Abstract
In this study, we evaluate the temporal variation in extrafloral nectaries (EFNs) secretion in different ontogenic stages of Alibertia verrucosa (Rubiaceae) fruits in a Neotropical savanna. We observe greater nectar secretion rate in fruits of intermediate size compared with young or ripe fruit, indicating that they are possibly more protected by ants. In addition, the nectar secretion was higher at night, a pattern that could be associated with an increase of herbivore pressure and water stress during the daylight hours. In fact, due to the high temperature and low humidity during the day in savannas, most herbivores display strong nocturnal activity, and plants can avoid nectar secretion in this period. Our results indicate that A. verrucosa can change the ants' attraction according to EFN secretion during the ontogenic stages of the fruits, probably secreting more nectar when the biotic defense are more necessary for the protection of the fruits and the plant as a whole.
1. Introduction
Over evolutionary time, the strong pressure exerted by herbivores has led plants to develop different chemical, structural, and biological strategies that can reduce plant damage by herbivores (Marquis Citation1984; Coley & Kursar Citation1996). The main and best-known defense mechanism against herbivory is direct defense, in which the plants have secondary compounds in several structures that make the herbivores’ ability to exploit these plants more difficult (Coley & Kursar Citation1996; Cortesero et al. Citation2000). However, plants also have indirect defenses by the third trophic level, particularly predator insect and parasitic wasps and flies (parasitoids), which are known to protect plants against herbivory (Rico-Gray & Oliveira Citation2007; Romero et al. Citation2008). This indirect and biotic defense is usually induced, where the plant releases volatile and/or nutritive substances that attract predators and herbivore parasitoids (Kessler & Baldwin Citation2001; Sabelis et al. Citation2001; Heil Citation2008).
In tropical ecosystems, ants have an important role in the biotic defense of plants (Rico-Gray & Oliveira Citation2007; Rosumek et al. Citation2009; Romero & Koricheva Citation2011). In this region, thousands of angiosperms and pteridophytes have extrafloral nectaries (EFNs) that secrete an energy-rich liquid that attracts ants (Rico-Gray & Oliveira Citation2007). Several authors have reported not only the increase in the rate of leaf herbivory, but also the reduction of fruit production in plants isolated from ants (Oliveira et al. Citation1999; Nascimento & Del-Claro Citation2010), which is reflected in the increase of EFN-plants' fitness visited by ants (Rico-Gray & Thien Citation1989; Oliveira et al. Citation1999; Chamberlain & Holland Citation2009). In fact, most studies involving the interaction between ants and EFN plants have focused only on the decrease of leaf herbivory (see Rico-Gray & Oliveira Citation2007 and references therein). In such cases, EFNs are extremely active in young leaves, which are more vulnerable to damage caused by herbivores (Heil et al. Citation2000; Korndörfer & Del-Claro Citation2006). During leaf ontogeny, EFNs decrease nectar secretions when the leaf begins to invest in physical defenses, such as cellulose, lignin, and silica (Dyer et al. Citation2001; Korndörfer & Del-Claro Citation2006).
However, it has been found that some plant species have EFN in their fruits (Rico-Gray Citation1989; Díaz-Castelazo et al. Citation2005; Holland et al. Citation2010). Since the function of EFN is traditionally related to the attraction of ants (Bentley Citation1977), it is expected that the presence of these structures on fruits may be related to the protection of fruits and seeds in formation. In addition, several studies have shown that plants invest in defense especially in reproductive (flowers) and dispersion (fruits) structures (Herrera Citation1982; Cipollini & Levey Citation1997; Dicke Citation1999; Thornburg et al. Citation2003; Carter et al. Citation2007), mainly by the fact that these structures are directly related to their reproductive success (Cipollini & Levey Citation1997; Holland et al. Citation2009). In fact, during fruit growth and maturation more energy is allocated in these structures and the more valuable it becomes (Cousens et al. Citation2008). Therefore, it is expected that plants invest more in defense in the later stages along ontogeny of the fruits, once ripe fruits are more apt to dispersion (Cipollini & Levey Citation1997). Thus, if the strategy of a plant species against herbivory is biotic defense, an increase in nectar secretion is expected during ontogenic stages of the fruits, increasing the visitation of protective ants.
