Abstract
The mushroom Boletus fraternus Peck. shows allelopathy and suppresses the growth of broad leaf plants in nature. According to a bioassay-guided fractionation of the fruiting body of the fungus, a rare nonprotein amino acid was isolated as a major allelochemical. The chemical structure of the compound was determined to be (2S,4R)-2-amino-4-methyl-hex-5-enoic acid (5-dehydrohomoleucine) by analysis of 1H- and 13C-nuclear magnetic resonance spectra and comparison with data from the literature. The allelochemical caused 50% inhibition of lettuce seedling radicle growth at a concentration of 34 ppm (w/v). Further, since radicle growth was directed away from the filter paper to prevent contact with the allelochemical at concentrations higher than 300 ppm (w/v), the fungus may use the allelochemical to protect its immediate environment from contamination by other plants.
Introduction
Allelopathy is a process by which plants, including microorganisms, produce special chemicals that can be either beneficial or harmful to other (or even their own) neighboring organisms. Mushrooms are classified as higher fungi, and although often considered plants, they differ from common plants in that they lack chlorophyll and must rely on organic material produced by other organisms for their nutrition. It is well known that many mushrooms can influence the plants around them either directly or indirectly (Lincoff Citation1981; Dangl & Jones Citation1998; Biemelt & Sonnewald Citation2006; Jonassona et al. Citation2006; Badri et al. Citation2009). In fact, Molisch, who defined the term ‘allelopathy’, described the effect of an edible mushroom (Agaricus campestris) on the growth of vetch, Vicia sativa (Molisch Citation1937). However, only a few studies on mushroom allelopathy have been published (Chaumont & Simeray Citation1985; Mo et al. Citation2004; Araya Citation2005, Citation2007; Endo et al. Citation2012; Otaka & Araya Citation2013). In our ongoing study on mushroom allelopathy, we have described the possible release routes of allelochemicals from mushrooms in the form of volatiles, tissue litters, leached substances, and exudates, similar to that reported for general plants (Araya Citation2005). Verification of this release of allelochemicals from mushrooms may help explain some aspects of vegetation patterns in mushrooms.
Mushrooms have fascinated researchers for a long time because of their unique natural products produced. Mushroom-derived compounds have a wide spectrum of biological activities, including antimicrobial, nematocidal, antioxidative, anticarcinogenic, and others (Zjawiony Citation2004; Li & Oberlies Citation2005; Lindequist et al. Citation2005; Paterson Citation2006; Quang et al. Citation2006; Liu Citation2007; Zhong & Xiao Citation2009). There are few reports, however, of the effects of these compounds on plant growth; thus, the compounds could be considered as candidates for classification as allelochemicals. One allelochemical-like characteristic described is a large ring pattern that appears in lawns and constitutes a well-known turf disease. Some mushrooms cause ring patterns that are commonly referred to as ‘fairy rings’. When the rings are small, there is little effect on the surrounding grass. However, the circles expand year by year. Grass growth occurs away from where the mushrooms come up but is suppressed inside the circles. These observations indicate that the mushrooms are producing a plant growth regulator. The bioactive substances have not been identified, but recently Kawagishi et al. reported that Lepista sordida, the fairy ring fungus, produces imidazole-4-carboxamide as a plant growth inhibitor (Choi, Abe, et al. Citation2010) and 2-azahypoxanthine as a plant growth stimulator (Choi, Fushim, et al. Citation2010). These compounds are likely to be important allelochemicals produced by the fungus.
In our sandwich method screening program for new allelochemicals from various mushroom species, we found several mushrooms that displayed strong inhibitory activity to lettuce seedling growth (Lactuca sativa cv. Great Lakes 366; Araya Citation2005, Citation2007). For example, the lyophilized fruit body of Boletus fraternus Peck. inhibited growth of lettuce seedlings. In fact, broad leaf annual plants are rarely found around this fungus. Secondary metabolites of this species have not been previously investigated. The aim of this study, therefore, was to determine the allelochemicals present in fruiting bodies of B. fraternus on the basis of a bioassay-guided fractionation. A major active substance was isolated from the fruiting bodies, and the structure was determined to be (2S,4R)-2-amino-4-methyl-hex-5-enoic acid by nuclear magnetic resonance (NMR) analysis.
