Abstract
In order to localize the main organic site of alkaline toxicity to plants, the injury of NaCl or NaHCO3 to tobacco (Nicotiana tabacum L.) seedlings was compared. The results showed that the injury effect of alkaline stress on tobacco were much stronger than salt stress, with respect to growth retardation, photosynthetic inhibition, and ionic unbalance. The root/shoot ratio, transpiration rate, and Na+ accumulation in the leaves were lower than other. Further investigation of the root ultrastructure showed that the endoplasmic reticulum and nuclear membrane were not visible or absent, while more small vacuoles and mitochondria with dilated or absent cristae were observed under the 100 mM NaHCO3 stress. These results indicate that under alkaline conditions, the endomembrane system of root cells is directly and seriously damaged. This possibly causes the failure of water absorption and ion compartmentalization.
Introduction
The alkalization of soil is a serious environmental problem in China and in other areas (Zhang & Mu Citation2009). Alkaline salt-affected soil is typically characterized by the accumulation of sodium carbonate and bicarbonate. Actually, the problem of soil alkalization due to NaHCO3 and Na2CO3 may be more severe than the problem of soil salinization caused by neutral salts such as NaCl and Na2SO4 (Shi & Wang Citation2005). Some studies examined the mechanisms of injury caused by neutral salt stress (Munns & Tester Citation2008). Injury from salt stress generally involves osmotic stress and ionic toxicity (Munns Citation2002). A two-phase growth response to salinity is common; the first phase of growth reduction is quickly apparent and is due to the salt outside the roots. The two-phase growth response to salinity starts when salt accumulates to toxic concentrations in the old leaves. When Na+ enters cells and accumulates to high levels, it becomes toxic to enzymes (Hasegawa et al. Citation2000). The main site of Na+ toxicity for most plants is the leaf blade rather than the roots, where Na+ accumulates after being deposited in the transpiration stream (Munns Citation2002).
In saline and sodic soils, the major solutes comprising dissolved mineral salt are the cations Na+, Ca2 +, Mg2 +, and K+ and the anions Cl−, ,
,
, and
(Shi & Wang Citation2005). When a salinized soil contains
, it causes injury to the plant not only through salt stress but also through alkali stress (Shi & Wang Citation2005). The high pH also disrupts ionic balance, especially the balance between potassium and sodium (Peng et al. Citation2004; Yang et al. Citation2008), causing K+/Na+ ratio imbalance. The leaf electrolyte leakage rate also suggests direct disruption of the structure of membrane selectivity (Shi & Sheng Citation2005; Shi & Wang Citation2005; Li et al. Citation2010). However, little attention has been focused on the main site of NaHCO3 toxicity for most plants. Therefore, this study aims to compare alkaline stress with saline stress, to determine rapid and accumulated growth response to alkaline salt, and to indentify the main site of NaHCO3 toxicity.
Materials and methods
Plant material and growth conditions
Seeds of tobacco (Nicotiana tabacum Linn.) were sown on cleaned quartz sands at 25°C in green house. The germinated seedlings were grown in Hoagland solution. When grown to about the four-leaf stage, similarly sized seedlings were chosen and cultivated in Hoagland solution with different salt concentrations added to measure morphological and physiological changes for one month in a greenhouse. The salt concentrations were 100 mM NaCl (pH 6.1), 200 mM NaCl (pH 6.0), and 100 mM NaHCO3 (pH 7.8) with Hoagland solution (pH 6.1) without added salts as the control. Each treatment has five to six seedlings as replications, and each seedling was planted in a single pot.
Gas exchange measurements
CO2 absorbance, stomatal conductance, and transpiration were measured at 30th day after initiation of the salt treatments on the second or third youngest fully expanded leaves. Measurements were made with a LI-COR 6400 portable photosynthetic system (LI-COR, Lincoln, NE). The photon flux density was 1200 µmol·m−2s−1 and 0 µmol·m−2s−1, which correspond to saturation light and dark conditions. Measurements were repeated five times for each blade, for five blades per treatment. Water use efficiency was analyzed as the relationship between net CO2 absorbance and transpiration from measurements of leaf gas exchange.
