Abstract
A total of 84 bacterial endophytes were isolated from seeds of 6 cultivars of ornamental hostas, and they were identified to 5 species based on morphological characteristics and 16S rDNA sequence analysis. Among them, the strain ‘Blu-v2’, which was isolated from the seeds of cultivar ‘Blue Umbrella’ and identified to be Bacillus amyloliquefaciens, showed highest antifungal activity and capacity to deter feeding by Fall armyworms (Spodoptera fruigiperda). Lipopeptides in cultures of Blu-v2 were determined using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) and its antifungal activities were verified. However, the lipopeptide preparation did not show toxicity to larvae of Fall armyworms. In a greenhouse experiment, Blu-v2 was inoculated into small plantlets of hosta (cultivar ‘Rainforest Sunrise’). The leaves of plants with bacteria (endophyte-infected = E+) and without bacteria (endophyte-free = E−) were used in seven-day feeding experiments employing fourth-instar larvae of Fall armyworms. We found that there was a significant decrease in the weights of larvae fed with E+ compared to E− plants; and the mortality rate of larvae fed with E− leaves was lower (3.33%) compared to that of larvae fed with E+ leaves (30%). Based on our studies, we suggest that endophytic B. amyloliquefaciens strain Blu-v2 has potential value as a biocontrol agent to reduce damage from fungal diseases and insect pests of hosta cultivars.
Introduction
Bacterial endophytes live inside plants for at least part of their life cycle without causing conspicuous symptoms (Stone et al. Citation2000). Although the presence of bacterial endophytes in plants is variable and occasionally transient, they are often capable of enhancing plant growth and improving their stress tolerance through production of various metabolites or modulation of host plant gene expression (Hardoim et al. Citation2008; Mei & Flinn Citation2010; Brader et al. Citation2014). Endophytic bacteria are of considerable interest for agricultural, horticultural and forestry applications. During the past several decades, numerous studies have been carried out on plant disease control, growth promotion and biomass improvement through use of bacterial endophytes, and many useful strains have been identified, resulting in several commercial products (Mei & Flinn Citation2010; Pérez-García et al. Citation2011; Alfonzo et al. Citation2012; Li et al. Citation2012a).
Bacillus amyloliquefaciens is one of the most common bacterial endophytes infecting many plants, and due to its beneficial effect of improving plant disease resistance, it has gained considerable attention (Sun et al. Citation2006; Chen et al. Citation2009a; Li et al. Citation2012b; Chen et al. Citation2013). Previous studies have demonstrated that various lipopeptides are among the main metabolites responsible for anti-pathogen activity of B. amyloliquefaciens (Sun et al. Citation2006; Ongena & Jacques Citation2008; Alfonzo et al. Citation2012). Besides antibiosis, lipopeptides may also stimulate ‘induced systemic resistance’ (ISR) in plants (Ongena & Jacques Citation2008; Choudhary & Johri Citation2009). Thus, endophytic B. amyloliquefaciens should be an ideal biocontrol agent against pathogens (Koumoutsi et al. Citation2004). The study of the complete genome of B. amyloliquefaciens FZB42 revealed that more than 8.5% of the genome is devoted to synthesizing various kinds of antibiotics and siderophores by pathways not involving ribosomes (Chen et al. Citation2009b). These results hint that B. amyloliquefaciens may have more ecological functions than has been previously suspected.
Hostas (Hosta spp.; Asparagaceae) are among the most popular perennial ornamental plants grown in urban landscapes. In the USA, the cultivation and production of hostas are a multimillion-dollar industry in which nurseries grow and sell over 500 cultivars of 10 different species and their hybrids. Gardeners grow hostas because of their attractive leaf shapes, texture and color, and low maintenance costs. However, there is a growing concern among the growers and nursery managers about the serious leaf damage caused by fungi, slugs, nematodes and foliar feeding insects (Wang & Jeffers Citation2000; Jagdale & Grewal Citation2008; Zhen & Agudelo Citation2012). At the same time, there is increasing reluctance to apply potentially toxic agrichemicals to control pests and pathogens in the residential environment.
In order to develop knowledge regarding the potential of endophytic microbes of hosta that might be used to protect hosta plants from fungal pathogens and insect pests, we initiated studies to identify protective bacterial endophytes of hostas. In this study, bacterial endophytes were isolated from seeds of ornamental hosta cultivars. The strain Blu-v2, which was isolated from hosta cultivar ‘Blue Umbrella’ and identified as B. amyloliquefaciens, was used to evaluate effects on pathogens and pests.
