (obtained from the Kew Botanical garden website www.kew.org/data/sid). Dispersal modes are found in Myster (Citation1993) and assume bird dispersal of oaks is minimal.
After nine days in the field, the percentage of seeds remaining in each Petri dish, which were not partially eaten and still looked viable, was scored. Evidence of seed predation was observed while collecting this data (e.g. chewed seeds and husks, small mammal feces: also seen in Blaney & Kotanen Citation2001) and 10 small, colored, toothpick pieces were placed in each of the dishes in all of the boxes to test for possible wind or rain splash removal of seeds. The toothpick controls were counted also after nine days in the field and found not to have been removed. Given this evidence, I made the assumption that the seeds had been removed by animals and did not germinate later, either because they were eaten or due to some other animal side effect. This assumption has been discussed in the literature for some years (see VanderWall et al. Citation2005 for a recent paper) but to date no study has produced statistically significant results to question its validity. In addition, attempts to use fish line glued to seeds or to tag seeds using isotopes, magnets, and fluorescent dyes lead to obvious experimental side effects of their own (also discussed in VanderWall et al. Citation2005). Only when the methodology has been proved effective, and germination after seed removal by animals has been shown to be statistically significant, should this design be reexamined.
Data were analyzed using a two-way analysis of variance (ANOVA) and means tests were conducted with the Tukey procedure (SAS Institute Citation1985) using individual species and plant community as the main effects. With five replicates of each treatment combination, all possible interactions among the two main effects could also be examined. All data were examined and found to be normally distributed and the Bonferroni test (Rice Citation1989) did not suggest that any significant results should be viewed with suspicion due to repeated ANOVA's. Data were also grouped together by dispersal vector – wind, bird, mammal – for interpretation.
Results
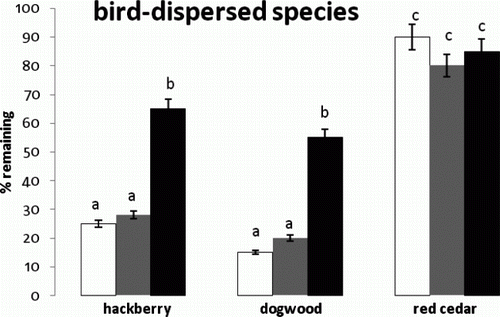
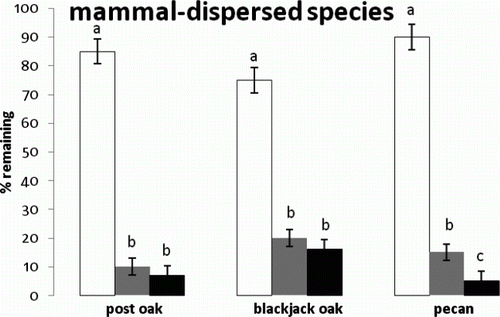
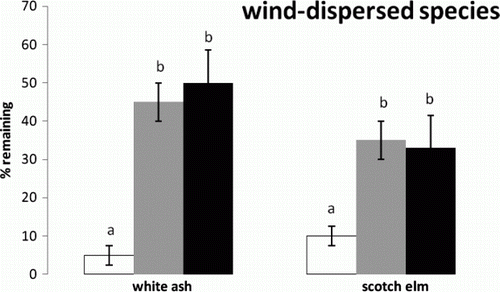
The average percentage of seed remaining in the field after nine days, for all species and plant communities, was 40%. Predation rates were not significantly different among the three plant communities nor were they significantly different among the nine species. The bird-dispersed species, however, were most different among species groups defined by dispersal vector. The interaction between species and plant community, however, was significant (F=5.43, df=16, P=0.023) so that the plant community effect was dependent on species. For both wind-dispersed tree species (), there was significantly more predation in the prairie and similar levels in the shrub and forest. Within the wind-dispersed species group, the lighter-seeded Scotch elm survived more in the prairie but less in the shrub and forest compared to white ash. For the heavier-seeded bird-dispersed species (hackberry and dogwood: ), there was significantly more predation in the prairie and shrub compared to the forest, with hackberry surviving a bit more than dogwood. The bird-dispersed red cedar was not taken by predators very much in any plant community. Finally, all three mammal-dispersed tree species showed significantly more predation in the shrub and forest compared to the prairie (). These differences were much greater than any plant community differences among the wind-dispersed or bird-dispersed species, approaching an order of magnitude. In addition, pecan showed significantly more predation in the forest compared to the other mammal-dispersed species.
