Abstract
We observed the abundance of leaf shelters, aphids, other herbivores, and predators on willow trees, Salix eriocarpa, from May to October 2003. There was a positive correlation between the growth rate of aphids and the number of ants per shoot, suggesting ant attendance to aphids. Although the mean abundance of leaf shelters per shoot was rather low (1.7–2.2) throughout the observation period, aphids preferred to use shoots with leaf shelters compared with those without leaf shelters. The abundance of ants was positively influenced by the presence of leaf shelters and aphids from May to August. The abundance of other herbivores was positively influenced by leaf shelters, but negatively influenced by aphid presence from May to August. Furthermore, leaf shelters had a positive effect on the abundance of predators from July to October. These data suggest that a relatively low abundance of naturally occurring leaf shelters per shoot influenced the arthropod communities on S. eriocarpa, and the effect of those leaf shelters on each type of arthropod varied according to the season.
1. Introduction
Herbivory frequently alters plant characters but rarely influences plant mortality. Herbivores may induce changes in certain plant traits, affecting the responses of other herbivores and predators on the same plant (Karban & Baldwin Citation1997; Ohgushi Citation2005). Thus, herbivore-induced indirect effects on arthropod communities can be either positive (Williams & Myers Citation1984; Damman Citation1987; Nakamura et al. Citation2003) or negative (Karban Citation1986; Strauss et al. Citation1996; Denno et al. Citation2000). For example, the amount of nicotine, known to have a defensive function in nature, in wild tobacco plants was increased in response to herbivory (Baldwin et al. Citation1994). In addition, the regrowth response of the willow to the stem gall midge, Rabdophaga rigidae, positively affected other herbivores by enhancing the availability of food resources (Nakamura et al. Citation2003).
Many herbivores alter leaves to produce leaf shelters (Fukui Citation2001). After being abandoned by their builders, leaf shelters are frequently used by other arthropods and enhance their performance (e.g. Damman Citation1987; Cappuccino Citation1993; Cappuccino & Martin Citation1994; Lill & Marquis Citation2003). On willow trees (Salix sp.), several aphid species are known to use abandoned leaf shelters (Nakamura & Ohgushi Citation2003), with aphid colonization often followed by aphid-attending ants (Way Citation1963; Buckley Citation1987). The shelters mediate aphid–ant interactions, resulting in negative effects on other arthropods since ants attending the aphids prey on other arthropod species (Flower & Macgarvin Citation1985; Floate & Whitham Citation1994; Nakamura & Ohgushi Citation2003). Nakamura and Ohgushi (Citation2003) experimentally examined how artificial shelters used by aphids that were attended by ants affected leaf beetles on a willow, Salix miyabeana, in July. They showed that the abundance of aphids increased with the abundance of artificial leaf shelters on a shoot as did the abundance of ants that entered leaf shelters to collect aphid honeydew. They concluded that shelter-making herbivores increased the abundance of both aphids and ants and decreased the survival rate of leaf beetle larvae.
The long-term seasonal effects of naturally occurring leaf shelters on the arthropod community on a plant would be important when considering ecological functions of leaf shelters, but such effects have not yet been studied. We hypothesized that such effects, if any, would depend on several factors, such as the seasonal changes of the number of leaf shelters, aphid–ant interactions, and co-occurring taxa in the arthropod community. In this study, we investigated the effects of naturally occurring leaf shelters on the willow tree Salix eriocarpa by focusing on the seasonal variation of the above factors.
2. Materials and methods
2.1. Study site
The field study was conducted on the banks of the Yamato River (34°57′N, 135°62′E) in Osaka, Japan, in 2003. The observation period was from May to late October (six months) and was arbitrarily divided into three periods (the first period [May to the end of June], the second period [July to the end of August], and the third period [September to the end of October]). We focused on one of the dominant willow tree species, Salix eriocarpa. One typhoon struck the site at the end of August. It caused floods and storms that partially damaged the study site. The average temperature was ca. 23°C in the first and the third periods, while it was ca. 27°C in the second period.
