Abstract
Red spider mite, Tetranychus macfarlanei, was identified as a causative parasite for Plumbago zeylanica in India. Present study was undertaken to evaluate the detrimental role of T. macfarlanei on P. zeylanica. The relative change in biomass, stem length, stem diameter, and leaf number was significantly reduced by mite infestation as compared with uninfected plants. A significant reduction of chlorophyll a, chlorophyll b, and total chlorophyll was observed in mite-infested plants. Mite infestation significantly hampered the normal physiological process of the plant, namely, CO2 assimilation, stomatal conductance, electrolyte leakage, cell membrane injury, and carboxylation efficiency. A significant increase in tissue H2O2 and lipid peroxidation coupled with depletion of antioxidant enzymes, namely superoxide dismutase and catalase indicated augmented oxidative stress associated with biotic interaction. Finally, a significant reduction of root-specific secondary metabolite, plumbagin, production highlighted the potential damage of T. macfarlanei in P. zeylanica. The experimental outcome could conclude that the aboveground symptoms due to mite infestation could also exert profound detrimental effect in the belowground tissues.
Introduction
Since times immemorial, medicinal plants have been used virtually by all the cultures and societies as a source of medicine. With an ever-increasing global preference, there is an obligatory demand for raw materials. Considering the growing importance of these plants, the initiative has been taken for their cultivation. With the increased cultivation of medicinal plants, pests and disease problems are also increasing. Mites and insects are frequently found on medicinal plants. Despite this, relatively little attention has been paid to the study of actual or potential pests and of the predatory mites associated with them. Pests can attack plants both above and below ground. The ensuing systemic defense response of the plant can affect even the most distant tissues. The metabolic profiles of roots can be altered upon shoot herbivory and vice versa (Erb et al. Citation2008). Therefore, it is important to identify the common pests and their detrimental role of a particular species of medicinal plant.
Plumbago zeylanica L. (Plumbaginaceae) is a commercially important medicinal plant native to warm tropical region of the world. This species grows wild in India. Plumbagin which is produced in the root of P. zeylanica identified as lead/scaffold for several pharmacological activities, namely microfilaricidal (Mathew et al. Citation2002), antimicrobial (Didry et al. Citation1994), anticancer (Kuo et al. Citation2006; Hazra et al. Citation2008), and antifertility (Bhargava Citation1984) activities. Aforementioned pharmacological properties of plumbagin synergized its demand in pharmaceutical industry. Synthetic approach of plumbagin production was not commercially promising. Therefore, the principle source of plumbagin is still remained in the roots of P. zeylanica collected from natural population, which resulted in an increased market demand of this plant. To meet with the demand, cultivation of this medicinal plant has been started in different parts of India. With increasing cultivation of P. zeylanica, pest and disease problems are also increasingly unabated and the farmers have experienced enormous crop loss. Tetranychus macfarlanei was identified as a common pest for P. zeylanica in four different geoclimatic locations in India.
Although T. macfarlanei is a little known species for medicinal plants, which was recorded in serious proportions on field crops and weed plants (Patil & Nandihalli Citation2008). This folivorous arthropod can alter physiological processes in the leaves of their host plants and inevitably affect the plants' productivity (Takemoto & Takabayashi Citation2012). These reactions can be better understood on the basis of mutual interaction between host and parasite. The detrimental effect of herbivores not only restricted within aboveground portion, but also transmitted to belowground and affects root's morphophysiological and biochemical parameters (Erb et al. Citation2011; Myster Citation2011). The objective of this research was to assess the foliar herbivory impact of T. macfarlanei on the biosynthesis of plumbagin in the P. zeylanica roots. An experiment was conducted at the Glass house, Norendrapore Ramakrishna Mission, India to find out the shoot to root interaction due to T. macfarlanei infection in P. zeylanica. Morpho-physiological, biochemical, and phytochemical parameters were measured as the index of host–parasite interaction.
Materials and methods
Identification of mite
The fields were visited during the month of June–August, 2010, when maximum infected plants were recorded in earlier years. A visual assessment of the mite infestation was made by using a hand lens (×20). Mites were randomly collected from infested P. zeylanica plants with the help of brush from in each location. These collections were made thrice in each location. The mites were preserved in 70% alcohol. For identification, mites were mounted in Heinze's medium. The mite specimens were identified by the mite biologist at the Department of Zoology, University of Calcutta, India by comparing them with existing specimens of T. macfarlanei.
Mite culture
An isogenic culture of T. macfarlanei was maintained in laboratory at 14 h L:10 h D photoperiod, 32 ± 2°C and 55 ± 5% relative humidity on freshly collected healthy P. zeylanica leaves maintained inside a wire mesh cage. The exhausted leaves were replaced by fresh leaves daily for providing enough nutrition to the mites. One male and one female mite were introduced onto each set. Approximately, 10 generations were completed before using for experiments. Only female mites were used in this study because they have long life span than males. Only unfertilized females produce male mites with very short life span. Therefore, female mites participate in a principle role for damaging.