Figure 1. Mean and standard error of percent seeds left for the interaction between wind-dispersed species and microsites. Means indicated with different letters were significantly different. Microsites are indicated as prairie (white), shrub (gray), and forest (black).
Discussion
There was a strong, significant pattern of small-seeded, wind-dispersed species suffering less predation as plant communities became more woody and closed, and this trend also existed for two out of the three bird-dispersed species. For the heavier mammal-dispersed species, however, the trend was reversed with more predation occurring in the forest. This is consistent with past studies in eastern deciduous forests recovering from agriculture where ants and other small insects took smaller seeds in open areas and squirrels and other small mammals took larger seeds in more closed areas where they found protection (Myster & Pickett Citation1993). Red cedar experienced low seed predation in this study, as it did in other field experiments in eastern deciduous forest (Myster & Pickett Citation1993). Other past experiments done in forested areas showed similar predations rates (Mittelbach & Gross Citation1984; Hulme Citation1994) and that predation of larger seeds increases as plant communities become more closed over time (Myster Citation1993; Blaney & Kotanen Citation2001) perhaps by providing more cover for seed predators.
Taken together, results suggest that trees and shrubs provide more cover than mowed prairie for predators capable of removing the larger seeds. Similar results were seen in a seed predation study across a forest-grassland ecotone in California where higher rates of seed predation were seen in the forest, medium rates in the ecotone, and the lowest rates in the grasslands for two oak species (Kennedy Citation2005; Kennedy & Diaz Citation2005). Other studies in forested areas (Hulme & Borelli Citation1999) have shown that predation of seeds of the sizes used in this study was mainly done by rodents, including deer mice (Peromyscus maniculatus). Litter is an important part of these plant communities and in a companion study (Myster, unpublished data), I found that an addition of local litter in each microsite changed predation rates for Q. stellata, decreasing rates in scrub and increasing rates in the forest.
Results support an escape hypothesis where oak seeds survive better if they disperse away from parent trees (Sork Citation1984), here especially into the prairie. Reduced tree seed predation under shrubs may be part of a more general effect of shrubs facilitating tree invasion together with other mechanisms like reducing herbaceous cover and vigor, shading and increasing soil moisture, soil nutrients, and relative humidity but also lowering both air and soil temperatures (Petranka & McPherson Citation1979). In addition, some shrubs like sumac (Rhus sp.) may have toxic chemicals in their leaves, rhizomes, and fruit which could inhibit tree seed germination and seedling growth as well as reduce arbuscular mycorrhizae availability to grasses (Petranka & McPherson Citation1979). The asexual habit of these shrubs helps their ramets establish in grass mats and many of the oaks in the Cross Timbers also send up root suckers (Myster, personal observation) to take advantage of this strategy and, perhaps, increase the probability of oak establishment. Once established, oak seedlings grow more under shrubs (as indicated by leaf chlorophyll content) and elm seedlings survive best and grow more under sumac (as indicated by leaf area ratio and leaf mass ratio: Myster Citation2009a, Citation2009b).
Whereas global warming may promote grasslands, fire suppression should help trees invade. Together these two anthroprogenic forces could then contribute to an ‘unstable equilibrium’ across this and other forest-grassland ecotones (Myster Citation2012b). Indeed in one study, fire decreased densities of J. virginiana, C. occidentalis, and U. americana, while increasing grass densities (Briggs et al. Citation2002). Water availability is also critical in the ecotone with diffuse competition for water particularly strong during drought years (Ross et al. Citation2003). In addition, (1) hailstorms may help trees, because grasslands lose more biomass (Peltzer & Wilson Citation2006), (2) trees may be facilitated when other trees shade and reduce wind (Baumeister & Callaway Citation2006), and (3) oaks may be able to allelopathically suppress other species by accumulation of their own tree leaf litter.