Materials and methods
General experimental procedures
1H- and 13C-NMR spectra were recorded using Alpha-600 or AL-300 NMR spectrometers (JEOL, Japan) in CD3OD. Optical rotations were recorded with a JASCO P-1020 using a quartz cell (50 mm × ϕ 3.5 mm i.d.). Column chromatography was carried out with a Funacel II (Funakoshi Co. Ltd., Japan). Thin layer chromatography (TLC) was carried out on Kieselgel 60 F254 (0.25 mm, Merck Millipore) or Cellulose F (Merck Millipore) plates. Ninhydrin reagent (Ninhydrin spray, Wako Pure Chemical Industries, Ltd. or Tokyo Chemical Industry Co., Ltd.) was sprayed on the TLC plates, and the plates were heated at 110°C on a hot plate to develop the color (Corning 4 × 5 Inch Top, PC-200 Hot Plate, Corning, USA).
Mushroom materials and extraction procedure
Fruiting bodies of B. fraternus (400 g) were collected at Showa, Fukushima, Japan, in September 2001. The mushroom was easily identified from its morphological features. The collected fruiting bodies were lyophilized after removal of soil and crushed. A 21.2 g sample was extracted with 99.5% EtOH (1.5 L × 2). The combined solution was concentrated to a syrup with an evaporator in vacuo, followed by extraction into 400 mL aqueous MeOH. The remaining MeOH was extracted further with hexane two times to give a hexane layer above the aqueous MeOH. The residual MeOH was then removed in an evaporator. After adding water to 400 mL, the solution was successively extracted with first EtOAc (400 mL × 2) and then BuOH (400 mL × 2). The removal of each of the solvents of the combined solutions yielded the following crude extracts: hexane (1.05 g), EtOAc (0.66 g), BuOH (1.14 g), and water (3.06 g).
Bioassay of lettuce seedlings
A modified method of Araya et al. (Citation2012) was used for the bioassay of lettuce seedling growth. In brief, aliquots of the four fractions (amount equivalent to 10 mg fruiting bodies) were applied separately to filter paper disks (27 mm diameter [precut by the maker], ADVANTEC No. 1) and transferred by micropipette into Petri dishes (27 mm i.d.). The dishes were placed in a vacuum oven at 40°C (Isotemp Vacuum Oven Model 280A, Fisher Scientific, USA), and the solvent was removed using a diaphragm vacuum pump. The disks were wetted with distilled water (1.0 mL), and five pre-germinated lettuce seeds (L. sativa cv. Great Lakes 366, Takii Seeds Co. Ltd., Japan) were placed on each paper disk. The seedlings were incubated within a moisture-saturated box placed in a dark incubator (BITEC-300L, Shimadzu, Japan or LTI-601SD, EYELA, Japan) for 48 h at 25°C. The length of the lettuce radicle and hypocotyl was measured in three independent experiments. The effects of each of the crude extracts on radicle and hypocotyl growth were expressed as the percentage of inhibition compared to the control. The ratio was calculated as below:
Purified allelochemical was prepared as 1, 3, 10, 30, 100, 300, and 1000 ppm (w/v) solutions and subjected to the lettuce seedling growth assay as described for the dose-response experiments.
Isolation of active compound by bioassay-guided fractionation based on starting material weight
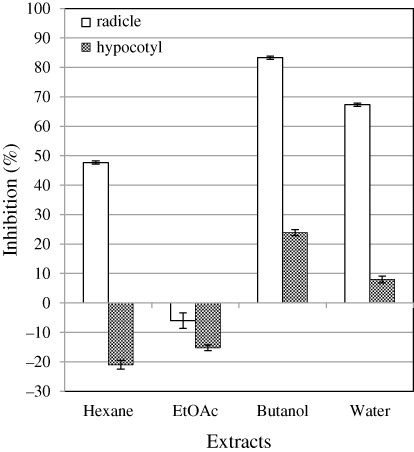
When the four extracts, hexane, EtOAc, BuOH, and water were tested in the lettuce seedling growth assay and correlated to starting material weight, the BuOH extract showed the greatest inhibition (). The 1H-NMR spectrum indicated that this fraction was composed of an almost pure compound 1 (>95%). Recrystallization of 1 failed with various solvents because of its low solubility in any of the solvents tested. The BuOH extract (1140 mg) was loaded over a microcrystalline cellulose column (Funacel II, ϕ 50 mm i.d. × 1500 mm), eluted with BuOH/AcOH/water = 8:1:1, and compound 1 (554 mg) was obtained in pure form.