2.2. Field observations
In the first period, we selected five trees for observation. We added five more trees in the last week of June. In the second period, three trees were lost during a typhoon at the end of August. Therefore, we added three more trees at the beginning of September. On each S. eriocarpa tree, we randomly selected five one-year-old shoots.
Seasonal variation of arthropods. We counted the number of arthropods per shoot weekly, categorizing the groups into aphids, ants, other herbivores, and generalist predators (spiders, predatory beetles, and stinkbugs). We identified aphids and ants to the species level.
Relationship between the number of ants and the growth rate of aphids on a shoot. We counted the number of ants and aphids on a shoot during the observation period. The growth rate of an aphid population was calculated for each week [(Xn + 1−Xn)/Xn; Xn = number of aphids at n-th observation].
Seasonal variation of shelters and arthropods in them. Shelter makers that we observed during the season were as follows: Sawflies, Phyllocolpa spp, made leaf rolls by inducing gall tissues. Larvae of Tortricidae made leaf shelters by rolling a single leaf (leaf roll) or by tying two or three leaves together with silken thread (leaf sandwich). Spiders also made leaf sandwiches. We counted the number of shelters per shoot every week. Pooled data were used for analyses. We planned to observe the same branches during all censuses. However, branches were sometimes lost due to flooding, typhoon, or strong wind. In such cases, we chose other branches. We carefully checked the inside of leaf shelters so as not to destroy them. We evaluated the overall effects of the abundance of shelters of the above three types.
2.3. Statistics
Spearman's correlation test was used to assess the relationship between the aphid population growth rate and the number of attending ants. The number of leaf shelters per shoot with shelter(s) was compared among the three periods by using the nonparametric Steel–Dwass multiple-comparison method. We used Ryan's method to test whether the ratio of leaf shelters used by each arthropod category to the total number of leaf shelters during each period was significantly different from that during the other periods. The number of aphid-infested shoots relative to the total number of shoots with leaf shelters was compared with that relative to the total number of shoots without leaf shelters in all periods. The Mann–Whitney U-test was used to compare the number of aphids on shoots with and without shelters. The effect of the number of shelters and aphids on the number of other arthropods in each season was analyzed using a generalized linear model (GLM) with Poisson distribution and log link function. When interaction factors were not significant, we recalculated the data using a GLM that excluded interaction factors. All analyses were performed using ‘R’ software (ver. 2.15.2) (R Core Team Citation2012).
3. Results and discussion
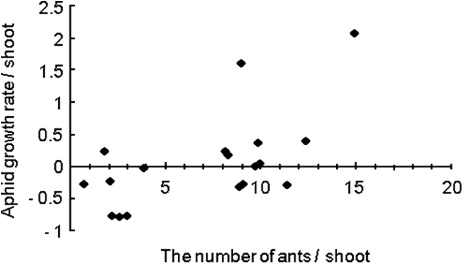
The seasonal changes in the abundance of aphids, ants, leaf shelters, other herbivores, and generalist predators (mainly spiders) per shoot are shown in . Among the other herbivores, willow leaf beetle, Plagiodera versicolora, was the dominant species. We observed 36,609 individual aphids consisting of Chaitophorus saliniger (91.9%), Tuberolachnus salignus (7.4%), and Aphid farinasa (0.7%). We commonly observed five ant species: Lasius sakagamii (87.6%), Ochetellus glaber (>1%), Leptothorax spinosior (>1%), Pristomyrmex pungens (>1%), and Formica japonica (>1%) (N = 1023). During field observation, we frequently found that the ant species L. sakagamii attended C. saliniger to collect honeydew. This ant attendance behavior is reported to have positive effects for the aphids whereby the ants reduce the incidence of lethal disease in the aphid population (Nielsen et al. Citation2009). In addition, the ants remove other natural enemies of aphids (Stadler & Dixon Citation2005). We found a significant correlation between the number of ants and the growth rate of aphids (Spearman rank correlation: r = 0.46, P < 0.05, N = 18; ), suggesting that attending ants protect aphids from predators. Nakamura and Ohgushi (Citation2003) found that on Salix miyabeana, C. saliniger was attended by the three ant species Camponotus japonicus, Lasius hayashi, and Myrmica jessensis. In addition, they found that numbers of aphids and ants were significantly correlated.