Plant material and growing conditions
P. zeylanica were propagated by cuttings with three to four nodes from the healthy mature P. zeylanica plants growing in Medicinal Plant Garden, Norendrapore Ramakrishna Mission, India. The leaves were removed from the cuttings with a sharp razor. Two hundred cuttings were maintained as per the protocol given by Ramassamy et al. (Citation2006) in the glass house of Norendrapore Ramakrishna Mission, India. At the end of 60 days, survived plants were transferred into 5–l earthen pots (1 plant/pot) containing a mixture of soil:farmyard manure (3:1, v/v) and supplemented with a commercial fertilizer (10.0 g/pot) (N:P:K:Mg, 16:9:12:2.5). Plants were grown in a glass house with daily irrigation. After 30 days, 120 healthy plants of uniform size were screened for the study. The mites (50 female mites/plant) were artificially introduced to the plants (60) on day 0 after transplantation. A set of uninfected plants (60) were kept as control. Each pot was covered individually using nylon mesh after artificial release of mites. The glass house condition was maintained with temperature of 30 ± 2°C and relative humidity of 60 ± 5% throughout the course of study. Pots were randomly moved every week to minimize position effects. The morphological, physiological, biochemical, and phytochemical parameters were recorded in day 0, 15, 30, 60, 90, and 120 after artificial release of mites. Ten plants from each of mite-infested and control groups were sacrificed on aforementioned intervals for assessing plant biomass, root parameters, and plumbagin estimation.
Growth and morphology
Stem growth characteristics were monitored by measuring length (from substrate surface to the apical meristem) and diameter (at the base) and by counting the number of matured leaves. The average initial height, diameter, and leaf number were found to be ∼ 5.6 cm, 8.1 mm, and 32, respectively. The relative increase in stem length, stem diameter, and number of leaves was calculated. Fresh weight (gfw) of roots and shoots was determined. The average initial fresh biomass of stems and roots were measured to be ∼ 19.2 and 7.6 g, respectively. Dry weight (gdw) was determined after drying plant material at 70°C (until a constant weight was achieved). Shoot to root dry mass ratio (S:R) was calculated.
Physiological parameters
The young, matured, and totally expanded leaf disks of similar age having about equal leaf surface were sampled for the estimation of physiological parameters of leaf. The solute leakage of the infected leaves was assessed to study the extent of membrane damage due to mite infection following the protocol of Masood et al. (Citation2006). Briefly, 1 g of young and mature leaf samples was kept in test tubes containing 20 ml deionized distilled water, and initial electrical conductivity of the medium (EC1) was measured by incubating the tubes at 32°C for 2 h. The final electrical conductivity (EC2) was measured to release all electrolytes by autoclaving the samples at 121°C for 20 min followed by cooling at 25°C. The electrolyte leakage was calculated as EC1/EC2 and expressed as a percentage. Cell membrane stability was computed on the same samples used for electrolyte leakage following the protocol given by Sudhakar et al. (Citation2001). The value was expressed as: injury (%) = 1 – (1 – T1/T2)/(1 – C1/C2), where T1 and T2 were the first and second conductivity measurements in mite-infested leaves, C1, and C2 are first and second conductivity measurements of the control. Leaf gas exchange was measured in an infrared gas analyzer (LCi, ADC, Hoddesdonm, UK) operating in an open system and with an air flow of 200 ml min−1. Leaf CO2 assimilation rate, stomatal conductance, intercellular CO2 concentration, and instantaneous carboxylation efficiency were evaluated (Neves et al. Citation2006; Sivritepe et al. Citation2009). For chlorophyll estimation, leaf samples were frozen in liquid nitrogen and powdered in a pre-cooled mortar and pestle. The chlorophyll was extracted with 80% acetone. Chlorophyll a, b, and total chlorophyll were determined on the basis of chlorophyll present in mg g−1fw using the protocol of Sadasivam and Manikam (Citation1992).
Leaf hydrogen peroxide content and lipid peroxidation
Fresh leaves (0.1 g) were powdered in liquid nitrogen and extracted with 100-mM potassium phosphate buffer (pH 6.4) containing 5 mM KCN, according to Cheeseman (Citation2006). The reaction was carried out at 25°C for 30 min and the absorbance was read at 560 nm. The H2O2 concentration was calculated from the standard curve and expressed as µmol g−1fw. Lipid peroxidation was determined by estimating the MDA content following the protocol of Rao and Sresty (Citation2000). The absorbance was read at 532 nm (correction was made by subtracting the absorbance at 600 nm for nonspecific turbidity) by using extinction coefficient of 155 mM−1 cm−1. The MDA concentration was expressed as nmol g−1fw.