(2S,4R)-2-amino-4-methyl-hex-5-enoic acid (1)
White powder, [α]D23: –18.9 (MeOH, c = 0.1), 13C-NMR (150 MHz, MeOH-d4) δC: 174.6 (C1), 143.9 (C5), 115.4 (C6), 54.7 (C2), 39.3 (C3), 36.1 (C4), 21.3 (C1’), 1H-NMR (600 MHz, MeOH-d4) δH: 5.72 (1H, ddd, J = 17.4, 10.2, 7.8 Hz, H-5), 5.14 (1H, ddd, J = 17.4, 1.8, 1.1 Hz, H-5a), 5.06 (1H, ddd, J = 10.2, 1.8, 0.7 Hz, H-5b), 3.49 (1H, dd, J = 9.4, 4.0 Hz, H-2), 2.36 (1H, br-sep, J = 7.2 Hz, H-4), 1.96 (1H, ddd, J = 18.9, 10.4, 4.4 Hz, H-3a), 1.67 (1H, ddd, J = 18.9, 9.4, 5.0 Hz, H-3b), 1.07 (3H, d, J = 5.4 Hz, H-1’).
TLC detection of 2-amino-4-methyl-hex-5-enoic acid from several species belonging to Boletaceae
Fruiting bodies of B. fraternus Peck., Boletus calopus Pers.: Fr., and Boletus pseudocalopus Hongo. were collected at Showa, Fukushima, Japan, in September 2006. Boletus hiratsukae Nagasawa and Suillus luteus (L.: Fr.) S.F. Gray fruiting bodies were harvested at Tsukuba, Ibaraki, Japan, in September 2004. Mushrooms of Leccinum extremiorientale (L. Vass.) Singer were collected at Nasushiobara, Tochigi, Japan, in 2007. All collected samples were lyophylized and stored at –20°C in a freezer. One gram of each sample was extracted with ethanol three times, and the combined solutions were evaporated in vacuo. The residues were dissolved in distilled water and loaded onto an Amberlite IR-120B (H+ form) column (5 mL). After washing the column with distilled water, the adsorbed compounds were eluted with 2N-NH4OH, and the solvent was removed in an evaporator. Residual extracts and isolated 2-amino-4-methyl-hex-5-enoic acid were spotted on a silica gel TLC plate (5 × 8 cm), and the plate was developed with BuOH/AcOH/water = 4:1:1. The amino acids (or amines) spots on the TLC plate were visualized by Ninhydrin reagent.
Results
Isolation of the allelochemical from B. fraternus fruiting bodies
B. fraternus Peck. suppresses the growth of broad leaf plants in nature, and the fruiting bodies had been previously shown to have allelopathic activity against lettuce seedling growth by the sandwich method (Araya Citation2005, Citation2007). The collected fruiting bodies were lyophilized after removal of soil, and the crushed material was used to produce four extracts (hexane, EtOAc, BuOH, and water) by extraction as described in the Methods. EtOAc To determine the most potent allelochemical by bioassay, the extracts, each prepared from 10 mg of fruiting bodies (dry weight [D. W.]), were tested for growth inhibition in the lettuce seedling assay. The amount of extract applied was based on the D.W. of fruiting body extracted to ensure the activity of each extract could be compared directly to each other. It can be seen in that the BuOH layer exhibited the strongest growth inhibitory activity. The active BuOH layer was almost pure on the basis of the 1H-NMR spectrum (>95%); however, further purification was attempted by recrystallization in various neutral organic solvents, but this was unsuccessful due too low solubility of the active component to any of the solvents. The BuOH extract was then chromatographed on a microcrystalline cellulose column, and pure compound 1 (554 mg) was obtained.
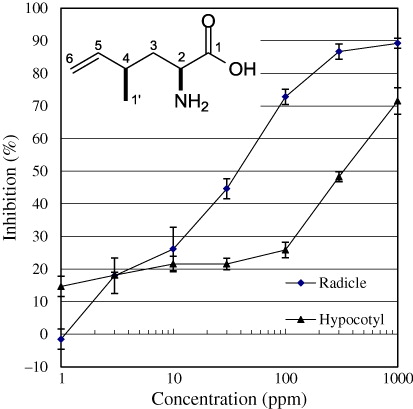
A dose-response relationship of 1 in terms of the inhibition of lettuce seedling growth was carried out. The compound inhibited radicle and hypocotyl growth of lettuce seedlings with IC50 values of 34 ppm (w/v) and 310 ppm (w/v), respectively.