Materials and methods
Bacterial endophytes isolation and identification
For bacterial endophyte isolation, a total of 180 mature seeds of 6 hosta cultivars (‘August Moon’, ‘Blue Umbrella’, ‘Halcyon’, ‘Sum and Substance’, ‘Hinkley Collection’ and ‘Dandy Lion’) (30 seeds for each cultivar) were selected at random and washed in running tap water and processed as follows: the seeds were surface-disinfected by sequentially dipping into 70% ethanol (2 min) and 0.5% sodium hypochlorite (2 min), then washed 3 times in sterile distilled water and dried on autoclaved paper towels (Li et al. Citation2012b). Finally, the seeds were placed in a Petri dish containing trypticase soy agar (TSA), incubated at 37°C, and checked for bacterial growth every other day for 20 d. The bacteria growing out of the seeds were transferred to fresh TSA plates. The endophyic isolates were sorted into morphotypes by first matching macroscopically similar colonies in color, shape, texture and growth rate, then by comparing microscopic characteristics such as cell shape, size, endospore production. Then, 3–5 isolates of each morphotype were selected at random and further identified based on 16S rDNA sequence analysis.
Antifungal activity of endophytes
The pathogenic fungi Alternaria alternata, Colletotrichum crassipes and Fusarium oxysporum were used in an antifungal activity test as follows: the bacterial endophytes were streaked to form a cross pattern on potato dextrose agar (PDA) plates and incubated at 37°C for 1 d. Then, the test fungi were inoculated between the cross arms and incubated at 25°C for 3–6 d, after which inhibition zones were determined. At the same time, nitrogen fixation ability and seed germination enhancing capacity (data not shown) were evaluated.
Based on these tests, an endophytic bacterium with strain designation ‘Blu-v2’, isolated from hosta ‘Blue Umbrella’, was selected to conduct the following experiments.
Greenhouse experiments
Small plantlets of hosta (cultivar ‘Rainforest Sunrise’) were transplanted into 6-inch standard pots containing a mixture of Canadian sphagnum peat with perlite (7:1 volume, Fafard, Canada). The pots were placed in a greenhouse at 17°C/16°C day/night cycle, 75% relative humidity, and a photoperiod of 15 h (300 µE). Plants were watered every other day with tap water and fertilized weekly with Peter's General Purpose 20–20–20 fertilizer (Grace Sierra Horticultural Products, Milpitas, CA).
Bacterial endophyte strain Blu-v2 was cultured in TSA plates at 37°C for 48 h, then washed with sterile distilled water and diluted to 1 × 105 CFUs (colony forming units) bacterial suspension. Then, the suspension was sprayed onto surfaces of small plantlets of hosta until they were wet (the treatment), and plantlets sprayed with sterile distilled water were used as the control. Spraying was done 3 times over 45 d (the 1st, 15th and 35th days after transplanting), and 10 replicates were made. To confirm bacterial colonization inside inoculated plants, plant leaves were excised on the 18th and 38th days after the first inoculation, and were washed under running tap water and surface-disinfected by sequentially dipping into 0.5% sodium hypochlorite (1 min) and 70% ethanol (2 min). After washing with sterile distilled water, the leaves were homogenized by grinding, and diluted suspensions were grown in TSA medium for detection of the strain inoculated. Colonization was confirmed by re-isolation of the strain Blu-v2 from plants.
Feeding tests
Larvae of Fall armyworms (Spodoptera fruigiperda) were obtained from a commercial supplier. Larvae at the second- to third-instar arrived in sterilized Bio-Serv Lepidoptera diet trays with special food, and remained in the lab at approximately 22°C until most had developed to the 4th-instar.
The effect of the bacterial endophyte on insect resistance of hosta was determined through assessing growth and survival of Fall armyworm (S. fruigiperda) larvae in ‘no-choice tests’ and in ‘choice tests’. In no-choice tests, one larva was weighed with a balance up to 0.01 mg and then placed into a 90 mm Petri dish containing a wet filter paper on the bottom, and leaves of endophyte-infected (E+) and endophyte-free (E−) plants were put into separate plates and changed every other day. To maintain humidity in plates, water was added to the filter paper as needed. Survival was assessed every day, and larvae were weighed after 7 d. In choice tests, one leaf blade of an E− plant and one leaf blade of an E+ plant of the same age were put into a 90 mm Petri dish together. Then, one 4–5th-instar larva was placed into the plate. After 36 h feeding, the area of the leaves eaten by the larva was measured by placing leaves on graph paper and counting grids (1 mm2) under the consumed part of the leaf. Thirty replicates were made for each treatment.