Enzyme extraction and activity assays
Samples of frozen leaves (0.1 g) were macerated in liquid nitrogen and extracted with 100 mM Tris–HCl buffer (pH 8.0) containing 30 mM DTT, 20% (v/v) glycerol, and 3% (w/v) PEG-6000 (Zimmermam et al. Citation2006). For superoxide dismutase (SOD) extraction, the pH of the buffer was adjusted to 7.0 and 30 mM DTT was replaced by 1 mM ascorbate. The crude extract was centrifuged at 14,000g for 30 min at 4°C and the supernatant was used as an enzymatic extract. The activity of SOD was determined by adding the leaf extract to a mixture containing 50 mM potassium phosphate buffer (pH 7.8), 0.1 mM EDTA, 13 mM L-methionine, 2 µM riboflavin, and 75 µM p-nitro blue tetrazolium chloride (NBT) in the dark. The reaction was carried out under illumination (using a 30 W fluorescent lamp) at 25°C for 6 min. The absorbance was measured at 540 nm. One SOD activity unit (U) was defined as the amount of enzyme required inhibiting 50% of the NBT photoreduction (Beauchamp & Fridovich Citation1971) and the activity is expressed as Ug fw−1 min−1. Catalase activity was determined after the reaction of the extract in the presence of 50-mM potassium phosphate buffer (pH 7.0) containing 20 mM H2O2. The reaction took place at 30°C and the absorbance at 240 nm was monitored for 300 s (Havir & Mchale Citation1987). The CAT activity was calculated according to the molar extinction coefficient of H2O2 (36 mM−1 cm−1) and is expressed as Ug fw−1 min−1. One CAT activity unit (U) was defined as µmoles of H2O2 consumed per minute.
Phytochemical analysis
Extraction and quantification of plumbagin were done following the protocol of Gangopadhyay et al. (Citation2010) in Dionex Ultimate 3000 HPLC system (Dionex, Germany), using a reverse phase C-18 column (250 × 4.6 mm, particle size 5 µ) by Acclaim 120, Germany, and UV detector. Briefly, the roots were separated from the sacrificed plants and washed thoroughly with tap water for removing adhering soil dust. The roots were dried (40 ± 5°C, 72 h), powdered, and macerated with methanol. The standard stock solution of plumbagin (Sigma–Aldrich, USA) was prepared at 1 mg ml−1 in HPLC grade methanol. The test solution was prepared by dissolving dried crude extract in HPLC grade methanol to give 1 mg ml−1 stock solution. All solutions were filtered through cellulose nylon membrane filter (0.45 µm) (PALL, Life Sciences). The aliquots of the filtrate were eluted with isocratic solvent mixture comprising acetonitrile:water (80:20, v/v) with the flow rate at 1 ml min−1 and detected at 410 nm. Plumbagin content was recorded as mg g−1fw. HPLC chromatograms were shown in supplementary data (Figure S1Footnote1).
Statistical analysis
The trial was laid out in completely randomized block design in two sets (release of mites or without mites) with 120 plants in specified growth stages counting from date of infection. Data were examined by analysis of variance to detect differences (p < 0.05) between the means and compared using independent T test at the same probability level using SPSS software (ver. 17.0.0; SPSS, Chicago, IL, USA).
Results and discussions
T. macfarlanei, commonly called red spider mite, is widespread throughout the Tropical and subtropical region of earth (Gupta Citation1976). It is exclusively phytophagous and causes substantial losses, especially to the herbs and small shrubs (Jose & Shah Citation1989). T. macfarlanei represents potential biotic stress to their host plants by piercing the plant tissues and sucking the plant sap, which resulted in characteristic damage symptoms. T. macfarlanei initially adheres to the lower surface of leaves of host plant and initiates detrimental effect to the whole plant.
Effect on growth and morphology
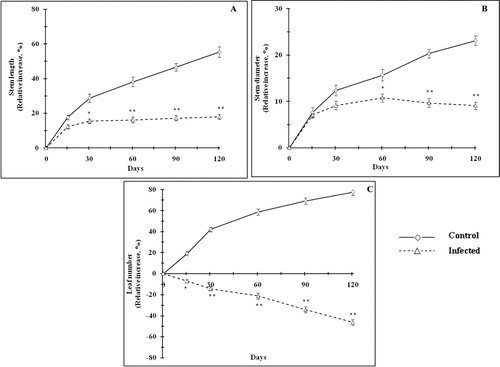
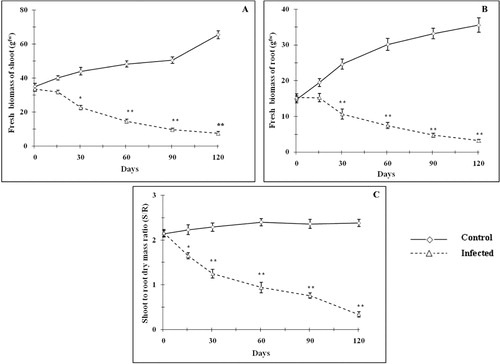
The plants were artificially infested with T. macfarlanei exhibited a sharp decline in relative increase in stem length (). Mite infestation in plants decreased the frequency of stem elongation with age as compared with uninfected plants. Day 30 onwards, the increase of stem length was arrested in infested plants and the relative increase of stem length (p < 0.01) remained almost parallel to x-axis. However, the stem length of controlled plants was gradually increased with time. The effect of mite infestation on stem diameter has been depicted in . From day 15 onwards, stem diameter enlargement was arrested in infected plant and stem diameter started to decrease (p < 0.05–0.01) 60 days onwards as compared with control sets. Pronounced reduction in plant height due to mite attack was reported by Pecinar et al. (Citation2009), while Farouk and Osman (Citation2011) observed that mite infestation resulted in the drying of the plant body, which often resulted in death. The number of leaves significantly reduced (p < 0.05–0.01) with age in mite-infested