Structure elucidation of the allelochemical (1)
Compound 1, the most potent allelochemical in the BuOH extract, was obtained as a white solid. The compound showed a positive purple spot on a TLC in a Ninhydrin test, suggesting a primary or secondary amine. The 1H- and 13C-NMR (in MeOH-d4) spectra showed signals assignable to an α-amino acid (δH 3.49 (dd); δC 174.6, 54.7), exo-methylene (δH 5.72 (ddd), 5.14 (ddd), 5.06 (ddd); δC 143.9, 115.4), a methine (δH 2.36 (br-sep); δC 36.1), a methylene (δH 1.96 (ddd), 1.67 (ddd); δC 39.3), and a methyl (δH 1.07 (d); δC 21.3. The planar structure was deduced to be 2-amino-4-methyl-hex-5-enoic acid. In addition, the presence of crosspeaks in 1H-1H-correlation spectroscopy (COSY) (H-2↔H-3ab; H-3ab↔H-4; H-4↔H-5; H-4↔H-1’; H-5↔H6ab) supported the structure.
In a previous study, two of four stereoisomers, 2S-(+)-2-amino-4-methyl-5-hexenoic acid from Streptomyces (Sp. UC5159) (Kelly et al. Citation1969) and 2S-(–)-2-amino-4-methyl-5-hexenoic acid from Boletus, section Ixocomus, group Nudi (Rudzats et al. Citation1972; Gellert et al. Citation1973), were isolated. Rudzats et al. reported the absolute stereochemistry was 2S,4S (Gellert et al. Citation1978); however, Snider and Dunčia corrected the stereochemistry to 2S,4R and determined 2S-(+)-2-amino-4-methyl-5-hexenoic acid as 2S,4S stereochemistry (Snider & Dunčia Citation1981). There are two symmetric carbons in 1, and the relative stereochemistry was determined by comparison of 1H-NMR in D2O with synthesized diastereomers pairs (racemic mixtures) (Snider & Dunčia Citation1981). The data of 1 were in accordance with a 2R*,4S* isomer. As optical rotation of 1 was negative, the absolute structure of 1 was elucidated as (2S,4R)-2-amino-4-methyl-5-hexenoic acid, as depicted in ), a rare nonprotein amino acid. The isolation of 1 in the present study is only the second time the compound has been isolated from Boletus.
Distribution of 2-amino-4-methyl-hex-5-enoic acid in other Boletaceae
As the only other isolation of (2S,4R)-2-amino-4-methyl-5-hexenoic acid was reported from a Boletus mushroom, section Ixocomus, group Nudi (Rudzats et al. Citation1972; Gellert et al. Citation1973), the compound was looked for in extracts by TLC from five other Boletaceae species: B. calopus Pers.: Fr., B. pseudocalopus Hongo., B. hiratsukae Nagasawa, S. luteus (L.: Fr.) S.F. Gray, L. extremiorientale (L. Vass.), with B. fraternus Peck. Each of the amino acid fractions, treated with Amberlite IR-120B (H+), were spotted on silica gel TLC. The same rate of flow (Rf) value and colored spots seen with (2S,4R)-2-amino-4-methyl-5-hexenoic acid were observed in B. hiratsukae Nagasawa and B. fraternus Peck. This result suggests that compound 1 is also produced by B. hiratsukae Nagasawa and B. fraternus Peck.
Discussion
In our screening of mushroom allelopathy by the sandwich method, we found various mushroom extracts that inhibited lettuce seedling growth (L. sativa cv. Great Lakes 366; Araya Citation2005, Citation2007). We began our investigation of allelochemicals produced by B. fraternus Peck. fruiting bodies for three reasons: the fungus suppresses growth of broad leaf plants in its immediate vicinity; the fruiting bodies exhibited allelopathy by the sandwich method in a laboratory experiment; and there have been no reports on secondary metabolites from this mushroom. From our study, a nonprotein amino acid, (2S,4R)-2-amino-4-methyl-5-hexenoic acid (1), was determined to be the major active compound, using a bioassay-guided fractionation based on the original weight of material. The isolation of compound (1) has now been reported independently two times (Rudzats et al. Citation1972; Gellert et al. Citation1973). Compound 1 inhibited growth of lettuce seedlings with an IC50 of 34 ppm for radicles and 310 ppm for hypocotyls. Compound 1 content was estimated to be more than 6% (D.W.), and the compound was detected in both the water layer and the BuOH extract. The strength of the allelopathic activity seen in the sandwich method could not be fully explained by the amount of the recovered active compound. The inhibition ratio at 540 ppm (roughly equal to 10 mg material; ) was higher than the total activity in the BuOH crude extract layer (equivalent to 10 mg material) () −86% versus 82% for radicles, and 48% versus 24% for hypocotyls. This indicated that there were no components other than compound 1 in the BuOH layer that had a significant influence on allelopathy.