Lipopeptide production and MALDI-TOF MS analysis
Blu-v2 was inoculated into a 250 ml flask containing 100 ml of Landy medium, and incubated at 30°C at 150 rpm for 36 h (Landy et al. Citation1948). Then, the culture was centrifuged at 10,000 × g for 20 min to remove bacterial cells, and the broth was adjusted to pH 2 with 6 M HCl to precipitate lipopeptides. Finally, the precipitate was readjusted to pH 7 with 4 M NaOH, and crude lipopeptides were extracted by ethanol and stored in a refrigerator for later use (Wang et al. Citation2010).
MALDI-TOF MS analysis of crude lipopeptides was performed using an ABI-MDS SCIEX 4800 MALDI-TOF/TOF mass spectrometer equipped with an N2 laser (337 nm). The sample was 5× serial diluted with matrix medium (a saturated solution of α-cyano-4-hydroxycinnamic acid in 50% aqueous acetonitrile containing 0.1% [vol/vol] TFA) before deposition. Data were acquired at reflector positive mode from 800 to 4000.
Anti-pathogen and anti-insect activity of lipopeptides
The pathogenic fungi A. alternata, C. crassipes, F. oxysporum were inoculated onto PDA plates as follows: the fungus was inoculated onto four corners of a square about 30 mm on a side. Then, 10 µl crude lipopeptide solution (1.6 mg/ml) was injected into the medium between two corners of the square, and the plate was incubated at 25°C for 3–6 d and the inhibition was observed.
Leaves of E− plants were dipped in a lipopeptide water solution (L+) and were used to rear larvae, and E− leaves dipped in water (L−) were used as controls. The feeding method was the same as above in no-choice tests.
Statistical analyses
Student's t-test was used to compare the difference in feeding scores in the choice experiments between endophyte-infected (E+) and uninfected plants (E−) leaves, and larval weight gains between larvae fed with E+ and E− as well as larvae fed with leaves dipped in lipopeptides (L+) and control leaves.
Results
Bacterial endophytes and their antifungal activity
Total 84 bacterial endophytes were isolated from 180 seeds of 6 hosta cultivars. They were sorted into six morphotypes and were further identified to five species based on morphological characteristics and 16S rDNA region analysis (). Among them, B. amyloliquefaciens was the dominant species, about 1/3 of the isolates belonging to it. We found that all B. amyloliquefaciens isolates, including August-M1 and Blu-v2, showed antifungal activity against three pathogenic fungi and were capable of growth on Norris Nitrogen-Free Media (). The isolates of other species did not exhibit antifungal activity. Among all isolates, Blu-v2, whose 16S rDNA region showed 99% identity to B. amyloliquefaciens in Genbank, showed better antifungal activity and seed germination enhancement than other strains, was selected for inoculation into hosta to evaluate its effects on fungal pathogens and insect.
Table 1. Characteristics of endophytic bacteria from hosta cultivars.
Strain Blu-v2 colonization confirmation
To confirm endophytic colonization of hosta seedlings with Blu-v2, on the 18th and 38th days after inoculating seedlings with Blu-v2, we attempted isolation of the bacterium from inoculated and non-inoculated plants. Blu-v2 was only re-isolated from the inoculated plants. Blu-v-2 was not obtained from non-inoculated controls.
Feeding tests
In feeding experiments, it was found that there was a significant increase in the weights of larvae fed with E− compared to E+ leaves after 7 d of feeding (t-test, p = .0057, df = 48), and the average weight of larvae fed with E− was 50.1 ± 20.6 mg, but only 35.61 ± 12.6 mg for larvae fed with E+ leaves. In addition, the mortality rate of larvae fed with E+ (30%) was higher than that of E− (3.33%).
In the choice experiments, we found that larvae tended to prefer E− leaves over E+ leaves, however, the difference between area consumed on leaf blades from E+ and E− leaves was not significant (t-test, p = .1281, df = 28). The mean area consumed on leaf blades from E+ leaves was 228 ± 38.4 cm2, while, the mean area consumed on leaf blades from E− plants was 309 ± 57.0 cm2.