P. zeylanica as compared with control plants (). A negative slope of relative increase of leaf number indicated the detrimental effect of mite infestation. Spots with light color punctures were noticed in the leaves where mites had invaded into the tissues. The damage of leaf surface resulted in disturbances in the normal physiological processes of leaf. This is in accordance with the observation of Heng-Moss et al. (Citation2004) and Sivritepe et al. (Citation2009) where discoloration of the infested leaves was gradually increased with time. The affected leaves may eventually turn pale green to yellow and finally were fallen off. Leaf damage in mite-attacked plants caused nutritional stress and the vegetative plant growth was ceased gradually with time. Infested plants were significantly shorter than healthy plants and the plants were looked pale and weaker due to drastic weight loss. Day 15 onwards, a significant reduction (p < 0.05–0.01) of fresh biomass of stem () and root () was observed as compared with uninfected plants. Mite infestation also significantly lowered S/R as compared with control plants (). It is evident from the results that T. macfarlanei infestation resulted significant increase in mite population with time by multiplying both sexually and asexually. A continuous withdrawal of nutrients from host for the lifecycle of T. macfarlanei adversely affects the growth and morphology of P. zeylanica. The longer period of damage is likely to result in greater depletion of nutrients. The aboveground mite infestation also induced response to belowground part. The significant reduction of root biomass is an indicative of stem–root interaction. The vascular network of the plant would be the physiological basis of shoot–root interactions (Atkins & Smith Citation2007). Lack of supply of nutrients from aboveground part to root through vascular network may be the responsible factor for affecting the root growth.
Effect on physiological parameters
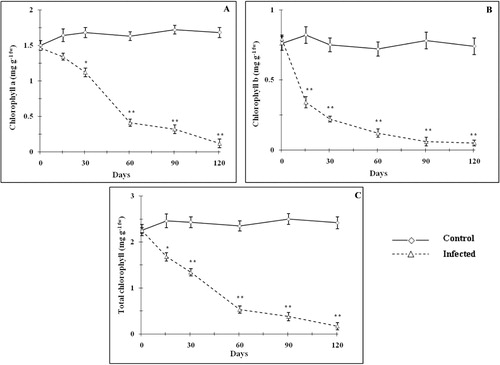
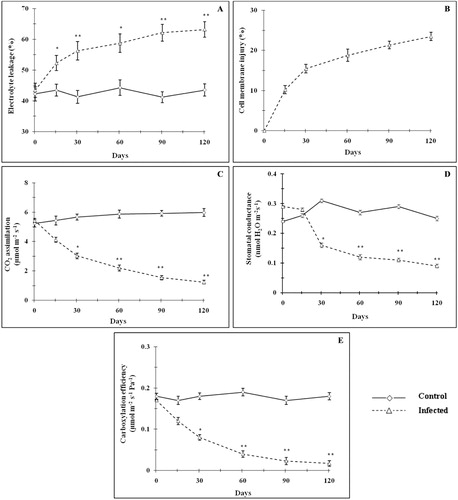
The content of chlorophyll a, chlorophyll b, and total chlorophyll decreased gradually (p < 0.05–0.01) with age after mite infestation as compared with control (). On day 120, a maximum reduction (p < 0.01) of 92.8, 93.2, and 92.9% was observed for chlorophyll a, chlorophyll b, and total chlorophyll, respectively. However, the ratio of chlorophyll a/chlorophyll b remained almost constant (data were not shown). The decrease in the chlorophyll content may be due to mechanical injury to the chloroplast during mite infestation and/or by the reactive oxygen species-mediated lipid peroxidation on chlorophyll pigments (Khattab Citation2007; Sivritepe et al. Citation2009). Electrolyte leakage (membrane permeability) was significantly increased () with mite feeding and it was accompanied with drastic augmentation of cell membrane injury (). Electrolyte leakage gradually increased (20.2–50.8%) with age and it attained maximum level (p < 0.01) in day 90. Cell membrane injury gradually increased (10.2% in day 15) to reach maximum injury level of 23.45% on day 120. The leaf CO2 assimilation rate decreased appreciably (24.1–79.4% between day 15 and 120) with age after mite infestation as compared with control (). Similarly, the stomatal conductance () and instantaneous carboxylation efficiency () were also reduced due to mite infestation. Tissue relative electrolyte leakage and cell membrane stability have long been studied to assess the membrane injury. The increased percentage of cell membrane injury and solute leakage was observed in T. macfarlanei-infested plants as compared with control. Free radical-mediated peroxidation of membrane lipid would be the cause of increased electrolyte leakage (Sivritepe et al. Citation2009). Destruction of chloroplast directly affects CO2 assimilation (Haile & Higley Citation2003; Bueno et al. Citation2009). Decrease in CO2 assimilation lowered the carbohydrate accumulation and consequent retroinhibition can be eliminated, since the mites continuously suck plant sap, thus preventing the leaves from storing carbohydrates (Patil & Nandihalli Citation2008; Sivritepe et al. Citation2009). Decrease in stomatal conductance allowed to differentiate among stomatal limitation or dark or light reactions impairment, respectively (Silva et al. Citation2010). The results found in this study pointed out to photosynthetic capacity reduction as a consequence of spider mite infestation due to decreased stomatal conductance. Physiological response of plants to spider mite injury is described as a reduction in gas-exchange parameters (Lakso et al. Citation1996; Sadras & Wilson Citation1997). It might be due to the major plant physiological response to T. macfarlanei injury that there was a closure of stomata (Candolfi et al. Citation1992; Sivritepe et al. Citation2009).