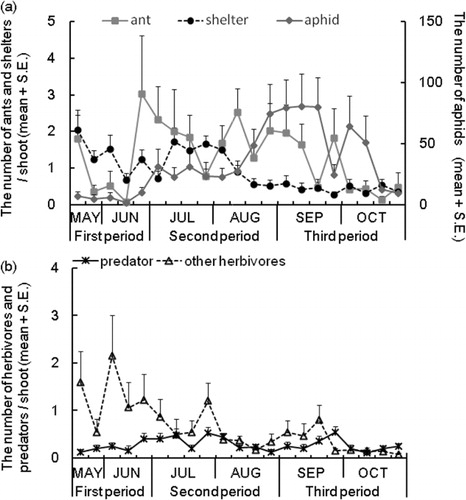
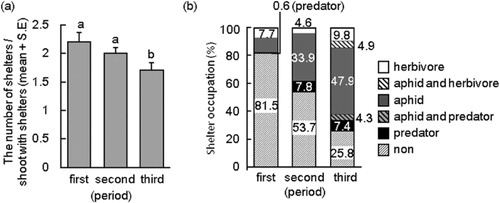
The number (M ± SE) of leaf shelters per shoot with shelters in the three periods was 2.2 ± 0.2 (first period), 2.0 ± 0.1 (second period), and 1.7 ± 0.2 (third period). The number in the third period was significantly smaller than the numbers in the first and second period (Steel–Dwass test, P < 0.001; first period, N = 76; second period, N = 174; third period, N = 98; a). In the first, second, and third periods, 81.5%, 53.7%, and 25.8%, respectively, of all leaf shelters were empty, and the values during the three periods were significantly different from each other (Ryan's test, P < 0.05 for all comparisons; first period, N = 168; second period, N = 348; third period, N = 163; b). This suggests that the use of leaf shelters increased throughout the observation period. In the first period, either aphids or other herbivores used the leaf shelters. In the second period, in addition to aphids and other herbivores, generalist predators occupied ∼8% of the shelters. The fraction of leaf shelters used by a combination of aphids and other users increased in the third period (b). These data demonstrate that (1) approximately two shelters per shoot were available to arthropods during the observation period; (2) a relatively high fraction of leaf shelters were empty, especially in the first and the second period; (3) the predominant users of leaf shelters were aphids. Nakamura and Ohgushi (Citation2003) found that leaf shelters on Salix miyabeana were predominantly used by the aphid C. saliniger.
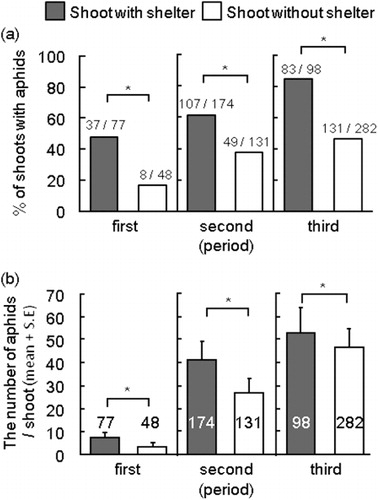
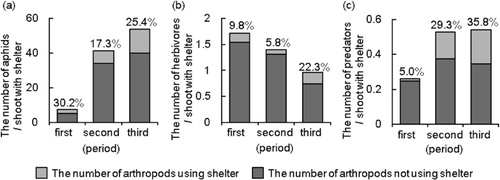
The fraction of aphid-infested shoots with leaf shelters was significantly higher than that without in all periods (Ryan's test: the first period, P < 0.001, shoots with and without shelter: N = 77, N = 48; the second period, P < 0.001, shoots with and without shelter: N = 174, N = 131; the third period, P < 0.001, shoots with and without shelter: N = 98, N = 282; a). The number of aphids per shoot was significantly higher on shoots with leaf shelters than that on shoots without throughout the observation period (Mann–Whitney U-test: first period, P = 0.001; second period, P < 0.001; third period, P < 0.001; b).