Measuring the biomass of roots, stems, and leaves
After the 30-day salt treatment, the whole plant was taken out from the cultural pot and washed with running water and then with distilled water thrice. The plants were divided into three parts (root, stem, and leaf), and then the fresh samples were dried in the oven for 30 min at 105°C and maintained at 80°C until a constant weight. This was measured as dry weight (DW).
Na+ and K+ content determination
The roots of the seedlings that received the 30-day treatment were rinsed with running water and then with deionized water thrice before they were dried with filter paper. The roots, stems, and old leaves were dried at 80°C to a constant weight. About 0.4 g of the dried powder samples were digested with HNO3 and HClO4 (4:1) at 400°C. The Na+ and K+ contents were determined with an Analytik Jena NOVAA-350 flame atomic absorption spectrometer (Germany).
Sample processing for transmission electron microscopy
Root-tip sections (1–2 mm) were cut with a sharp blade and then immersed in 5% glutaraldehyde in 0.05 M phosphate buffer at pH 6.8. Root-tip sections were repeated three times from three different seedlings. These sections were fixed overnight in 5% glutaraldehyde at 4°C and, subsequently, rinsed thrice with 0.05 M phosphate buffer at 10 min intervals. The tissues were then post fixed in 1% OsO4 in the same buffer at 4°C for 1.5 h and dehydrated with a graded series of ethanol (50, 70, 90, and 100%); ethanol was later replaced by acetone. The samples were embedded in 812 resin and polymerized at 70°C for 24 h. Ultrathin sections (70–90 nm in thickness) were cut with a diamond knife and placed on 200-mesh copper grids. The grids were stained with 2% uranyl acetate for 20 min followed by lead citrate for 5 min. Then the sections were viewed on an H7650 (Hitachi, Japan) transmission electron microscope. Changes of ultrastructure in the elongation zone of the root were examined through sections perpendicular to the axes. Sample for transmission electron microscope was replicated three times.
Statistical analyzes
All data experiments were based on five replicated measurements. Data were analyzed by one-way analysis of variance using the statistical software SPSS 14.0 (SPSS Inc., Chicago, USA). Multiple comparisons of means of data treatments within the plants were performed using Duncan's test at the 0.05 significance level. Gas exchange characteristics, biomass, and ion content were represented by means and standard errors (S.E.).
Results
Leaf gas exchange responses to salt and alkaline stress
The CO2 absorbance, stomatal conductance, intercellular CO2, transpiration rate, and dark respiration rate of tobacco were affected significantly by different salinity of the culture medium (). The lower photosynthetic rate in salt-treated leaves was associated with the reduced stomatal conductance and transpiration rate. Under 100 mM NaHCO3 treatment, CO2 absorbance and stomatal conductance were greatly reduced compared with the other treatments. However, water use efficiency was markedly higher in neutral salt stress. Compared with the control treatments, the water use efficiency of tobacco leaves was increased 1.65 and 2.24 times under 100 mM NaCl and 200 mM NaCl, respectively. However, under alkaline salt (NaHCO3) stress, the water use efficiency was decreased significantly. These results show that under NaCl and NaHCO3 stress of the same concentration, NaHCO3 stress had a stronger influence on the photosynthetic organs than under NaCl stress. Injury was also more severe with NaHCO3 stress than with NaCl stress.
Table 1. Gas exchange characteristics of tobacco treated with different salt treatments.