Nevertheless, once nectar is costly to the plant, its secretion may not be continuous (Southwick Citation1984; Pyke Citation1991; Heil et al. Citation2000). In regions with high temperature and low humidity during the day (e.g. Neotropical savanna), most herbivores display strong nocturnal activity due to their ecophysiological limitations (Fagundes et al. Citation1996; Strauss et al. Citation2009; Byk & Del-Claro Citation2010). Therefore, a change in the nectar secretion during periods with high herbivory and/or high levels of water stress can reduce energy loss in ‘superfluous’ EFN secretion, and reduce the risk of EFN fungal infections (Heil et al. Citation2000; Holland et al. Citation2010; Yamawo et al. Citation2012a).
In this study, we evaluate the temporal variation in EFN secretion in different ontogenic stages of Alibertia verrucosa S. Moore (Rubiaceae) fruits in a Brazilian Neotropical savanna. Since the reproductive success of A. verrucosa is directly related to its fruit dispersal performed by birds and mammals (J.C.F.F. and T.J.I. unpublished observations), it is expected that bigger and riper fruits are more often dispersed. In this context, we hypothesized that the bigger and riper the fruits of A. verrucosa, the greater the EFN secretion and, indirectly, the biotic defenses provided by ants. Moreover, we also hypothesized that due to nocturnal foraging of most herbivores and water stress during the daylight hours in the Neotropical savanna, the EFN secretion of A. verrucosa is higher at night, which attracts more ants.
2. Materials and methods
2.1. Study area
We conducted this study at Estação Ecológica Serra das Araras (EESA) (15°38′S and 57°12′W, elev. 217 m), located between the municipalities of Porto Estrela, Cáceres, and Barra do Bugres, west of the state of Mato Grosso, Brazil. The reserve is a Conservation Unit of Integral Protection under the administration of the Brazilian Institute for the Environment and Natural Resources (IBAMA). According to the Köppen classification, the climate is Tropical savanna (Aw) with an average annual temperature of 28°C, humidity 70%, and 1.400 mm of precipitation. It has two well-defined seasons, a rainy season between November and April and dry season between May and October. The reserve area covers 28.700 ha of continuous forest with different physiognomies. However, the study area is characterized as a Brazilian Neotropical savanna, a xeromorphic forest also known as cerrado. The terrain is undulating with altitudinal variation of 500 m between the plateaus, riverside terrains, and mountains (Zardo et al. Citation2010). Field observations were conducted in October 2011, at the end of the dry season and at the end of the A. verrucosa fruiting period.
2.2. Species studied
A. verrucosa S. Moore is a treelet species of the Rubiaceae family, which reaches up to 8 m tall and exhibits a dense, broad, and highly branched canopy. This species is commonly found in gallery forests, seasonal forests, and cerrado in the central region of the Brazilian Neotropical savanna. Fruiting period of A. verrucosa is between July and November. Fruits have fleshy mesocarp and rough epicarp, and nearly 30 mm in diameter. Interestingly, this species has only one EFN at the superior extremity of each fruit (Silva-Jr & Pereira Citation2009). In an analogous manner to Tocoyena formosa (Rubiaceae), after pollination and fall of the corolla, the floral nectaries of A. verrucosa continue secreting nectar, acting functionally as EFN (Santos & Del-Claro Citation2001).
2.3. Data collection
In order to evaluate the temporal variation in nectar secretion in fruits of A. verrucosa, we randomly selected 41 fruits in 35 individuals. All selected plants were at least 35 m apart. To quantify the amount of nectar secreted, we excluded the possible nectar consumers (winged and nonwinged) covering each fruit with paper bags and putting nontoxic grease in the fruit peduncle. Paper bags have the additional function of preventing desiccation of nectar secreted. Fruits were isolated from their visitors for 24 hours, and we measured the volume of nectar (by graduates microcapillary) every 4 hours (n=7 measurements). We quantified the nectar volume using 1-µL microcapillaries (graduated by 0.1 µL divisions) (Heil et al. Citation2000; Dáttilo et al. Citation2012). To evaluate the variation in nectar secretion during fruit ontogeny, we collected the fruits used previously and measured the diameter of each fruit. Later, we also evaluated the investment in carbon during ontogeny fruits of A. verrucosa by the dry weight of each fruit method. For this, the fruits were kept in an oven at 72°C for 24 hours (or until constant weight) and afterward, we measured their dry weight with a precision balance. Here, we used the fruit size as a measure to classify the different ontogenic stages of the A. verrucosa fruits. This classification was confirmed by the color and smell of fruits during their development.