It is noteworthy that all the lettuce seedling radicles tested at 300 ppm grew upward so as not to come in contact with the solution in the filter paper containing compound 1, in defiance of gravity given the negative geotropism of radicles. This observation suggests that an allelopathic agent can be used to protect the soil surrounding it from competition by neighboring plants by releasing allelochemicals which cause the radicles of neighboring plant seeds to turn upward. In addition, Negishi et al. reported that B. fraternus Peck. and other mushrooms possess methyl mercaptan-capturing properties (Negishi et al. Citation2001) that may represent a mechanism for detoxifying allelochemicals released by neighboring organisms.
Compound 1 has also been isolated from Boletus, section Ixocomus, group Nudi (0.04%, D.W.; Rudzats et al. Citation1972; Gellert et al. Citation1973), and its presence in B. hiratsukae Nagasawa was suggested in the TLC experiment of the present study. However, the content of the allelochemical in these mushrooms is too low to show allelopathy in the sandwich assay. (2S,4R)-2-amino-4-methyl-hex-5-enoic acid was isolated as the major contributing allelochemical of B. fraternus, and it may be specific to some Boletus. Further investigation of alleochemicals in other mushrooms has identified: cryptoporic acids in Cryptoporus volvatus fruiting bodies (Otaka et al. Citation2013) and 2-hydroxyalbicanol in Polyporus arcularius fungal culture broth (Otaka & Araya Citation2013). These results indicate that various mushrooms produce allelochemicals with diverse chemical structures that may play a role in the construction of some natural ecosystems.
Acknowledgments
This work was supported by Foundation of Meiji University and Foundation of National Institute for Agro-Environmental Sciences. The authors wish to express their sincere gratitude to Professor John Miller in the Biological Science, Victoria University of Wellington for the critical review of the manuscript.
References
- Araya H. 2005. Allelopathic activities in litters of mushrooms. In: Clark JM, Ohkawa H, editors. New discoveries in agrochemicals. Washington, DC: ACS; p. 63–72.
- Araya H. 2007. Fruiting bodies of mushrooms as allelopathic plants. In: Fujii Y, Hiradate S, editors. Allelopathy – new concepts and methodology. Enfield: Science Publisher; p. 341–352.
- Araya H, Otaka J, Nishihara E, Fujii Y. 2012. First isolation and identification of salicylate from Betula grossa var. ulmifolia – a potent root growth inhibitor. Allelopathy J. 30:153–158.
- Badri DV, Weir TL, Lelie D, Vivanco1 JM. 2009. Rhizosphere chemical dialogues: plant–microbe interactions. Curr Opin Biotech. 20:642–650. 10.1016/j.copbio.2009.09.014
- Biemelt S, Sonnewald U. 2006. Plant–microbe interactions to probe regulation of plant carbon metabolism. J Plant Physiol. 163:307–318. 10.1016/j.jplph.2005.10.011
- Chaumont JP, Simeray J. 1985. Propriétés allélopathiques de 114 extraits de carpophores de champignons sur la germination de semences de radis [Allelopathic effects of 114 mushroom fruiting bodies on the germination of radish seeds]. Revue D Ecologie Et De Biologie Du Sol. 22:331–339.