The effects of leaf shelters and aphids on the number of ants were both significantly positive in the first and second periods (). Why the interaction of aphid and leaf shelter showed a negative association only in the second period remains unknown. In the third period, the effect of aphids on the number of ants was positive, while the effect of shelters was negative (). These results suggest that the effects of the presence of leaf shelters vary according to the season. Causative factors of these variations are unknown, and their clarification would require further studies.
Table 1. Results of the GLM for the effect of shelters and aphids on the number of ants on Salix eriocarpa.
The presence of leaf shelters positively influenced the number of herbivores on S. eriocarpa shoots throughout the observation period (). In contrast, the presence of aphids negatively influenced the number of herbivores in the first and second periods (). No effect of aphids was detected in the third period. Nakamura and Ohgushi (Citation2003) showed that fewer larvae of the leaf beetle P. versicolora survived on shoots with artificial shelters inhabited by ant-attending aphids than on shoots without the shelters. Their observation period was one week. In the present study, we observed naturally occurring shelters on willow shoots for ca. 6 months and found that herbivores preferred shoots with naturally occurring shelters compared with those without.
Table 2. Results of the GLM for the effect of shelter and aphids on the number of herbivores on Salix eriocarpa.
We investigated the effects of leaf shelters and aphids on the number of generalist predators (; ). The presence of shelters positively influenced the number of predators in the second and the third periods. There was no significant effect of aphids on the number of predators during any period. Thus, the effect of shelters on the carnivorous arthropod community also showed seasonal variation.
Table 3. Results of the GLM for the effect of shelters and aphids on the number of predators on Salix eriocarpa.
Leaf shelters have been reported to affect microhabitat conditions (Hunter & Willmer Citation1989; Cappuccino Citation1993; Cappuccino & Martin Citation1994; Larsson et al. Citation1997; Martinsen et al. Citation2000; Fukui Citation2001). In this study, the presence of approximately two leaf shelters per shoot affected the distribution of aphids, ants, other herbivores, and generalist predators. Although aphids preferred shoots with leaf shelters, a substantial number of the aphids were found outside shelters (a). Substantial numbers of other herbivores and predators were also found outside shelters (b and c). Therefore, some unknown factor(s) associated with the presence of leaf shelters affected the distributions of aphids, ants, other herbivores, and predators. For example, in response to shelters built by herbivores, shoots might have changed their quality for arthropods. Further, if individual shoots have different food quality, the distributions of builders and other arthropods would be affected by such differences. Further studies are needed to clarify the effects of leaf shelters on the arthropod assemblages on willow trees.
Acknowledgments
This research was financially supported in part by Global Center of Excellence Program A06 ‘Formation of a Strategic Base for Biodiversity and Evolutionary Research: from Genome to Ecosystem’ from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, by the Core-to-Core project from Japan Science and Technology Agency, Japan, by a Grant-in-Aid for Scientific Research (S) from MEXT, Japan, by the Foundation for Riverfront Improvement and Restoration, and by Grants for Excellent Graduate Schools from MEXT, Japan.