2.4. Data analysis
From the fruit diameter, we estimated the fruit volume by approaching their shape to a sphere. We related the fruit volume with the dry weight of each fruit to determine if the carbon allocation is proportional to growth using simple linear regression (SLR). Initially, in order to test our hypothesis that the greater the fruit, the greater the EFN secretion, we investigated the relationship between dry weight of the fruits (predictor variables) and EFN secretion (response variable) using SLR. However, as the SLR did not fit to the data, we used a posteriori, a model of quadratic regression. This model was the best fit for the data, using the ‘curve fitting’ in the BioStat 5.3 software (Ayres et al. Citation2007). Finally, to determine whether the amount of nectar secreted varied throughout the day, we used a repeated measures analysis of variance (ANOVA). We performed ANOVA analysis in the SYSTAT software version 13.0 (Wilkinson Citation1998).
3. Results
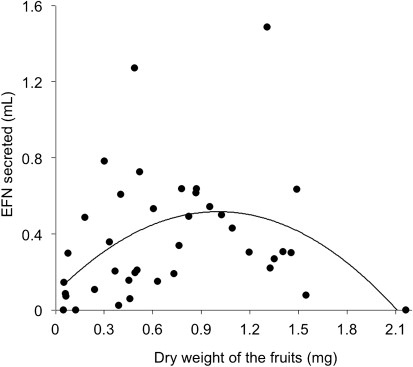
In this population of A. verrucosa in the Neotropical savanna, the fruit diameter was 16.26±5.65 mm (mean±SD) and dry weight was 0.716±0.509 mg. As expected, the fruit volume was positively correlated with its dry weight (weight= 0.994×volume), and therefore, weight gain was proportional to growth. In addition, we observed that during fruit ontogeny of A. verrucosa, there are different patterns of nectar secretion. In fact, we did not observe a linear relationship between dry weight of fruits and volume of secreted nectar (r 2 =0.02; P=0.351). However, a posteriori, the quadratic regression was the best fit for the data [nectar secreted=0.1+(0.6890×weight) – (0.3568×weight2); r 2 =0.27; P<0.001] (). This indicates that the fruits of intermediate size produce greater amount of nectar secreted, with little nectar secreted in young fruits, and the absence of nectar secretion in ripe fruits ().
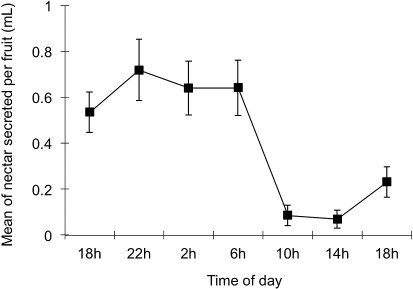
We also observed a large variation in the amount of nectar secreted by the A. verrucosa fruits (mean±SD: 0.421±0.687 mL), with EFN producing nectar from 0 mL to 3.91 mL at the highest peaks of secretion. In fact, we observed a large variation in the amount of nectar secreted over 24-hour period (repeated measures ANOVA: F=7547; GL=6; P=0.001). Nectar production was significantly higher during the night (between 18 and 6 hours), decreasing drastically in the morning (10 hours), and remaining constant throughout the day ().
4. Discussion
Several authors have demonstrated that the damage caused by predators is greater in young fruits, mainly due to their physical properties, which make them softer (Reuveni & Reuveni Citation1995; Alford Citation2007; Fernández et al. Citation2008; Wise & Hébert Citation2010). However, sometimes the predators do not have a special preference for young fruit (Clarke Citation1992; Lenzi et al. Citation2006; Silva et al. Citation2007), mainly because in some plant species the young fruits contain high levels of tannins compared with ripe fruits (Dement & Mooney Citation1974). Particularly in our study, we consistently observed an unidentified species of Coreidae (Hemiptera) with the rostrum inserted only in young fruits not tended by ants, probably feeding on the seed. Some plant species may abort their fruit damaged by herbivores or seed-sucking, avoiding subsequent spending of energy (Alford Citation2007; Boieiro et al. Citation2012). As we demonstrated, only a small amount of carbon (energy) was invested in young fruits. Thus, this smaller amount of energy allocated in young fruits can also justify the lower energetic investment in defenses (e.g. nectar) at this stage of fruit development.