Results showed a significant difference in post-dispersal seed loss between species and plant community where lighter wind-dispersed seeds survived best in the forest and heavier mammal-dispersed seeds survived best in the prairie. Mixed shrub areas had intermediate levels, thus preserving this trend of a decrease in survivorship with increasing woody cover. This trend was most dramatic among the heaviest mammal-dispersed species except for red cedar. The suggestion here is that seed predators are not preventing oaks (which dominate this forest) and other mammal-dispersed tree species, which form canopies in eastern deciduous forests, from advancing across this ecotone. It may also be possible that yearly fluctuations in predator numbers and behaviors, especially in the shrub transitional zone, could be helping to maintain it. Finally the results and other similar past studies after agriculture in eastern deciduous forests (see Myster Citation1993) suggest that these tree species can disperse into the ecotone and that most of their seeds would germinate if they could escape predation (Myster Citation1994). Whereas seed mechanisms (e.g. dispersal, predation, and germination) may not be barriers to tree advancement, seedling mechanisms, and tolerances to, for example, temperature and soil moisture could be key in controlling this ecotone (Myster Citation2009a, Citation2009b) by determining how, or if, individual trees replace the resident grasses (Myster Citation2007, Citation2012a).
Conclusion
Combining these results with other similar studies, I suggest that Oak invasion into this ecotone is slowed down by mammalian seed dispersal (Myster, personal observation) as in old fields which border eastern deciduous forest (Myster & Pickett Citation1992), but Oak invasion can be helped by root sprouting if it as common in the ecotone as in the adjoining closed-canopy forest (Clark & Hallgren Citation2003). Likewise resprouting after a tornado may help trees to persist. If tree seeds can be dispersed into grassy patches, however, they will suffer low seed predation compared to shrub patches and underneath trees, where they are likely to germinate (Myster Citation1994). After germination, those tree seedlings that can escape herbivory have a major advantage. Oak seedlings growing under trees have a low mortality, but high mortality if growing under shrubs and in grass patches, perhaps due to intense below-ground competition for water (Myster Citation2009b). Taken together then the slow oak invasion, and ecotonal change, that has been observed for decades may be due, in part, to poor oak dispersal and the lack of patches which have high survivorship and growth for both oak seeds and oak seedlings.
Acknowledgements
I would like to thank C. Stansberry of Oklahoma State University-Stillwater for his help in finding the study site and in the execution of this project. I also thank L. Svensson and B. Collins for helpful comments on a previous draft of the manuscript.
References
- Aguilera , MO and Lauenroth , WK. 1995 . Seedling establishment in adult neighbourhoods-intraspecific constraints in the regeneration of the bunchgrass Bouteloua gracilis . J Ecol , 81 : 253 – 161 .
- Allen , CD and Breshears , DD. 1998 . Drought-induced shift of a forest-woodland ecotone: rapid landscape response to climate variation . Proc Nat Acad Sci , 95 : 14839 – 42 .
- Arevalo , JR. 2002 . Distribution of trees and saplings at the edge of Cross Timbers forests, Oklahoma USA . Nat Areas J , 22 : 99 – 107 .
- Baumeister , D and Callaway , RM. 2006 . Facilitation by Pinus flexilis during succession: a hierarchy of mechanisms benefits other plant species . Ecology , 87 : 1816 – 30 .
- Blaney , CS and Kotanen , PM. 2001 . Post-dispersal losses to seed predators: an experimental comparison of native and exotic old field plants . Can J Bot , 79 : 284 – 92 .
- Briggs , JM , Knapp , AK and Brock , BL. 2002 . Expansion of woody plants in Tallgrass prairie: a fifteen-year study of fire and fire-grazing interactions . Am Midl Nat , 147 : 287 – 94 .
- Bullock , JM , Clear Hill , B , Silvertown , J and Sutton , M. 1995 . Gap colonization as a source of grassland community change: effects of gap size and grazing on the rate and mode of colonization by different species . Oikos , 72 : 273 – 82 .