Effect on oxidation status
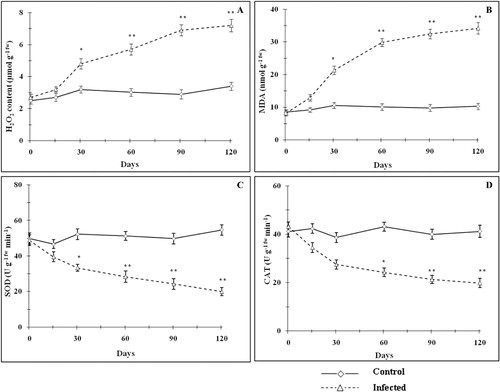
It has been reported that biotic stress can produce oxidative stress due to over production of reactive oxygen species (ROS) (War et al. Citation2012). ROS disrupts normal metabolic process through peroxidation of lipid, denaturation of proteins, and nucleic acids. O2− anion radical produced under normal or under stress conditions is scavenged by SOD, and this reaction results in the formation of H2O2 and O2 which is the first line of defense (Asada Citation2006; Caregnato et al. Citation2010). This detoxification mechanism is especially important in chloroplasts and mitochondria, where O2− is produced by metabolic imbalance under stress conditions (Shigeoka et al. Citation2002; Jain et al. Citation2006). CAT is indispensable for the plant's antioxidant defense system to detoxify photorespiratory H2O2 (Bauwe et al. Citation2010; Jaleel et al. Citation2010). If considered in terms of mass flow photorespiration, it is thought to be the second most important process after photosynthesis itself (Cavalcanti et al. Citation2004). Therefore, scavenging of this H2O2 is vital for maintenance of cellular redox state. In this study, leaf H2O2 concentration increased significantly (p < 0.01) 30 days onwards and reached maximum (∼2.1 fold) in mite-infested plants in comparison to the control (). Increased lipid peroxidation (MDA levels) is an index of augmented oxidative stress. Mite-infested plants exhibited a significantly higher (p < 0.05–0.01) MDA levels ranging between ∼1.4 and 3.3 fold between day 30 and 120 (). Antioxidant enzymes, such as SOD and CAT, showed similar patterns due to mite infestation. Leaf SOD activity was arrested significantly (p < 0.05–0.01) by 15.4–63.6% between 30 and 120 days (). The CAT activity was decreased significantly (p < 0.05–0.01) after 60 days and reached to lowest value by 51.8% on day 120 as compared with control plants of same age (). The inhibition of the CAT and SOD activities may be a consequence of enhanced oxidative stress due to biotic stress caused by mite infestation (Menard et al. Citation1999).
Effect on plumbagin production in root
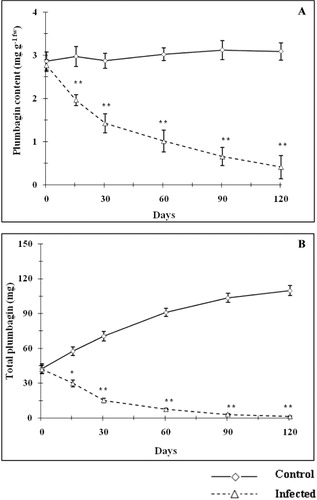
Changes in plant metabolite due to biotic interaction were reported by several workers (Blossey & Hunt-Joshi Citation2003; Ament et al. Citation2004; Moller et al. Citation2007). In this study, it was noticed that mite infestation on aerial part could exhaust the root reserves and completely damaged the underground roots. This result is in accordance with the findings of Blossey and Hunt-Joshi (Citation2003), Van Dam et al. (Citation2003), and Bezemer and Van Dam (Citation2005). Plumbagin is the key bioactive phytochemical synthesized in the root of Plumbago species (Gangopadhyay et al. Citation2011a, Citation2011b). Intracellular plumbagin accumulation was significantly reduced (p < 0.05) in the root of P. zeylanica on 30 days onwards and reached to minimum quantity of 0.41 mg g−1fw as compared with control plant (3.09 mg g−1fw) of same age (). The total plumbagin content was reduced significantly due to mite infestation (). A maximum of 98.8% reduction of total plumbagin production was observed on day 120. Bezemer et al. (Citation2003, Citation2004) found that shoot attack leads to a reduction of terpenoids bio-production in the root of G. herbaceum. A similar observation was recorded in maize (Rasmann & Turlings Citation2007). Therefore, it would be postulated that herbivores themselves manipulate plant defenses in their favor (Erb et al. Citation2008), which could also result even in the distant tissues. This could simply be suppression of defense responses (Musser et al. Citation2002) and/or activation of defenses that are ineffective against the herbivore itself. Such ‘decoy strategies’ could have major significance in shoot–root interactions (Erb et al. Citation2008).