Accumulated responses of growth to salt and alkaline stress
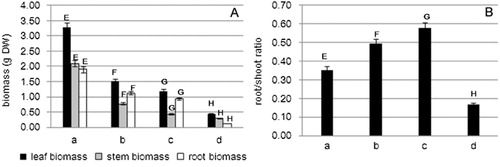
Different salt treatments produced remarkable differences in growth (). With the treatment of 100 mM NaCl, 200 mM NaCl, and 100 mM NaHCO3, the biomass of roots, stems, and leaves was reduced. Biomass reduction under the three treatments always reached significant values (P <0.05). In the 100 mM NaHCO3 treatment, the biomass was reduced more evidently than in the other treatments. However, different parts of the seedling also showed different levels of response to different salt stresses (). The root/shoot ratios of NaCl-treated plants were all higher than controls, and the ratios were increased with increasing concentration of NaCl. However, under increasing NaHCO3 stress, the root/shoot ratio decreased significantly compared with the other treatments. This shows that the growth suppression of belowground parts was more severe than those of the stems and leaves. NaHCO3 stress had a severe effect on the root growth of tobacco.
Accumulated responses of ion to salt and alkaline stress
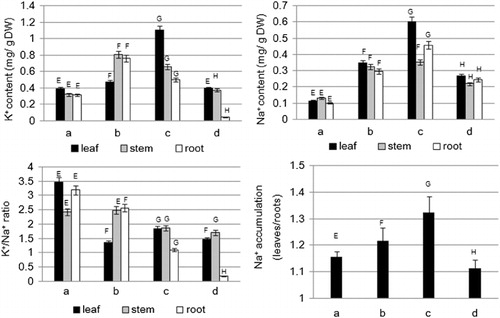
The ion content of the leaves, stems, and roots from the different treatments was determined (). The K+ and Na+ content of the leaves, stems, and roots increased significantly with increasing NaCl levels. However, under NaHCO3 stress, the K+ content of the leaves (0.39 mg g−1 DW) and stems (0.37 mg g−1 DW) was not significantly changed compared with that of the sample in the control treatment (0.39 mg g−1 DW, 0.32 mg g−1 DW, respectively). At the same time, the K+ content of the roots (0.04 mg g−1 DW) decreased more significantly under NaHCO3 stress than in the other solutions. The Na+ content of the leaves, stems, and roots was increased significantly by 0.27 mg g−1 DW, 0.22 mg g−1 DW, and 0.24 mg g−1 DW, respectively, at 100 mM NaHCO3 stress compared with the control treatment (0.11 mg g−1 DW, 0.13 mg g−1 DW, and 0.10 mg g−1 DW, respectively). The K+/Na+ content ratio decreased significantly under NaCl and NaHCO3 stresses, and the magnitude of decrease was from 3.45 to 0.18. The K+/Na+ ratio was minimal up to 0.18 on the root of the plants from NaHCO3 stress. The K+/Na+ ratio decreased significantly under the NaHCO3 stress environment as a combined effect of increased Na+ content and decreased K+ content. The leaf Na+ content/root Na+ content was used as an indicator of salt accumulation in old leaves. In the 100 and 200 mM NaCl treatments, the Na+ accumulated ratio was 1.22 and 1.32, respectively, whereas that in the control treatment was 1.16. A clear accumulation of Na+ was found under neutral salt stress with increased salinity stress. However, the Na+ accumulated ratio was 1.11 in the 100 mM NaHCO3 treatment, which has no significant difference with control (P >0.05).