References
- Baldwin IT, Schmelz EA, Ohnmeiss TE. 1994. Wound-induced changes in root and shoot jasmonic acid pools correlate with induced nicotine synthesis in Nicotiana-sylvestris spegazzini and comes. J Chem Ecol. 20:2139–2157. doi:10.1007/BF02066250
- Buckley RC. 1987. Interactions involving plants, Homoptera, and ants. Annu Rev Ecol Syst. 18:11–135. doi:10.1146/annurev.es.18.110187.000551
- Cappuccino N. 1993. Mutual use of leaf shelters by lepidopteran larvae on paper birch. Ecol Entomol. 18:287–292. doi:10.1111/j.1365-2311.1993.tb01103.x
- Cappuccino N, Martin M. 1994. Eliminating early-season leaf-tiers of paper birch reduces abundance of mid-summer species. Ecol Entomol. 19:399–401. doi:10.1111/j.1365-2311.1994.tb00258.x
- Damman H. 1987. Leaf quality and enemy avoidance by the larvae of a pyralid moth. Ecology. 68:88–97. doi:10.2307/1938808
- Denno RF, Peterson MA, Gratton C, Cheng J, Langellotto GA, Huberty AF, Finke DL. 2000. Feeding-induced changes in plant quality mediate interspecific competition between sap-feeding herbivores. Ecology. 81:1814–1827. doi:10.1890/0012-9658(2000)081[1814:FICIPQ]2.0.CO;2
- Floate KD, Whitham TG. 1994. Aphid–ant interaction reduces chrysomelid herbivory in a cottonwood hybrid zone. Oecologia. 97:215–221. doi:10.1007/BF00323152
- Flower SV, Macgarvin M. 1985. The impact of hairy wood ants Formica lugubris, on the guild structure of herbivorous insects on birch Betula pubescens. J Anim Ecol. 54:847–855. doi:10.2307/4382
- Fukui A. 2001. Indirect interactions mediated by leaf shelters in animal–plant communities. Popul Ecol. 43:31–40. doi:10.1007/PL00012013
- Hunter MD, Willmer PG. 1989. The potential for interspecific competition between two abundant defoliators on oak: leaf damage and habitat quality. Ecol Entomol.14:267–277. doi:10.1111/j.1365-2311.1989.tb00956.x
- Karban R. 1986. Interspecific competition between folivorous insects on Erigeron glaucus. Ecology. 67:1063–1072. doi:10.2307/1939829
- Karban R, Baldwin IT. 1997. Induced responses to herbivory. Chicago: Univ. of Chicago Press.
- Larsson S, Häggstroem H, Denno RF. 1997. Preference for protected feeding site by larvae of the willow-feeding leaf beetle Galerucella lineola. Ecol Entomol. 22:445–452. doi:10.1046/j.1365-2311.1997.00083.x
- Lill JT, Marquis RJ. 2003. Ecosystem engineering by caterpillars increases insect herbivore diversity on white oak. Ecology. 84:682–690. doi:10.1890/0012-9658(2003)084[0682:EEBCII]2.0.CO;2
- Martinsen GD, Floate KD, Waltz AM, Wimp GM, Whitham TG. 2000. Positive interactions between leafrollers and other arthropods enhance biodiversity on hybrid cottonwoods. Oecologia. 123:82–89. doi:10.1007/s004420050992
- Nakamura M, Ohgushi T. 2003. Positive and negative effects of leaf shelters on herbivorous insects: linking multiple herbivore species on a willow. Oecologia. 136:445–449. doi:10.1007/s00442-003-1285-5
- Nakamura M, Miyamoto Y, Ohgushi T. 2003. Gall initiation enhances the availability of food resources for herbivorous insects. Funct Ecol. 17:851–857. doi:10.1111/j.1365-2435.2003.00786.x
- Nielsen C, Agrawal AA, Hajek AE. 2009. Ants defend aphids against lethal disease. Biol Letts. 6:205–208. doi:10.1098/rsbl.2009.0743
- Ohgushi T. 2005. Indirect interaction webs: herbivore-induced effects through trait change in plants. Annu Rev Ecol Evol Syst. 36:81–105. doi:10.1146/annurev.ecolsys.36.091704.175523
- R Core Team. 2012. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. ISBN 3-900051-07-0. Available from: http://www.R-project.org/.
- Stadler B, Dixon AFG. 2005. Ecology and evolution of aphid–ant interactions. Annu Rev Ecol Evol Syst. 36:345–372. doi:10.1146/annurev.ecolsys.36.091704.175531
- Strauss SY, Coner JK, Rush SL. 1996. Foliar, herbivory affects floral characters and plant attractiveness to pollinators: implications for male and female plant fitness. Am Nat. 147:1098–1107. doi:10.1086/285896
- Way MJ. 1963. Mutualism between ants and honeydew producing Homoptera. Annu Rev Entomol. 8:307–344. doi:10.1146/annurev.en.08.010163.001515
- Williams KS, Myers JH. 1984. Previous herbivore attack of red alder may improve food quality for fall webworm larvae. Oecologia. 63:166–170. doi:10.1007/BF00379873