Some studies show that in certain cases, the EFN secretion can be induced by jasmonic acid (Heil et al. Citation2001), a phytohormone which often increases during fruit development (Lopez et al. Citation1987; Czapski & Saniewski Citation1992; Creelman & Mullet Citation1997). In this context, such studies could support our initial hypothesis in which the EFN secretion can be higher in bigger and riper fruits of A. verrucosa. However, we observed that during fruit ontogeny of A. verrucosa, there are different patterns of nectar secretion, in which bigger and riper A. verrucosa fruits produced little or no nectar. The increase of nectar secretion in fruits of intermediate size demonstrates that the energy allocated in the fruit defense is proportional to the energy investment of plant in the fruit until some point in the fruit ontogeny. So why not protect fruit which invested much energy? The optimal defense hypothesis can be used to explain this pattern. Firstly, bigger fruits of A. verrucosa could be sturdier to specific herbivores of young A. verrucosa fruits, which could not access the seeds inside the bigger fruits. In fact, we did not observe Coreidae attacking bigger and riper fruits, even in the absence of ant foraging. It is possible that chemical modifications in the seed render them less attractive, or the thickness of the epicarp and mesocarp represents a physical barrier for herbivorous inaccessibility to the seed. Thus, with lower predation pressure by seed-sucking in bigger and riper fruits, reduction of investment in defense of these fruits would also be expected. Furthermore, there could be an exchange of defenses; when the fruit reaches an almost ripe stage, where more carbon is allocated in physical defenses causing hardening of fruit epicarp. This pattern could be similar to that observed in leaves that invest more in physical defense (e.g. leaf toughness) when they become fully expanded (Dyer et al. Citation2001; Korndörfer & Del-Claro Citation2006). However, the allocation of fruit mass in A. verrucosa was proportional to the fruit volume. Quantitative defenses, which imply an increase in the hardness of the fruit to the allocation of lignin or cellulose (Coley et al. Citation1985), are inevitably associated with a disproportionate increase in the investment carbon in relationship to the fruit volume, detectable by measuring of dry weight, which was not observed in this study.
However, the hypothesis of exchange defenses cannot be completely ruled out, since physical defenses or other chemical defenses could be involved in the defense of ripe fruits. In this case, the presence of such chemicals would be detectable only with the use of laboratory chemical analysis. Finally, the bigger fruits may be subject to predators and vertebrate dispersers (e.g. birds and mammals). In such cases, the presence of ants foraging on fruits may be nonfunctional, as ants may be inefficient in defending the host plant against some vertebrates (Hölldobler & Wilson Citation1990; Stapley Citation1998; Rico-Gray & Oliveira Citation2007). Moreover, in some cases, the presence of ants may even be undesirable, as they may frighten some possible disperses (Madden & Young Citation1992; Stapley Citation1998; Thomas Citation1988). A recent study performed by Yamawo et al. (Citation2012b) showed that the plants can gradually shift from direct to indirect defense against herbivory during leaf aging. Here, we show that the likely change in defense tactics during the ontogenic stages of the fruits of A. verrucosa supports the ‘optimal defense theory’ (McKey Citation1974, Citation1979; Rhoades Citation1979), in which, plant tissues most closely linked to fitness, such as fruits, should be most defended at high and different levels. Similarly, working in the Sonoran Desert of North America, Holland et al. (Citation2009) showed that the EFN production in Pachycereus schottii (Cactaceae) is greater in fruits than in buds. In fact, the framework of the optimal defense hypothesis has been corroborated by different studies involving ontogenetic or diurnal patterns in the secretion of nectar or other indirect defenses (Heil et al. Citation2000; Radhika et al. Citation2008; Rostás & Eggert Citation2008).
We observed higher nectar secretion in A. verrucosa fruits at nocturnal period, possibly mediated by both biotic and abiotic factors. Optimal defense theory can also help to explain this pattern, one most herbivores in regions with high temperature (e.g. Neotropical savanna) tend to be strictly nocturnal (Fagundes et al. Citation1996; Strauss et al. Citation2009; Byk & Del-Claro Citation2010). Moreover, due to the plants reduce greatly the nectar secretion when this resource is removed in low frequency (Koptur Citation1983; Pyke Citation1991; Heil et al. Citation2000), the ants' visitation on EFN of A. verrucosa may also have night foraging in arid and semi-arid regions (Briese & Macauley Citation1980; Oliveira et al. Citation1995). However, abiotic factors could also influence the nectar secretion of A. verrucosa. In fact, in regions with high temperatures throughout the day, most plants suffer water stress and can avoid nectar production during this period (Holland et al. Citation2010; Kulloli et al. Citation2011; Yamawo et al. Citation2012a).
