Abstract
The effect of fan-forced wind on the severity and growth of Fusarium oxysporum f. sp. radicis-lycopersici (FORL) was examined in this study. The discoloration severity of the total root system was significantly reduced in plants treated with air blasting for 30 min at a wind speed of 4 m/s compared with the control. In addition, the number of colony-forming units of FORL per gram of fresh root weight was significantly reduced (p ≤ 0.05) in plants treated with air blasting at a wind speed of 4 m/s for 30 min, and the root extracts of these plants had a significantly lower production of FORL budding cells. Booster wind treatments significantly reduced the severity and growth of FORL compared with single and control treatments. Furthermore, RT-PCR analysis indicated that the expression of defense-related genes was induced in the leaves of seedlings treated with air blasting at a wind speed of 4 m/s.
1. Introduction
Fusarium oxysporum f. sp. lycopersici (FOL) and F. oxysporum f. sp. radicis-lycopersici (FORL) are two forma specialis of F. oxysporum affecting tomato production worldwide (Armstrong & Armstrong Citation1981; Steinkellner et al. Citation2005). FORL was initially reported in Japan (1969) and California (1971) (Benhamou et al. Citation1989; Fazio et al. Citation1999). It is a soil-inhabiting fungus that invades plant roots and vascular tissue and is one of the most economically important and destructive pathogens of tomato. FORL causes severe damage to both field and greenhouse-grown tomato plants, resulting in stunted seedlings and drooping and yellowing leaves. Infected plants frequently wilt and die (Rowe & Farley Citation1977; Jarvis & Shoemaker Citation1978; Horinouchi et al. Citation2007, Citation2008). Although the use of Fusarium-resistant tomato cultivars can provide some degree of protection against this pathogen, the emergence of its new resistant races is a continuing problem (Dekker Citation1979). The most effective method of controlling FORL to date has been soil disinfection using methyl bromide. However, the use of this fumigant was outlawed in 2005 because it caused severe environmental problems (Meadows Citation2013).
Plants are exposed to stress from biological (biotic stress) and non-biological sources (abiotic stress). Feeding damage caused by insects and pathogen infection are examples of biological stress, while certain environmental stimuli such as light and temperature, and physical stimuli such as friction and contact between plants caused by wind are examples of abiotic stress. The exposure of plants to biotic and abiotic stresses can cause a protective response, such as the accumulation of lignin and the activation of defense-related enzymes such as peroxidase and phenylalanine ammonia-lyase (Hrazdina & Parsons Citation1982; Hahlbrook & Scheel Citation1989). Wind is a major environmental factor that affects expansion and growth of plants, causing suppression of plant height (Biddington Citation1986). Wind can change the number of pores and the thickening of the cuticle layer of the cell wall and stem (Todd et al. Citation1972). At the same time, the plant defense response is activated by wind as a physical stimulus. Disease control using artificial blast processing has been reported in rice for rice blast disease (pathogen: Pyricularia oryzae) (Taguchi et al. Citation2014, Citation2015). Wind blowing at various speeds, i.e. 3–5 m/s, for a period of 4–5 days was reported to suppress rice blast disease. The induction of resistance in kidney bean plants resulting from the blowing process has been reported to occur through the accumulation of lignin and an increase in peroxidase activity compared with non-blown plants (Cipollini Citation1997, Citation1998). Additionally, the lesion area in kidney bean leaves caused by anthracnose (pathogen: Colletotrichum lindemuthianum) was decreased by applying wind at a speed of 3 m/s for 1-h intervals (Cipollini Citation1997). The induction of resistance by blast processing was reported to be due to the activation of the elicitor cytosol and the protein kinase signaling pathways that increase the calcium ion (Ca2+) as a secondary messenger (Knight et al. Citation1992).