- Cierjacks , A , Iglesias , JE , Wesche , K and Hensen , I. 2007 . Impact of sowing, canopy cover and litter on seedling dynamics of two Polylepis species at upper tree lines in central Ecuador . J Trop Ecol , 23 : 309 – 18 .
- Clark , SL and Hallgren , SW. 2003 . Dynamics of oak (Quercus marilandica and Q. stellata) reproduction in an old-growth Cross Timbers forest . Southeast Nat , 2 : 559 – 74 .
- Clements , FC. 1905 . Research methods in Ecology , Lincoln , NE : University Publishing Co .
- Collins , SL and Adams , DE. 1983 . Succession in grasslands: Thirty-two years of change in a central Oklahoma tall-grass prairie . Vegetatio , 51 : 181 – 90 .
- Collins , SL and Klahr , SC. 1991 . Tree dispersion in oak-dominated forests along an environmental gradient . Oecologia , 86 : 471 – 7 .
- Collins , SL and Uno , GE. 1985 . Seed predation, seed dispersal and disturbance in grasslands: a comment . Am Nat , 125 : 866 – 72 .
- Dauby , RK and Hik , DS. 2007 . Variability, continquency and rapid change in recent subartic alpine tree line dynamics . J Ecol , 95 : 352 – 63 .
- Engle , DM , Stritzke , JF and McCollum , FT. 1991 . Vegetation management in the Cross Timbers: response of understory vegetation to herbicides and burning . Weed Technol , 5 : 406 – 10 .
- Francaviglia , R. 2000 . The Cast Iron forest: a natural and cultural history of the North American Cross Timbers , Austin , TX : University of Texas Press .
- Grubb , PJ. 1977 . The maintenance of species richness in plant communities: the importance of the regeneration niche . Bio Rev Cam Phil Soc , 52 : 107 – 111 .
- Haught , JE and Myster , RW. 2008 . Effects of species, density, season and prairie-type on post-dispersal seed removal in Oklahoma . Am Midl Nat , 159 : 482 – 8 .
- Hoagland , BW , Butler , IH , Johnson , FL and Glenn , S. 1999 . “ The Cross Timbers ” . In Savannas, Barrens and Rock Outcrop plant communities of North America , Edited by: Anderson , RC , Fralish , JS and Baskin , JM . 231 – 45 . Cambridge : Cambridge University Press .
- Hoffmann , W , Orthen , AB and Franco , AC. 2004 . Constraints to seedling success of savanna and forest trees across the savanna-forest boundary . Oecologia , 140 : 252 – 60 .
- Hulme , PE. 1994 . Post-dispersal seed predation in grassland: its magnitude and sources of variation . J Ecol , 82 : 645 – 52 .
- Hulme , PE and Borelli , T. 1999 . Variability in post-dispersal seed predation in deciduous woodland: relative importance of location, seed species, burial and density . Plant Ecol , 145 : 149 – 156 .
- Johnson , KS , Branson , CC , Curtis , NM , Ham , WH , Marcher , MV and Roberts , JF. 1972 . Geology and earth resources of Oklahoma , Dallas , TX : Educational publication I: Oklahoma Geologic survey, Steck-Warlick .
- Kennedy , PG. 2005 . Post-dispersal seed predation varies by habitat not acorn size for Quercus chrysolepis (Fagaceae) and Lithocarpus densiflora (Fagaceae) in central coastal California . Madrono , 52 : 30 – 4 .
- Kennedy , PG and Diaz , JM. 2005 . The influence of seed dispersal and predation on forest encroachment into a California grassland . Madrono , 52 : 21 – 9 .
- Knapp , AK , Briggs , JM , Hartnett , DC and Collins , SL. 1998 . Grassland dynamics: long-term ecological research in Tallgrass prairie , Oxford : Oxford University Press .
- Lindroth , RL and Batzli , GO. 1984 . Food habits of the meadow vole (Microtus pennsylvanicus) in bluegrass and prairie habitats . J Mammal , 65 : 600 – 6 .