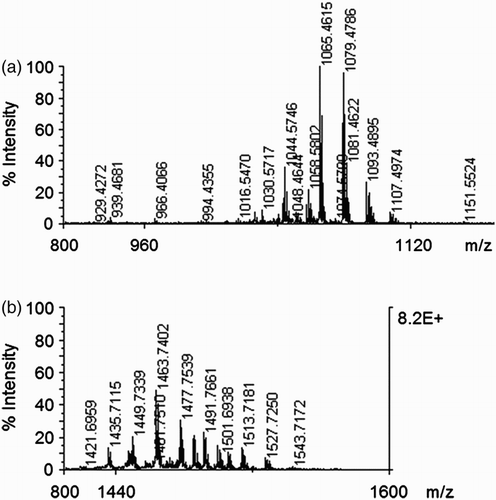
MALDI-TOF MS identification of lipopeptide products of Blu-v2
The product patterns determined by MALDI-TOF mass spectrometry for the crude lipopeptides from cultures of Blu-v2 are shown in . The mass spectra show that there were three well-resolved groups of peaks at m/z values between 1000 and 1060, between 1070 and 1150, and between 1420 and 1550. The groups of peaks could be attributed to the isoform ensembles of surfactins, iturins, and fengycins by comparing their mass data with those previously obtained by MS analysis of the lipopeptide products of numerous B. amyloliquefaciens and B. subtilis strains (Vater et al. Citation2002; Koumoutsi et al. Citation2004).
Anti-pest activity of lipopeptides
Crude lipopeptides from cultures of Blu-v2 showed antagonistic activity against three test fungi. However, the larval feeding test did not show a significant difference in weight gains between larvae fed with leaves dipped in crude lipopeptides and with control leaves (t-test, p = .3289, df = 24).
Discussion
Endophytes of five species were isolated from hosta seeds of multiple cultivars (), which indicated that hostas associate with diverse endophytic bacteria and that those bacteria transmit through seeds. Among these species, Bacillus amyloliquefaciens is the dominant endophyte. It was isolated from five hosta cultivars, but not from cultivar ‘Sum and Substance’. Similarly, B. amyloliquefaciens has been reported to be an endophyte of numerous plants (Sun et al. Citation2006; Choudhary & Johri Citation2009; Chen et al. Citation2013; White et al. Citation2014).
Previous studies have demonstrated that some isolates of B. amyloliquefaciens can improve plant disease resistance (Sun et al. Citation2006; Chen et al. Citation2013). Consistent with this, we found that all B. amyloliquefaciens isolates from hosta showed antifungal activity (). Surfactins, iturins, and fengycins represent the well-known biosurfactant families produced by B. amyloliquefaciens and B. subtilis strains (Vater et al. Citation2002; Koumoutsi et al. Citation2004). Numerous studies have demonstrated that these lipopeptides possess antagonistic activity against various pathogens (Sun et al. Citation2006; Ongena & Jacques Citation2008; Alfonzo et al. Citation2012). In the present study, it was found that crude lipopeptides from cultures of Blu-v2 showed antifungal activity, and the MALDI-TOF MS analysis indicated that strain Blu-v2 produced surfactins, iturins, and fengycins at the same time (). Therefore, lipopeptides are the likely antifungal active metabolites of Blu-v2.
In a greenhouse experiment, Blu-v2 was inoculated into small plantlets of hosta by spraying plants with a suspension of the bacterium. We verified the infection state of our experimental plants by re-isolation of Blu-v2 from inoculated plants; un-inoculated controls did not show presence of Blu-v2. It seems clear that Blu-v2 readily colonizes hosta plants. The likely sites of bacterial entry into the plant include meristems (shoot and root) and stomata (White et al. Citation2014). We did not determine precisely where Blu-v2 was entering hosta tissues.
Our results in the ‘no choice’ feeding tests indicated that larvae fed with E+ leaves gained less weight than larvae fed with E− leaves (t-test, p = .0057, df = 48); consistent with this, the mortality rate of larvae fed E+ leaves was 30%, while larvae fed E− leaves showed only 3.33% mortality during the course of the experiment. Similarly, some previous studies found that fungal endophyte-infected plants reduced insect feeding and inhibited insect development compared to endophyte-free plants. For example, Boning and Bultman (Citation1996) found that S. frugiperda weighed less and took longer to develop into adults when fed fungal endophyte-infected versus endophyte-free grass. Crawford et al. (Citation2010) found that S. frugiperda larval survival was significantly higher on endophyte-free than on fungal endophyte-infected plants in two native grass species, and S. frugiperda had significantly greater mass when reared on endophyte-free plants. In contrast, other studies found that some endophytes did not appear to reduce feeding by insects; e.g. Crawford et al. (Citation2010) found that larvae raised on Lolium arundinaceum did not differ in mass when reared on fungal endophyte-infected versus endophyte-free plants. Clement et al. (Citation2005, Citation2011) also concluded that endophyte infection does not always confer resistance to insect herbivores. They suggested that the outcome of an interaction, involving plant, endophyte and insect, is influenced by host species or genotype, endophyte species or genotype, and the insect species.