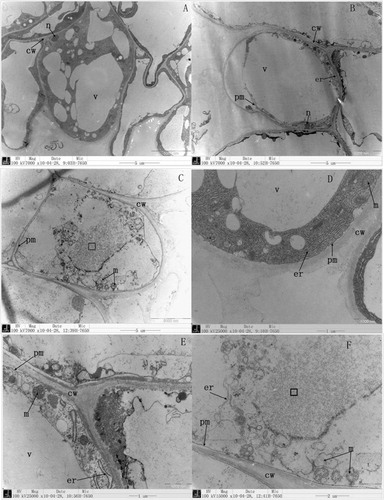
Root ultrastructure to high salt and alkaline stress
Observation of epidermal cells using transmission electron microscope showed a marked difference among the different salt treatments (). In the control () and 100 mM NaCl treatment (), the central vacuole was prominent, and the vacuole membrane was clearly distinguishable. However, in the 100 mM NaHCO3 treatment (), the central vacuole membrane was not clearly distinguishable, and the cytoplasm had lysed regions. In the control treatment and 100 mM NaCl-treated roots, the endoplasmic reticulum was visible, the nucleus was found to have densely packed nucleoli, and the mitochondria showed intact membranes and cristae ( and ). However, in the 100 mM NaHCO3-treated roots (), the endoplasmic reticulum and nuclear membrane were not visible or absent. More small vacuoles and mitochondria were observed, and even the mitochondria were globular with dilated or absent cristae. In the 100 mM NaCl-treated plants, the endoplasmic reticulum was reduced, and the mitochondria were increased, but the mitochondrial cristae were clearly distinguishable. These results show that the cell endomembrane system was seriously injured with 100 mM NaHCO3.
Discussions
Soils are classified as saline when the electrical conductivity is 4 dS/m or greater, which is equivalent to approximately 40 mM NaCl. Osmotic stress occurs in most plants at approximately 40 mM NaCl (Munns & Tester Citation2008). For arabidopsis (salt-sensitive species), continued exposure to 100 mM NaCl does not allow the completion of its life cycle (Sickler et al. Citation2007), but many dicotyledonous halophytes require a relatively high NaCl concentration (100–200 mM) for optimum growth (Flowers et al. Citation1977). At NaCl concentration of 300 mM, the photosynthesis system of tobacco leaves can be seriously endangered (Badawi et al. Citation2004a, Citation2004b; Cao et al. Citation2006). The stress caused by 150 mM NaCl on tobacco could reduce the fresh weight by 20% and can significantly increase the Na+/K+ ratio in the leaf (Cao et al. Citation2006). The effect of alkaline salt stress was stronger than that of neutral salt stress at the same salt concentrations (Shi & Wang Citation2005). We observed that when tobacco was treated with 150 mM NaHCO3, the plant cannot survive for a long time.
In vitro studies have shown that Na+ starts to inhibit many enzymes at concentrations approaching 100 mM (Greenway & Osmond Citation1972). Several enzymes are sensitive to lower concentrations (Flowers & Dalmond Citation1992), but high concentrations of Na+ were found in leaves that are still functioning normally. This result suggests that the compartmentation and transportation of Na+ are an essential mechanism in plants salt tolerances. In neutral salt stress, plants can regulate the root/shoot ratio to keep absorbing water from the salt solution. However, at NaHCO3 solution concentrations below 100 mM, the decrease in the root/shoot ratio and the transpiration rate caused difficulty in Na+ transport. At low to moderate salinity levels, plants could usually maintain a high K+/Na+ ratio to protect the activity of the enzyme in the root cytoplasm by extruding Na+ ions out of the cell and by vacuolar compartmentation of Na+ ions (Yamaguchi & Blumwald Citation2005). In highly concentrated NaCl stress, the root cells also show several damage characteristics (Niu et al. Citation1996; Pareek et al. Citation1997; Rahman et al. Citation2001; Bennici & Tani Citation2009). The findings of Rahman indicated that in 1% NaCl-treated rice seedlings, the stroma of the plastids, the matrix of the mitochondria, the nucleoplasm, and the cytoplasm all appear to have a comparable high density (Rahman et al. Citation2001). These studies suggest that the efflux and compartmentalization of Na+ have specific limits. In high salt stress, the concentrations of Na+ in the cytoplasm increased and, ultimately, affected the cell structure. In 100 mM NaCl stress, the structure of the tobacco root cell was not destroyed, but the endomembrane system of the root cells was severely damaged. Na+ extrusion or compartmentation was triggered by H+-ATPase from the plasma membrane or the vacuolar membrane. The integrity and functionality of the endomembrane system is very important, while the leaf electrolyte leakage rate observed during alkaline stress suggested the disruption of the structure of membrane selectivity (Shi & Yin Citation1993; Shi & Sheng Citation2005; Shi & Wang Citation2005; Li et al. Citation2010).