In summary, our results indicate that A. verrucosa can change its energy allocation to defense against predation during ontogeny of its fruits. While in small fruits, where little energy was allocated, there is a low nectar production, fruits of intermediate size secreted higher amounts of nectar. This can increase the ants' visitation and consequently, the biotic defense offered by ants. However, in ripe and more energetically expensive fruits there is a strong decrease in nectar secretion, possibly in order to allow access to dispersers. Lastly, A. verrucosa may be altering the ants' visitation through increase in nectar secretion at night. This increase in ants' visitation may be crucial to reduce the herbivores foraging on A. verrucosa fruits in the Neotropical savanna. We suggest future studies to evaluate how ants' behavior, herbivores, and nectar seasonality influence this tritrophic interaction involving plant–ant–herbivore.
Acknowledgements
We are grateful to Víctor Rico-Gray, Ryan Smith, Kléber Del-Claro, Anselmo Nogueira, Haydée Yáñez, and two anonymous reviewers for their helpful comments and suggestions on earlier versions of this manuscript. J.C.F.F. was granted a master's degree fellowship from the CNPq. WD is grateful for financial support by the CNPq grant No. 237339/2012-9 and CONACYT.
References
- Alford DV. 2007. Pests of fruit crops: a colour handbook. London: Manson Publishing.
- Ayres M, Ayres-Júnior M, Ayres DL, Santos AA. 2007. BIOSTAT – Aplicações estatísticas nas áreas das ciências biomédicas [Statistical applications in the biomedical sciences]. Belém: Ong Mamirauá.
- Bentley BL. 1977. Extrafloral nectaries and protection by pugnacious bodyguards. Annu Rev Ecol Syst. 8(1):407–427. 10.1146/annurev.es.08.110177.002203
- Boieiro M, Rego C, Serrano ARM, Espadaler X. 2012. Seed production and pre-dispersal reproductive losses in the narrow endemic Euphorbia pedroi (Euphorbiaceae). Plant Ecol. 213(4):581–590. 10.1007/s11258-012-0023-7
- Briese DT, Macauley BJ. 1980. Temporal structure of an ant community in semi-arid Australia. Aust J Ecol. 5(2):121–134. 10.1111/j.1442-9993.1980.tb01236.x
- Byk J, Del-Claro K. 2010. Nectar and pollen-gathering Cephalotes ants provide no protection against herbivory: a new manipulative experiment to test ant protective capabilities. Acta Ethol. 13(1):33–38. 10.1007/s10211-010-0071-8
- Carter C, Healy R, O'Tool NM, Saqlan-Naqvi SM, Ren G, Park S, Beattie GA, Horner HT, Thornburg RW. 2007. Tobacco nectaries express a novel NADPH oxidase implicated in the defense of floral reproductive tissues against microorganisms. Plant Physiol. 143:389–399. 10.1104/pp.106.089326
- Chamberlain SA, Holland JN. 2009. Quantitative synthesis of context dependency in ant-plant protection mutualisms. Ecology. 90(9):2384–2392. 10.1890/08-1490.1
- Cipollini ML, Levey DJ. 1997. Secondary metabolites of Xeshy vertebrate–dispersed fruits: adaptive hypothesis and implications for seed dispersal. Am Nat. 150(3):346–372. 10.1086/286069
- Clarke PJ. 1992. Predispersal mortality and fecundity in the grey (Avicennia marina) in southeastern Australia. Aust J Ecol. 17:161–168. 10.1111/j.1442-9993.1992.tb00794.x
- Coley PD, Bryant PJ, Chapin FS. 1985. Resource availability and plant antiherbivore defense. Science. 230(4728):895–899. 10.1126/science.230.4728.895
- Coley PD, Kursar TA. 1996. Antiherbivore defenses of young tropical leaves: physiological constraints and ecological trade-offs. In: Mulkey SS, Chazdon RL, Smith AP, editors. Tropical forest plant ecophysiology. New York: Chapman & Hall Press; p. 305–335.
- Cortesero AM, Stapel OJ, Lewis WJ. 2000. Understanding and manipulating plant attributes to enhance biological control. Biol Control. 17(1):35–49. 10.1006/bcon.1999.0777
- Cousens R, Dytham C, Law R. 2008. Dispersal in plants: a population perspective. New York: Oxford University Press.