- Mittelbach , GG and Gross , KL. 1984 . Experimental studies of seed predation in oldfields . Oecologia , 56 : 109 – 116 .
- Myster , RW. 1993 . Tree invasion and establishment in old fields at Hutcheson Memorial Forest . Bot Rev , 59 : 251 – 72 .
- Myster , RW. 1994 . Contrasting litter effects on old field tree germination and emergence . Vegetatio , 114 : 169 – 74 .
- Myster , RW. 2007 . “ Introduction ” . In Post-agricultural succession in the Neotropics , Edited by: Myster , RW . 1 – 21 . Berlin : Springer-Verlag .
- Myster , RW. 2009a . Controls on Shumard Oak (Quercus shumardii) establishment into the Cross Timbers ecotone of Oklahoma: implications for restoration . Rest Ecol , 17 : 893 – 9 .
- Myster , RW. 2009b . Tree survivorship, growth, and allocation in the Cross Timbers ecotone of Oklahoma . Plant Ecol , 205 : 193 – 9 .
- Myster , RW. 2012a . Plant replacing plants: the future of community modeling and research . Bot Rev , 78 : 2 – 9 .
- Myster , RW. 2012b . Ecotones between forest and grassland , Berlin : Springer-Verlag .
- Myster , RW and Malahy , MP. 2010 . Spatial heterogeneity of Tornado damage and resprouting in the Cross timbers ecotone of Oklahoma . J Plant Ecol , 3 : 157 – 63 .
- Myster , RW and Pickett , STA. 1992 . Effects of palatability and dispersal mode on spatial patterns of tree seedlings in old fields . Bull Torrey Bot Club , 119 : 145 – 51 .
- Myster , RW and Pickett , STA. 1993 . Effects of litter, distance, density and vegetation patch type on post dispersal tree seed predation in old fields . Oikos , 66 : 381 – 8 .
- Peltzer , DA and Wilson , SD. 2006 . Hailstorm damage promotes aspen invasion into grassland . Can J Bot , 84 : 1142 – 7 .
- Petranka , JW and McPherson , JK. 1979 . The role of Rhus copallina in the dynamics of the forest-prairie ecotone in North-central Oklahoma . Ecology , 60 : 956 – 65 .
- Reader , RJ. 1990 . Control of seedling emergence by ground cover: a potential mechanism involving seed predation . Can J Bot , 69 : 2084 – 7 .
- Rice , WR. 1989 . The sequential Bonferroni test . Evolution , 43 : 223 – 5 .
- Ross , AL , Foster , BL and Loving , GS. 2003 . Contrasting effects of plant neighbors on invading Ulmus rubra seedlings in a successional grassland . Ecoscience , 10 : 525 – 31 .
- SAS Institute . 1985 . User's guide: statistics , 5th ed , Cary , NC : SAS Institute, Inc .
- Schwartz , D , de Foresta , H , Mariotti , A , Balesdent , J , Massimba , JP and Girardin , C. 1996 . Present dynamics of the savanna-forest boundary in the Congolese Mayombe: a pedological, botanical and isotopic (13C and 14C) study . Oecologia , 106 : 516 – 24 .
- Scholes , RJ and Archer , SR. 1997 . Tree-grass interactions in Savannas . Ann Rev Ecol Syst , 28 : 517 – 44 .
- Smith , TB , Wayne , RK and Girman , DJ. 1997 . A role for ecotones in generating rainforest biodiversity . Science , 276 : 1855 – 7 .
- Sork , VL. 1984 . Examination of seed dispersal and survival in red oak, Quescus rubra (Fagaceae), using metal-tagged acorns . Ecology , 65 : 1020 – 2 .
- Therrell , MD and Stahle , DW. 1998 . A predictive model to locate ancient forests in the Cross Timbers of Osage county, Oklahoma . J Biogeogr , 25 : 847 – 54 .
- VanderWall , SB , Kuhn , KM and Beck , MJ. 2005 . Seed removal, seed predation, and secondary dispersal . Ecology , 86 : 801 – 6 .
- Walter , H. 1973 . Vegetation of the earth , Berlin : Springer-Verlag .