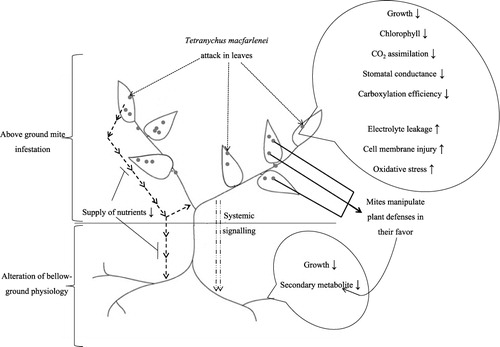
In conclusion, pest-mediated interactions can have detrimental effect on the productivity of whole plants. Overall symptoms caused by T. macfarlanei were depicted schematically in . The aboveground herbivore infestation does not confined within the site of infection, but having profound effect even in the distant tissues. In present study, T. macfarlanei introduction in P. zeylanica leaves caused detrimental effect on morpho-physiological and biochemical profiles of aboveground parts. The effect was further extended to the belowground tissues and adversely affected the growth and secondary metabolism.
Supplemental_Material.jpg
Download JPEG Image (47.7 KB)Acknowledgments
The authors are thankful to Dr. Manas Ghose, Dean of Faculty Centre of Ramakrishna Mission Vivekananda University for his cordial support. The authors also acknowledge the Department of Pharmaceutical Technology, Jadavpur University, Kolkata, India for providing the necessary facilities for HPLC. The authors would also like to thank the reviewers for their time and valuable comments.
Notes
1 Supplemental Content may be viewed online at http://dx.doi.org/10.1080/17429145.2013.865795.
References
- Ament K, Merijn RK, Sabelis MW, Haring MA, Schuurink RC. 2004. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol. 135:2025–2037. 10.1104/pp.104.048694
- Asada K. 2006. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 141:391–396. 10.1104/pp.106.082040
- Atkins CA, Smith PMC. 2007. Translocation in legumes: assimilates, nutrients, and signaling molecules. Plant Physiol. 144:550–561 10.1104/pp.107.098046
- Bauwe H, Hagemann M, Fernie AR. 2010. Photorespiration: players, partners and 1 origin. Trends Plant Sci. 15:330–336. 10.1016/j.tplants.2010.03.006
- Beauchamp C, Fridovich I. 1971. Superoxide dismutase: improved assay applicable to acrylamide gels. Anal Biochem. 44:276–287. 10.1016/0003-2697(71)90370-8
- Bezemer TM, Van Dam NM. 2005. Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol Evol. 20:617–624. 10.1016/j.tree.2005.08.006
- Bezemer TM, Wagenaar R, Van Dam NM, Van Der Putten WH, Wäckers FL. 2004. Above- and below-ground terpenoid aldehyde induction in cotton, Gossypium herbaceum, following root and leaf injury. J Chem Ecol. 30:53–67. 10.1023/B:JOEC.0000013182.50662.2a
- Bezemer TM, Wagenaar R, Van Dam NM, Wäckers FL. 2003. Interactions between above-and belowground insect herbivores as mediated by the plant defense system. Oikos. 101:555–562. 10.1034/j.1600-0706.2003.12424.x
- Bhargava, SK. 1984. Effect of plumbagin on reproductive function on male dog. Indian J Exp Biol. 22:153–156.
- Blossey B, Hunt-Joshi TR. 2003. Belowground herbivory by insects: influence on plants and aboveground herbivores. Annu Rev Entomol. 48:521–547. 10.1146/annurev.ento.48.091801.112700
- Bueno AF, Bueno RCOF, Nabity PD, Higley LG, Fernandes OA. 2009. Photosynthetic response of soybean to two spotted spider mite (Acari: Tetranychydae) injury. Braz Arch Biol Technol. 52:825–834. 10.1590/S1516-89132009000400005
- Candolfi MP, Boller RE, Wermelinger B. 1992. Influence of two spotted spider mite, Tetranychus urticae, on gas exchange of pinot noire grapevine leaves. Vitis. 31:205–212.