The growth inhibition effect of alkaline salt stress was stronger than that of neutral salt stress at the same salt concentrations (El-Samad & Shaddad Citation1996; James et al. Citation2002; Nuttall et al. Citation2003; Shi & Sheng Citation2005). At low to moderate salinity levels, osmotic stress may be the main stress caused by NaHCO3 impact on plants, which is similar to NaCl stress. However, at high levels (100 mM), Na+ accumulation in leaves was not observed, and the transpiration rate and the root/shoot ratio were significantly decreased. The significant reduction in the transpiration rate and the root/shoot ratio not only increased the difficulties of plants to absorb water but also reduced the probability of Na+ to be transported to the aerial parts of plants through the flow of water. These characteristics of tobacco cannot be possibly explained by the mechanism of Na+ accumulation in the leaves and cannot explain the fact that the formation of bare land was easier in sodic soil. Further analysis on the root cell structure shows that the endomembrane system of the root cells was directly and seriously damaged. This result possibly caused the failure of water absorption and ion compartmentalization. At the plant level, the damaged roots seriously affected the transpiration rate and Na+ transport with water to the leaves.
Acknowledgments
The study was supported by the Special Fund for Basic Scientific Research of Central Colleges (DL12BA05, DL11BA15) and the National Science Foundation of China (31000176/C030301).
References
- Bennici A, Tani C. 2009. Ultrasturctural effects of salinity in Nicotiana bigelovii var. bigelovii callus cells and Allium cepa roots. Caryologia. 62:124–133.
- Badawi GH, Kawano N, Yamauchi Y, Shimada E, Sasaki R, Kubo A, Tanaka. 2004b. Over-expression of ascorbate peroxidase in tobacco chloroplasts enhances the tolerance to salt stress and water deficit. Physiol Plantarum. 121:231–238. 10.1111/j.0031-9317.2004.00308.x
- Badawi GH, Yamauchi Y, Shimada E, Sasaki R, Kawano N, Tanaka K, Tanaka K. 2004a. Enhanced tolerance to salt stress and water deficit by overexpressing superoxide dismutase in tobacco (Nicotiana tobacum) chloroplasts. Plant Sci. 166:919–928. doi: 10.1016/j.plantsci.2003.12.007
- Cao WH, Liu J, Zhou QY, Cao YR, Zheng SF, Du DX, Zhang JS, Chen SY. 2006. Expression of tobacco ethylene receptor NTHK1 alters plant responses to salt stress. Plant Cell Environ. 29:1210–1219. doi: 10.1111/j.1365-3040.2006.01501.x
- El-Samad HMA, Shaddad MAK. 1996. Comparative effect of sodium carbonate, sodium sulphate, and sodium chloride on the growth and related metabolic activities of pea plants. J Plant Nutr. 19:717–728. doi: 10.1080/01904169609365155
- Flowers TJ, Dalmond D. 1992. Protein synthesis in halophytes: the influence of potassium, sodium and magnesium in vitro. Plant Soil. 146:153–161. doi: 10.1007/BF00012008
- Flowers TJ, Troke PF, Yeo AR. 1977. The mechanism of salt tolerance in halophytes. Annu Rev Plant Physiol. 28:89–121. doi: 10.1146/annurev.pp.28.060177.000513
- Greenway H, Osmond CB. 1972. Salt responses of enzymes from species differing in salt tolerance. Plant Physiol. 49:256–259. doi: 10.1104/pp.49.2.256
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. 2000. Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Bio. 51:463–499. doi: 10.1146/annurev.arplant.51.1.463
- James SA, Bell DT, Robson AD. 2002. Growth response of highly tolerant eucalyptus species to alkaline pH, bicarbonate and low iron supply. Aust J Exp Agr. 42:65–70. doi: 10.1071/EA00154
- Li R, Shi F, Fukuda K. 2010. Interactive effects of various salt and alkali stresses on growth, organic solutes, and cation accumulation in a halophyte Spartina Alterniflora (Poaceae). Environ Exp Bot. 68:66–74. doi: 10.1016/j.envexpbot.2009.10.004
- Munns R. 2002. Comparative physiology of salt and water stress. Plant Cell Environ. 25:239–250. doi: 10.1046/j.0016-8025.2001.00808.x
- Munns R, Tester M. 2008. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911
- Niu X, Damsz B, Kononowicz AK, Bressan RA, Hasegawa PM. 1996. NaCl-induced alterations in both cell structure and tissue-specific plasma membrane H + -ATPase gene expression. Plant Physiol. 111:679–686.