Although many researchers have reported the induction of disease resistance resulting from the blowing process, only a few plant species have been treated. In this study, tomato plants, which have not been reported as test plants for the purpose of examining the induction of resistance using the blowing process, were used to study the mechanism of disease resistance. Additionally, to confirm the induction of systemic resistance acquired through wind treatment, the expression of genes associated with the defense response to pathogenic proliferation in tomato was investigated.
2. Materials and methods
2.1. Plants
Tomato seeds ‘cv. House Momotaro’ (Takii Seed Co., Ltd., Japan), which is a popular cultivar used mainly in greenhouses in Japan and is susceptible to FORL, were used in all the experiments. All seeds were surface disinfected using 70% ethanol for 1 min, then 1% sodium hypochlorite solution for 5 min and rinsed three times in sterile distilled water prior to sowing. Seeds were pre-germinated for 3 days. Plastic pots (6 cm in diameter) were filled with Star Bed potting soil (Zen-Noh, Tokyo, Japan) containing clay, peat, zeolite and composted plant material. Tomato seeds were individually sown and cultivated at 25°C for 25 days in growth chambers under 12 h light (300 μmol m−2 s−1) : 12 h dark conditions. These tomato seedlings were used in the following experiments.
2.2. Preparation of pathogen inoculum
Fusarium oxysporum f. sp. radicis-lycopersici RJN1, obtained from a tomato plant exhibiting Fusarium crown and root rot symptoms, was used as the pathogen. This pathogen was cultured on potato dextrose agar medium for 5–7 days in the dark at 25°C. Five mycelial discs (5 mm in diameter) of this isolate collected from the edges of 5-day-old cultures were transferred into 100 ml of potato dextrose broth in 300-ml Erlenmeyer flasks and incubated for 7 days at 25°C in a rotary shaker (NR-150, Taitec Co., Ltd., Japan) at 110 rpm. To obtain budding cells, the fungal cultures were filtered through three layers of sterile gauze cloth. The resulting fungal suspension was adjusted to 105 budding cells/ml using 10 ml of sterile distilled water and used as the inoculum source.
2.3. Blast processing and disease severity evaluation
An electric fan (Matsushita Electric Industrial Co., type FC302J, blade diameter of 60 cm) was used for controlling the disease. Tomato seedlings (25 days after planting) were subjected to air blasting for 5 days. During this period, a rotating blower (180-degree rotation range) was used with 4 strokes/min. Plants were inoculated by soil drenching with 30 ml of conidial suspension. The following treatment groups were included: A, using different wind speeds (processing time: 30 min, and processing period: 5 days) at 0 m/s (control), 1, 2, 3 and 4 m/s; B, using different processing times (at wind speed of 4 m/s, and processing period of 5 days) for 0 min (control), 30, 60, 120 and 240 min; C, non-treated plants (control), plants treated with wind processing without booster treatment (one time wind exposure at wind speed of 4 m/s, processing time of 30 min and processing period of 5 days) and plants treated with booster wind treatment (three times wind exposure at wind speed of 4 m/s, processing time of 30 min and processing period of 5 days) and sampled at 30, 63 and 95 days from the first wind treatment (at wind speed of 4 m/s, processing time of 30 min and processing period of 5 days). Tomato plants were grown at 25°C for 25 days in the growth chamber under 12 h light (300 μmol m−2 s−1) : 12 h dark conditions. The development of symptoms was evaluated every 7 days after pathogen inoculation. The discoloration of the vascular tissue was assessed starting from 50 days after planting as an index of the proportion of the browning of the vascular tissue at the bottom of the tomato stem using a scale of 0–4: 0, 0% (no vascular discoloration); 1, <25%; 2, 25–50%; 3, 51–75%; 4, >75% vascular discoloration. The discoloration severity of the root system was assessed as an index of the proportion of the discolored area of the total root system using a scale of 0–4: 0, 0% (no root discoloration); 1, <10%; 2, 10–40%; 3, 41–70%; 4, >70% discoloration of the total root system.