- Caregnato FF, Clebsch CC, Rochaa RF, Feistauer LBH, Oliveira PL, Junior ADD, Moreira JCF. 2010. Ozone exposure differentially affects oxidative stress parameters in distinct Phaseolus vulgaris L. varieties. J Plant Interact. 5:111–115. 10.1080/17429140903518455
- Cavalcanti FR, Oliveira JTA, Martins-Miranda AS, Viegas RA, Silveira JAG. 2004. Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt-stressed cowpea leaves. New Phytol. 163:563–571. 10.1111/j.1469-8137.2004.01139.x
- Cheeseman JM. 2006. Hydrogen peroxide concentrations in leaves under natural conditions. J Exp Bot. 57:2435–2444. 10.1093/jxb/erl004
- Didry N, Dubrevil L, Pinkas M. 1994. Activity of anthraquinonic and naphthoquinonic compounds on oral bacteria. Pharmazie. 49:681–683
- Erb M, Robert CAM, Turlings TCJ. 2011. Induction of root-resistance by leaf-herbivory follows a vertical gradient. J Plant Interact. 6:133–136. 10.1080/17429145.2010.545958
- Erb M, Ton J, Degenhardt J, Turlings TCJ. 2008. Interactions between arthropod-induced aboveground and belowground defenses in plants. Plant Physiol. 146:867–874. 10.1104/pp.107.112169
- Farouk S, Osman MA. 2011. The effect of plant defense elicitors on common bean (Phaseolus vulgaris L.) growth and yield in absence or presence of spider mite (Tetranychus urticae Koch) infestation. J Stress Physiol Biochem. 7:5–22.
- Gangopadhyay M, Dewanjee S, Bhattacharyya S, Bhattacharya S. 2010. Effect of different strains of Agrobacterium rhizogenes and nature of explants on Plumbago indica hairy root culture with special emphasis on root biomass and plumbagin production. Nat Prod Commun. 5:1913–1916.
- Gangopadhyay M, Dewanjee S, Bhattacharya S. 2011a. Enhanced plumbagin production in elicited Plumbago indica hairy root cultures. J Biosci Bioeng. 111:706–710. 10.1016/j.jbiosc.2011.02.003
- Gangopadhyay M, Dewanjee S, Chakraborty D, Bhattacharya S. 2011b. Role of exogenous phytohormones on growth and plumbagin accumulation in Plumbago indica hairy roots and conservation of elite root clones via. synthetic seeds. Indian Crop Prod. 33:445–450. 10.1016/j.indcrop.2010.10.030
- Gupta SK. 1976. Contribution to the knowledge of tetranychid mites (Acarina) with descriptions of three new species. Origin Insects. 10:327–351. 10.1080/00305316.1976.10432332
- Haile FJ, Higley LG. 2003. Changes in soybean gas-exchange after moisture stress 1 and spider mite injury. Environ Entomol. 32:433–440. 10.1603/0046-225X-32.3.433
- Havir EA, Mchale NA. 1987. Biochemical and development characterization of multiples forms of catalase in tobacco-leaves. Plant Physiol. 84:450–455. 10.1104/pp.84.2.450
- Hazra B, Sarkar R, Bhattacharya S, Ghosh PK, Chel G, Dinda B. 2008. Synthesis of plumbagin derivatives and their inhibitory activities against ehrlich ascites carcinoma and Leishmania denovani Promastigotes in vitro. Phytother Res. 16:133–137. 10.1002/ptr.867
- Heng-Moss TM, Sarath G, Baxendale FP, Novak D, Bose S, Ni X, Quisenberry S. 2004. Characterization of oxidative enzyme changes in buffalo grasses challenged by Blissus occiduus. J Econ Entomol. 97:1086–1095. 10.1603/0022-0493(2004)097[1086:COOECI]2.0.CO;2
- Jain N, Koopar R, Saxena S. 2006. Effect of accelerated ageing on seeds of radish (Raphanus sativus L.). Asian J Plant Sci. 5:461–464. 10.3923/ajps.2006.461.464
- Jaleel CA, Salem MA, Hasanuzzaman M, Nahard K. 2010. Plant growth regulator interactions results enhancement of antioxidant enzymes in Catharanthus roseus. J Plant Interact. 5:135–145. 10.1080/17429140903377456
- Jose VT, Shah AH. 1989. Carryover of spider mite, Tetranychus macfarlanei through alternate host plants in cotton growing areas of south and central Gujarat, India. In: Channabasavanna GP, Viraktamath CA, editors. Progress in Acarology. New Delhi: Oxford and IBH Publishing; p. 29–31.
- Khattab H. 2007. The defense mechanism of cabbage plant against phloem-sucking aphid (Brevicoryne brassicae L.). Aust J Basic Appl Sci. 1:56–62.
- Kuo PL, Hsu YL, Cho CY. 2006. Plumbagin induces G2-M arrest and autophagy by inhibiting the AKT/mammalian target of rapamycin pathway in breast cancer cells. Mol Cancer Ther. 5:209–3221. 10.1158/1535-7163.MCT-06-0478
- Lakso AN, Mattii GB, Nyrop JP, Denning SS. 1996. Influence of European red mite on leaf and whole cannopy carbon dioxide exchange, yield, fruit size, quality, and return cropping in ‘Starkrimson Delicious’ apple trees. J Am Soc Hortic Sci. 121:954–958.