- Choi JH, Abe N, Tanaka H, Fushimi K, Nishina Y, Morita A, Kiriiwa Y, Motohashi R, Hashizume D, Koshino H, Kawagishi H. 2010. Plant-growth regulator, imidazole-4-carboxamide produced by fairy-ring forming fungus Lepista sordida. J Agric Food Chem. 58:9956–9959. 10.1021/jf101619a
- Choi JH, Fushimi K, Abe N, Tanaka H, Maeda S, Morita A, Hara M, Motohashi R, Matsunaga J, Eguchi Y, et al. 2010. Disclosure of the “fairy” of fairy-ring forming fungus Lepista sordida. Chem Bio Chem. 11:1373–1377. 10.1002/cbic.201000112
- Dangl J, Jones JDG. 1998. Plant–microbe interactions – affairs of the plant: colonization, intolerance, exploitation and co-operation in plant–microbe interactions. Curr Opin Plant Biol. 1:285–287. 10.1016/1369-5266(88)80047-5
- Endo Y, Minowa A, Kanamori R, Araya H. 2012. A rare α-pyrone from bitter tooth mushroom, Sarcodon scabrosus (Fr.) Karst. Biochem Syst Ecol. 44:286–288. 10.1016/j.bse.2012.06.018
- Gellert E, Halpern B, Rudzats R. 1973. Amino acids and steroids of a New Guinea Boletus. Phytochemistry. 12:689–692. 10.1016/S0031-9422(00)84465-9
- Gellert E, Halpern B, Rudzats R. 1978. The absolute configuration of the new amino acid 2-amino-4-methyl-hex-5-enoic acid from a new guinea Boletus. Phytochemistry. 17:802. 10.1016/S0031-9422(00)94235-3
- Jonassona S, Castroa J, Michelsen A. 2006. Interactions between plants, litter and microbes in cycling of nitrogen and phosphorus in the arctic. Soil Biol Biochem. 38:526–532. 10.1016/j.soilbio.2005.05.024
- Kelly RB, Martin DG, Hanka LJ. 1969. 2-Amino-4-methyl-5-hexenoic acid, a naturally occurring antimetabolite antibiotic. Can J Chem. 47:2504–2506. 10.1139/v69-414
- Li C, Oberlies NH. 2005. The most widely recognized mushroom: chemistry of the genus Amanita. Life Sci. 78:532–538. 10.1016/j.lfs.2005.09.003
- Lincoff GH. 1981. National Audubon society field guide to North American mushrooms. New York (NY): Chanticleer Press Inc.; p. 11–13.
- Lindequist U, Niedermeyer THJ, Jülich WD. 2005. The pharmacological potential of mushroom. eCAM. 2:285–299.
- Liu JK. 2007. Secondary metabolites from higher fungi in China and their biological activity. Drug Discov Ther. 1:94–103.
- Mo MH, Ma HM, Xiao QF. 2004. Study of the allelopathic effects of the ethanol-soluble extracts of Lactarius hatsudake on Oryza sativa and Echinoloa crusgalli. Acta Ecol Sin. 24:2951–2954.
- Molisch H. 1937. Der Einfluβ einer Pflanze auf die andere Allelopathy [The influence of one plant on another: allelopathy]. Jena: Fischer; p. 41.
- Negishi O, Negishi Y, Aoyagi Y, Sugawara T, Ozawa T. 2001. Mercaptan-capturing properties of mushrooms. J Agr Food Chem. 49:5509–5515. 10.1021/jf010534z
- Otaka J, Araya H. 2013. Two new isodrimene sesquiterpenes from the fungal culture broth of Polyporus arcularius. Phytochem Lett. 6:598–601. 10.1016/j.phytol.2013.07.010
- Otaka J, Taiko S, Goseki S, Miketa M, Araya H. 2013. Isolation and bioassay studies of Cryptoporic acids from Cryptoporus volvatus fruiting bodies. Mushroom Sci Biotech. 21:113–122.
- Paterson RRM. 2006. Ganoderma – a therapeutic fungal biofactory. Phytochemistry. 67:1985–2001. 10.1016/j.phytochem.2006.07.004
- Quang DN, Hashimoto T, Asakawa Y. 2006. Inedible mushroom: a good source of biologically active substances. Chem Rec. 6:79–99. 10.1002/tcr.20074
- Rudzats R, Gellert E, Halpern B. 1972. Constituents of a new guinea Boletus – isolation and identification of a new unsaturated α-amino acid. Biochem Biophys Res Commun. 47:290–292. 10.1016/S0006-291X(72)80041-X
- Snider BB, Dunčia JV. 1981. Stereospecific synthesis of both diastereomers of (±)-2-amino-4-methyl-5-hexenoic acid. J Org Chem. 46:3223–3226. 10.1021/jo00329a015
- Zhong JJ, Xiao JH. 2009. Secondary metabolites from higher fungi: discovery, bioactivity, and bioproduction. Adv Biochem Eng Biotech. 113:79–150.
- Zjawiony JK. 2004. Biologically active compounds from Aphyllophorales (Polypore) fungi. J Nat Prod. 67:300–310. 10.1021/np030372w