- Creelman RA, Mullet JE. 1997. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol. 48(1):355–381. 10.1146/annurev.arplant.48.1.355
- Czapski J, Saniewski M. 1992. Stimulation of ethylene production and ethylene-forming enzyme in fruits of the non-ripening nor and rin tomato mutants by methyl jasmonate. J Plant Physiol. 139(3):265–268. 10.1016/S0176-1617(11)80334-2
- Dáttilo W, Martins RL, Uhde V, Noronha JC, Florêncio FP, Izzo TJ. 2012. Floral resource partitioning by ants and bees in a jambolan Syzygium jambolanum (Myrtaceae) agroforestry system in Brazilian Meridional Amazon. Agroforestery Syst. 85(1):105–111. 10.1007/s10457-012-9489-5
- Dement WA, Mooney HA. 1974. Seasonal variation in the production of tannins and cyanogenic glucosides in the chaparral shrubs, Heteromeles arbutifolia. Oecologia 15(1):65–76. 10.1007/BF00345228
- Díaz-Castelazo C, Rico-Gray V, Ortega F, Angeles G. 2005. Morphological and secretory characterization of extrafloral nectaries in plants of coastal Veracruz, Mexico. Ann Bot. 96(7):1175–1189. 10.1093/aob/mci270
- Dicke M 1999. Evolution of induced indirect defense of plants. In: The ecology and evolution of inducible defenses. Princeton: Princeton University Press; p. 62–88.
- Dyer LA, Dodson CD, Beihoffer J, Letourneau DK. 2001. Tradeoffs in antiherbivore defenses in Piper cenocladum: ant mutualists versus plant secondary metabolites. J Chem Ecol. 27(3):581–592. 10.1023/A:1010345123670
- Fagundes M, Zanuncio JC, Lopes FS, Marco-Jr P. 1996. Comunidades de lepidópteros noturnos desfolhadores de eucalipto em três regiões do cerrado de Minas Gerais [Communities nocturnal Lepidoptera, defoliators Eucalyptus in three Cerrado regions in Minas Gerais]. Rev Bras Zool. 13(3):763–771. 10.1590/S0101-81751996000300024
- Fernández M, Lobo J, Chacón E, Quesada M. 2008. Curculionid beetles in aborted flower buds and immature fruits of Ceiba pentandra (Bombacaceae). Plant Ecol. 194(1):1–4.
- Heil M. 2008. Indirect defence via tritrophic interactions. New Phytol. 178(1):41–61. 10.1111/j.1469-8137.2007.02330.x
- Heil M, Fiala B, Baumann B, Linsenmair KE. 2000. Temporal, spatial and biotic variations in extrafloral nectar secretion by Macaranga tanarius. Funct Ecol. 14(6):749–757. 10.1046/j.1365-2435.2000.00480.x
- Heil M, Thomas K, Hilpert A, Fiala B, Boland W, Linsenmair KE. 2001. Extrafloral nectar production of the ant-associated plant, Macaranga tanarius, is an induced, indirect defensive response elicited by jasmonic acid. Proc Natl Acad Sci USA. 98(3):1083–1088. 10.1073/pnas.98.3.1083
- Herrera CM. 1982. Defense of ripe fruit from pests: its significance in relation to plant–disperser interactions. Am Nat. 120(2):218–247. 10.1086/283984
- Holland JN, Chamberlain SA, Horn KC. 2009. Optimal defence theory predicts investment in extrafloral nectar resources in an ant–plant mutualism. J Ecol. 97(1):89–96. 10.1111/j.1365-2745.2008.01446.x
- Holland JN, Chamberlain SA, Horn KC. 2010. Temporal variation in extrafloral nectar secretion by reproductive tissues of the senita cactus, Pachycereus schottii (Cactaceae), in the Sonoran Desert of Mexico. J Arid Environ. 74(6):712–714. 10.1016/j.jaridenv.2009.10.008
- Hölldobler B, Wilson EO. 1990. The ants. Cambridge: Harvard University Press.