In choice experiments, we found that larvae tended to prefer E− plant leaves over E+ leaves. The same phenomenon was observed by Clement et al. (Citation2011), where it was shown that the aphid Rhopalosiphum padi preferred fungal endophyte-free grass over fungal endophyte-infected grass in choice experiments. Crawford et al. (Citation2010) also found that both in the field and in experimental trials, herbivores showed a significant preference for endophyte-free plant material for the majority of native grasses. Our results suggest that development of a program to select bacterial endophytes to improve hosta resistance to herbivores and pathogens could yield positive results if enough endophytes are screened.
Yun et al. (Citation2013) found that the lipopeptide surfactin showed aphicidal activity. However, in our experiment, we did not see a significant detrimental effect on larvae fed with leaves dipped in lipopeptides (L+) (t-test, p = .3289, df = 24). It has been reported that lipopeptide antagonism is influenced by host species, in some plant species lipopeptides showed direct antagonism to phytopathogens, but in other plant species, they acted as plant resistance inducers (Tran et al. Citation2007; Ongena & Jacques Citation2008). Because we did not see direct effects of lipopeptides on the Fall armyworms, it seems likely that the lipopeptides produced by Blu-v2 acted as resistance inducers. In addition, presence of calcium oxalate crystals in plant tissues has been associated with reduced feeding and higher mortality for chewing insects (Doege & Korth Citation2003). Although we could not quantify it, a microscopic examination of tissues scraped from leaves and stems of plants infected with Blu-v2 and non-infected controls suggests that infected plants may have larger and more numerous calcium oxalate needle crystals (raphides) than endophyte-free hosta plants. It will require additional experimentation to determine whether increased calcium oxalate crystals or another mechanism accounts for anti-insect effects of Blu-v2.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
The authors acknowledge the John and Christina Craighead Foundation, United States Department of Agriculture NIFA Multistate Project W3147, the New Jersey Agricultural Experiment Station and the National Natural Science Foundation of China (31360128) for fund support for this project.
References
- Alfonzo A, Piccolo SL, Conigliaro G, Ventorino V, Burruano S, Moschetti G. 2012. Antifungal peptides produced by Bacillus amyloliquefaciens AG1 active against grapevine fungal pathogens. Ann Microbiol. 62:1593–1599.
- Boning RA, Bultman TL. 1996. A test for constitutive and induced resistance by tall fescue (Festuca arundinacea) to an insect herbivore: impact of the fungal endophyte, Acremonium coenophialum. Am Midl Nat. 136:328–335.
- Brader G, Compant S, Mitter B, Trognitz F, Sessitsch A. 2014. Metabolic potential of endophytic bacteria. Curr Opin Biotechol. 27:30–37.
- Chen XH, Koumoutsi A, Scholz R, Borriss R. 2009b. More than anticipated-production of antibiotics and other secondary metabolites by Bacillus amyloliquefaciens FZB42. J Mol Microbiol Biotechnol. 16:14–24.
- Chen XH, Scholz R, Borriss M, Junge H, Mögel G, Kunz S, Borriss R. 2009a. Difficidin and bacilysin produced by plant-associated Bacillus amyloliquefaciens are efficient in controlling fire blight disease. J Biotechnol. 140:38–44.
- Chen YT, Yuan Q, Shan LT, Lin MA, Cheng DQ, Li CY. 2013. Antitumor activity of bacterial exopolysaccharides from the endophyte Bacillus amyloliquefaciens sp. isolated from Ophiopogon japonicus. Oncol Lett. 5:1787–1792.
- Choudhary DK, Johri BN. 2009. Interactions of Bacillus spp. and plants with special reference to induced systemic resistance (ISR). Microbiol Res. 164:493–513.
- Clement SL, Elberson LR, Bosque-Pérez NA, Schotzko DJ. 2005. Detrimental and neutral effects of wild barley Neotyphodium fungal endophyte associations on insect survival. Entomol Exp Applicata. 114:119–125.