- Nuttall G, Armstrong RD, Connor DJ. 2003. Evaluating physicochemical constraints of calcarosols on wheat yield in the Victorian southern Mallee. Aust J Agr Res. 54:487–497. doi: 10.1071/AR02168
- Pareek A, Singla SL, Grover A. 1997. Short-term salinity and high temperature stress-associated ultrastructural alterations in young leaf cells of Oryza sativa L. Ann Bot-London. 80:629–639. doi: 10.1006/anbo.1997.0494
- Peng YH, Zhu YF, Mao YQ, Wang SM, Su WA, Tang ZC. 2004. Alkali grass resists salt stress through high [K+] and an endodermis barrier to Na+. J Exp Bot. 55:939–949. doi: 10.1093/jxb/erh071
- Rahman MS, Matsumuro T, Miyake H, Takeoka Y. 2001. Effects of salinity stress on the seminal root tip ultrastructures of rice seedlings (Oryza sativa L.). Plant Prod Sci. 4:103–111. doi: 10.1626/pps.4.103
- Shi DC, Sheng Y. 2005. Effect of various salt-alkaline mixed stress conditions on sunflower seedlings and analysis of their stress factors. Environ Exp Bot. 54:8–21. doi: 10.1016/j.envexpbot.2004.05.003
- Shi DC, Wang DL. 2005. Effects of various salt-alkaline mixed stresses on Aneurolepidium Chinense (Trin.) Kitag. Plant Soil. 271:15–26. doi: 10.1007/s11104-004-1307-z
- Shi DC, Yin LJ. 1993. Difference between salt (NaCl) and alkaline (Na2CO3) stresses on Puccinellia tenuiflora (Griseb.) Scribn et Merr. plants. Acta Bot Sin. 35:144–149.
- Sickler CM, Edwards GE, Kiirats O, Gao Z, Loescher W. 2007. Response of mannitol producing Arabidopsis thaliana to abiotic stress. Funct Plant Biol. 34:382–391. doi: 10.1071/FP06274
- Yamaguchi T, Blumwald E. 2005. Developing salt-tolerant crop plants: challenges and opportunities. Trends Plant Sci. 10(12), 615–620. doi: 10.1016/j.tplants.2005.10.002
- Yang CW, Shi DC, Wang DL. 2008. Comparative effects of salt stress and alkali stress on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte Suaeda Glauca (Bge.). Plant Growth Regul. 56:179–190. doi: 10.1007/s10725-008-9299-y
- Zhang JT, Mu CS. 2009. Effects of saline and alkaline stresses on the germination, growth, photosynthesis, ionic balance and anti-oxidant system in an alkali-tolerant leguminous forage Lathyrus Quinquenervius. Soil Sci Plant Nutr. 55:685–697. doi: 10.1111/j.1747-0765.2009.00411.x