- Kessler A, Baldwin IT. 2001. Defensive function of herbivore-induced plant volatile emissions in nature. Science 291(5511):2141–2144. 10.1126/science.291.5511.2141
- Korndörfer AP, Del-Claro K. 2006. Ant defense versus induced defense in Lafoensia pacari (Lythraceae), a myrmecophilous tree of the Brazilian cerrado. Biotropica 38(6):786–788. 10.1111/j.1744-7429.2006.00200.x
- Koptur S. 1983. Flowering phenology and floral biology of Inga (Fabaceae: Mimosoideae). Syst Bot. 8(4):354–368. 10.2307/2418355
- Kulloli SK, Chandore AN, Aitawade MM. 2011. Nectar dynamics and pollination studies in three species of Lamiaceae. Curr Sci. 100:509–516.
- Lenzi M, Soares J, Orth AF. 2006. Predação de Opuntia monacantha (Willd.) Haw. (Cactaceae) por Cactoblastis cactorum (Lepidoptera: Pyralidae) em restingas da Ilha de Santa Catarina, sul do Brasil [Predation of Opuntia monacantha (Willd.) Haw. (Cactaceae) by Cactoblastis cactorum (Lepidoptera: Pyralidae) in a sand bank area of Santa Catarina island, south Brazil]. Biotemas. 19:35–44.
- Lopez R, Dathe W, Bruckner C, Miersch O, Sembdner G. 1987. Jasmonic acid in different parts of the developing soybean fruit. Biochem Physiol Pfl. 182:195–201.
- Madden D, Young TP. 1992. Symbiotic ants as an alternative defense against giraffe herbivory in spinescent Acacia drepanolobium. Oecologia 91(2):235–238. 10.1007/BF00317789
- Marquis RJ. 1984. Leaf herbivores decrease fitness of a tropical plant. Science 226(4674):537–539. 10.1126/science.226.4674.537
- McKey D. 1974. Adaptive patterns in alkaloid physiology. Am Nat. 108(961):305–320. 10.1086/282909
- McKey D. 1979. The distribution of secondary compounds within plants. In: Herbivores: their interactions with secondary plant metabolites. New York: Academic Press; p. 55–133.
- Nascimento EA, Del-Claro K. 2010. Ant visitation to extrafloral nectaries decreases herbivory and increases fruit set in Chamaecrista debilis (Fabaceae) in a Neotropical savanna. Flora 205(11):754–756. 10.1016/j.flora.2009.12.040
- Oliveira PS, Klitzke C, Vieira E. 1995. The ant fauna associated with the extrafloral nectaries of Ouratea hexasperma (Ochnaceae) in an area of cerrado vegetation in Central Brazil. Entomol Mon Mag. 131:77–82.
- Oliveira PS, Rico-Gray V, Díaz-Castelazo C, Castilho-Guevara C. 1999. Interaction between ants, extrafloral nectaries and insect herbivores in Neotropical coastal and dunes: herbivore deterrence by visiting ants increases fruit set in Opintia stricta (Cactacea). Funct Ecol. 13(5):623–631. 10.1046/j.1365-2435.1999.00360.x
- Pyke GH. 1991. What does it cost a plant to produce floral nectar? Nature 350(6313):58–59. 10.1038/350058a0
- Radhika V, Kost C, Bartram S, Heil M, Boland W. 2008. Testing the optimal defence hypothesis for two indirect defences: extrafloral nectar and volatile organic compounds. Planta 228(3):449–457. 10.1007/s00425-008-0749-6
- Reuveni M, Reuveni R. 1995. Efficacy of foliar sprays of phosphates in controlling powdery mildews in field-grown nectarine, mango trees and grapevines. Crop Prot. 14(4):311–314. 10.1016/0261-2194(94)00009-W
- Rico-Gray V. 1989. The importance of floral and circum-floral nectar to ants inhabiting dry tropical lowlands. Biol J Linn Soc. 38(2):173–181. 10.1111/j.1095-8312.1989.tb01572.x
- Rico-Gray V, Thien LB. 1989. Effect of different ant species on the reproductive fitness of Schomburgkia tibicinis (Orchidaceae). Oecologia 81(4):487–489. 10.1007/BF00378956
- Rico-Gray V, Oliveira PS. 2007. The ecology and evolution of ant–plant interactions. Chicago: The University of Chicago Press.
- Rhoades DF 1979. Evolution of plant chemical defense against herbivores. In: Rosenthal GA, Berenbaum MR. Herbivores: their interaction with secondary plant metabolites. New York: Academic Press; p. 4–53.