- Masood A, Shah NA, Zeeshan M, Abraham G. 2006. Differential response of antioxidant enzymes to salinity stress in two varieties of Azolla (Azolla pinnata and Azolla filiculoides). Environ Exp Bot. 58:216–222. 10.1016/j.envexpbot.2005.08.002
- Mathew N, Paily KP, Vanamil AP, Balaraman KK. 2002. Macrofilaricidal activity of the plant Plumbago indica/ rosea in vitro. Drug Dev Res. 56:33–39. 10.1002/ddr.10056
- Menard R, Larue JP, Silue D, Thouvenot D. 1999. Glucosinolates in cauliflower as biochemical markers for resistance against drowny mildew. Phytochemistry. 52:29–35. 10.1016/S0031-9422(99)00165-X
- Moller IM, Jensen PE, Hansson A. 2007. Oxidative modifications to cellular components in plants. Annu Rev Plant Biol. 58:459–481. 10.1146/annurev.arplant.58.032806.103946
- Musser RO, Hum-Musser SM, Eichenseer H, Peiffer M, Ervin G, Murphy JB, Felton GW. 2002. Herbivory: caterpillar saliva beats plant defences-a new weapon emerges in the evolutionary arms race between plants and herbivores. Nature. 416:599–600. 10.1038/416599a
- Myster RW. 2011. Above-ground vs. below-ground interactive effects of mammalian herbivory on tallgrass prairie plant and soil characteristics. J Plant Interact. 6:283–290. 10.1080/17429145.2010.541290
- Neves AD, Oliveira RF, Parra JRP. 2006. A new concept for insect damage evaluation based on plant physiological variables. An Acad Bras Ciênc. 78:821–835.
- Patil RS, Nandihalli BS. 2008. Estimation of loss in brinjal due to red spider mites. Karnataka J Agr Sci. 21:456–457.
- Pecinar I, Stevanovic B, Rector BG, Petanovic R. 2009. Morphological injury of cut-leaf teasel, Dipsacus laciniatus L. (Dipsacaceae) induced by the eriophyid mite Leipothrix dipsacivagus Petanovic et Rector (Acari: Eriophyoidea). J Plant Interact. 4:1–6. 10.1080/17429140802361015
- Ramassamy V, Jayachandran V, Rajakumaran K. 2006. Effect of phytoextracts and auxins on the stem cuttings of Plumbago zeylanica L. Seaweed Res Utiln. 28:105–112. 10.1016/S0168-9452(00)00273-9
- Rao KVM, Sresty TVS. 2000. Antioxidative parameters in the seedlings of pigeon pea (Cajanus cajan L. Millspaugh) in response to Zn and Ni stresses. Plant Sci. 157:113–128. 10.1016/S0168-9452(00)00273-9
- Rasmann S, Turlings TCJ. (2007). Simultaneous feeding by aboveground and belowground herbivores attenuates plant-mediated attraction of their respective natural enemies. Ecol Lett. 10:926–936. 10.1111/j.1461-0248.2007.01084.x
- Sadasivam S, Manikam A. 1992. Biochemical method for agricultural sciences. New Delhi: Willey Eastern Limited.
- Sadras VO, Wilson LJ. 1997. Growth analysis of cotton crops infested with spider mites: light interception and radiation-use-efficiency. Crop Sci. 37:481–491. 10.2135/cropsci1997.0011183X003700020029x
- Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K. 2002. Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot. 53:1305–1319. 10.1093/jexbot/53.372.1305
- Silva EN, Ferreira-Silva SL, Fontenele AV, Ribeiro RV, Viegas RA, Silveira JAG. 2010. Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J Plant Physiol. 167:1157–1164. 10.1016/j.jplph.2010.03.005
- Sivritepe N, Kumral NA, Erturk U, Yerlikaya C, Kumral A. 2009. Responses of grapevines to two- spotted spider mite mediated biotic stress. J Biol Sci. 9:311–318. 10.3923/jbs.2009.311.318
- Sudhakar C, Lakshmi A, Giridarakumar S. 2001. Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci. 161:613–619. 10.1016/S0168-9452(01)00450-2
- Takemoto H, Takabayashi J. 2012. Exogenous application of liquid diet, previously fed upon by pea aphids Acyrthosiphon pisum (Harris), to broad bean leaves induces volatiles attractive to the specialist parasitic wasp Aphidius ervi (Haliday). J Plant Interact. 7:78–83. 10.1080/17429145.2011.625475
- Van Dam NM, Harvey JA, Wackers FL, Bezemer TMV, Putten WH, Vet LEM. 2003. Interactions between above ground and below ground induced responses against phytophages. Basic Appl Ecol. l4:63–77. 10.1078/1439-1791-00133
- War AR, Paulraj MG, War MY, Ignacimuthu S. 2012. Differential defensive response of groundnut germplasms to Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). J Plant Interact. 7:45–55. 10.1080/17429145.2011.587898
- Zimmermam P, Heinlein C, Orendi G, Zentgra U. 2006. Senescence-specific regulation of catalases in Arabidopsis thaliana (L.) Heynh. Plant Cell Environ. 129:1049–1060. 10.1111/j.1365-3040.2005.01459.x