- Clement SL, Hu J, Steward AV, Wang B, Elberson LR. 2011. Detrimental and neutral effects of a wild grass-fungal endophyte symbiotum on insect preference and performance. J Insect Sci. 11:77.
- Crawford KM, Land JM, Rudgers JA. 2010. Fungal endophytes of native grasses decrease insect herbivore preference and performance. Oecologia. 164:431–444.
- Doege SJ, Korth KL. 2003. The role of natural calcium oxalate crystals in plant defense against chewing insects. Inquiry. 4:88–94.
- Hardoim PR, Overbeek LS, Elsas JD. 2008. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 16:463–471.
- Jagdale GB, Grewal PS. 2008. Influence of the entomopathogenic nematode Steinernema carpocapsae infected host cadavers or their extracts on the foliar nematode Aphelenchoides fragariae on Hosta in the greenhouse and laboratory. Biol Control. 44:13–23.
- Koumoutsi A, Chen XY, Henne A, Liesegang H, Hitzeroth G, Franke P, Vater J, Borriss R. 2004. Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J Bacteriol. 186:1084–1096.
- Landy M, Warren GH, Roseman SB, Colio LG. 1948. Bacillomycin: an antibiotic from Bacillus subtilis active against pathogenic fungi. Proc Soc Exp Biol Med. 67:539–541.
- Li HY, Shen M, Zhou ZP, Li T, Wei YL, Lin LB. 2012a. Diversity and cold adaptation of endophytic fungi from five dominant plant species collected from the Baima Snow Mountain, Southwest China. Fungal Divers. 54:79–86.
- Li SB, Fang M, Zhou RC, Huang J. 2012b. Characterization and evaluation of the endophyte Bacillus B014 as a potential biocontrol agent for the control of Xanthomonas axonopodis pv. dieffenbachiae –Induced blight of Anthurium. Biol Control. 63:9–16.
- Mei C, Flinn BS. 2010. The use of beneficial microbial endophytes for plant biomass and stress tolerance improvement. Recent Pat Biotechnol. 4:81–95.
- Ongena M, Jacques P. 2008. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends microbial. 16:115–125.
- Pérez-García A, Romero D, Vicente A. 2011. Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr Opin Biotechnol. 22:187–193.
- Stone JK, Bacon CW, White JF. 2000. An overview of endophytic microbes: endophytism defined. In: Bacon CW, White JF, editors. Microbial endophytes. New York: Marcel-Dekker; p. 3–30.
- Sun L, Lu Z, Bie X, Lu F, Yang S. 2006. Isolation and characterization of a co-producer of fengycins and surfactins, endophytic Bacillus amyloliquefaciens ES-2, from Scutellaria baicalensis Georgi. World J Microbiol Biotechnol. 22:1259–1266.
- Tran H, Ficke A, Asiimwe T, Höfte M, Raaijmakers JM. 2007. Role of the cyclic lipopeptide massetolide A in biological control of Phytophthora infestans and in colonization of tomato plants by Pseudomonas fluorescens. New Phytol. 175:731–742.
- Vater J, Kablitz B, Wilde C, Franke P, Mehta N, Cameotra SS. 2002. Matrix assisted laser desorption ionization-time of flight mass spectrometry of lipopeptide biosurfactants in whole cells and culture filtrates of Bacillus subtilis C-1 isolated from petroleum sludge. Appl Environ Microbiol. 68:6210–6219.
- Wang B, Jeffers SN. 2000. Fusarium root and crown rot: A disease of container-grown Hostas. Plant Dis. 84:980–988.
- Wang Y, Lu Z, Bie X, Lv F. 2010. Separation and extraction of antimicrobial lipopeptides produced by Bacillus amyloliquefaciens ES-2 with macroporous resin. Eur Food Res Technol. 231:189–196.
- White JF, Torres MS, Sullivan RF, Jabbour RE, Chen Q, Tadych M, Irizarry I, Bergen MS, Havkin-Frenkel D, Belanger FC. 2014. Microscopy research and technique: Occurrence of Bacillus amyloliquefaciens as a systemic endophyte of vanilla orchids. Microsc Res Tech. doi:10.1002/jemt.22410.
- Yun DC, Yang SY, Kim YC, Kim IS, Kim YH. 2013. Identification of surfactin as an aphicidal metabolite produced by Bacillus amyloliquefaciens G1. J Korean Soc Appl Biol Chem. 56:751–753.
- Zhen F, Agudelo P. 2012. A protocol for assessing resistance to Aphelenchoides fragariae in Hosta Cultivars. Plant Dis. 96:1438–1444.