- Romero GQ, Souza JC, Vasconcellos-Neto J. 2008. Anti–herbivore protection by mutualistic spiders and the role of plant glandular trichomes. Ecology 89(11):3105–3115. 10.1890/08-0267.1
- Romero GQ, Koricheva J. 2011. Contrasting cascade effects of carnivores on plant fitness: a meta-analysis. J Anim Ecol. 80(3):696–704. 10.1111/j.1365-2656.2011.01808.x
- Rostás M, Eggert K. 2008. Ontogenetic and spatio-temporal patterns of induced volatiles in Glycine max in the light of the optimal defence hypothesis. Chemoecology. 18(1):29–38. 10.1007/s00049-007-0390-z
- Rosumek FB, Silveira FAO, Neves FD, Barbosa NPD, Diniz L, Oki Y, Pezzini F, Fernandes GW, Cornelissen T. 2009. Ants on plants: a meta-analysis of the role of ants as plant biotic defenses. Oecologia. 160(3):537–549. 10.1007/s00442-009-1309-x
- Sabelis MW, Jansen A, Kant MR. 2001. The enemy of my enemy is my ally. Science. 291(5511):2104–2105. 10.1126/science.1059939
- Santos JC, Del-Claro K. 2001. Interactions between ants, herbivores and extrafloral nectaries in Tocoyena formosa (Rubiaceae) in cerrado vegetation. Revista Brasileira de Zoociências. 3:77–92.
- Silva FR, Begnini RM, Scherer KZ, Cortês-Lopes B, Castellani TT. 2007. Predação de sementes de Syagrus romanzoffiana (Cham.) Glassman (Arecaceae) por insetos na ilha de Santa Catarina, SC [Seed predation of Syagrus romanzoffiana (Cham.) Glassman (Arecaceae) by insects in Santa Catarina Island, SC]. Rev Bras Bioc. 5:681–683.
- Silva-Jr MC, Pereira BAS. 2009. 100 árvores do cerrado – Matas de Galeria: guia de campo [100 trees of Cerrado – Gallery forests: field guide]. Brasília: Rede de sementes do cerrado.
- Stapley L. 1998. The interaction of thorns and symbiotic ants as an effective defence mechanism of swollen–thorn acacias. Oecologia. 115(3):401–405. 10.1007/s004420050534
- Strauss SY, Stanton ML, Emery NC, Bradley CA, Carleton A, Dittrich-Reed DR, Ervin OA, Gray LN, Hamilton AM, Rogge JH, et al. 2009. Cryptic seedling herbivory by nocturnal introduced generalists impacts survival, performance of native and exotic plants. Ecology. 90(2):419–429. 10.1890/07-1533.1
- Southwick EE. 1984. Photosynthate allocation to floral nectar: a neglected energy investment. Ecology. 65(6):1775–1779. 10.2307/1937773
- Thomas DW. 1988. The influence of aggressive ants on fruit removal in the tropical tree, Ficus capensis (Moraceae). Biotropica. 20(1):49–53. 10.2307/2388425
- Thornburg RW, Carter C, Powell A, Mittler R, Rizhsky L, Horner HT. 2003. A major function of the tobacco floral nectary is defense against microbial attack. Plant Syst Evol. 238:211–218.
- Wilkinson L. 1998. SYSTAT: the system for statistics. Evanston, IL: SYSTAT Inc.
- Wise MJ, Hébert JB. 2010. Herbivores affect natural selection for floral–sex ratio in a field population of horsenettle, Solanum carolinense. Ecology 91(4):937–943. 10.1890/09-1373.1
- Yamawo A, Hada Y, Suzuki N. 2012a. Variations in direct and indirect defenses against herbivores on young plants of Mallotus japonicus in relation to soil moisture conditions. J Plant Res. 125(1):71–76. 10.1007/s10265-011-0407-0
- Yamawo A, Suzuki N, Tagawa J, Hada Y. 2012b. Leaf aging promotes the shift in defence tactics in Mallotus japonicus from direct to indirect defence. J Ecol. 100(3):802–809. 10.1111/j.1365-2745.2011.01934.x
- Zardo DC, Carneiro AP, Lima LG, Santos-Filho M. 2010. Comunidade de artrópodes associada à serrapilheira de cerrado e mata de galeria, na Estação Ecológica Serra das Araras – Mato Grosso, Brasil [Arthropod communities associated with litter of Cerrado and gallery forest at the Estação Ecológica Serra Das Araras – Mato Grosso, Brazil]. Rev Uniara. 13:105–113.