2.4. Monitoring of FORL in tomato roots
The FORL population in the tomato roots was estimated starting from 50 days after planting. The roots were washed separately using tap water, surface sterilized (0.5% NaOCl for 3 min) and then homogenized in sterile distilled water (1 : 10 w/v) using a blender (Model AM, Ace Homogenizer, Nihonseiki Kaisha Ltd., Japan) at 8000 rpm for 5 min. The homogenized roots were filtered through two layers of gauze cloth, diluted 10–1000-fold and plated on Komada's selective medium (Komada Citation1975). Plates were incubated for approximately 5 days. The FORL population in the roots (cfu/g fresh weight) was calculated by counting the number of colonies on the medium.
2.5. Effect of root extracts on the germination and proliferation of FORL
The roots of infected tomato plants were collected at the end of the experiment (24 h after monitoring the FORL population). The roots were washed separately using tap water, surface sterilized (0.5% NaOCl for 3 min) and then homogenized in sterile distilled water (1 : 10 w/v) using a blender (Homogenizer TYPE BA, Terao force) at 8000 rpm for 5 min. The filtrates of homogenized roots were centrifuged at 3000 rpm for 10 min. The supernatant solutions were collected and filtered using 0.22-µm Millipore (‘Millex-HV’, Millipore Co., Bedford, MA). The effect of root extracts on pathogen proliferation was evaluated by counting newly formed budding cells in the root extracts. Stem extracts (9 ml) and 1 ml of FORL budding cells (1.0 × 106 budding cells/ml) were mixed in a 100-ml Erlenmeyer flask. The mixture in the flask was cultured with shaking at 110–120 rpm for 7 days at 25°C. During the culture period, 100 µl of root extract was collected each day, and the number of budding cells formed was determined using a hemocytometer.
2.6. Analysis of defense-related genes expression
Tomato seedlings were prepared as described previously and subjected to blast processing using a wind speed of 4 m/s for 30 min per day over a 5-day period. The control was set to 0 m/s (no fan). Total RNA was extracted from tomato leaves 24 h after wind treatment following Elsharkawy et al. (Citation2012a) with some modifications. The leaves were randomly collected and immersed immediately in liquid nitrogen in 1.5-ml tubes and stored at −80°C until use. The samples were crushed using an electric drill and were homogenized using the following extraction buffer: 100 mM Tris-HCl (pH 9.5), 10 mM EDTA (pH 8.0), 2% lithium dodecyl sulfate, 0.6 M NaCl, 0.4 M trisodium citrate and 5% 2-mercaptoethanol. Following centrifugation at room temperature, the resulting aqueous phase was re-extracted using a chloroform/isoamyl alcohol (24 : 1) mixture. The supernatant was collected and extracted using water-saturated phenol, guanidium thiocyanate, sodium acetate (pH 4.0) and chloroform. The upper aqueous phase was precipitated using isopropanol. The precipitated RNA was collected, washed, air-dried briefly and dissolved in RNase-free water. After treatment with RNase-free DNase, the DNase was inactivated according to the manufacturer's instructions (Takara Bio Inc.). Approximately 1 µg of total RNA was reverse-transcribed into single-stranded cDNA using a mixture of oligo-dT primer, RNase inhibitor (20 U µL−1) and RTase (50 U µL−1) according to the manufacturer's instructions (Toyobo, Osaka, Japan). An aliquot of the obtained cDNA was amplified using RT-PCR, as described by Elsharkawy et al. (Citation2013), to monitor the expression of a set of defense-related genes: PR-1, Acht, Bcht and Actin (Hyakumachi et al. Citation2013) and PAL and LOX (Vanitha & Umesha Citation2011). The gene-specific primers used in these experiments are listed in .
Table 1. List of primers used in RT-PCR analysis.
2.7. Data analysis
The experiments were repeated at least three times. The data were subjected to an analysis of variance using EKUSERU-TOUKEI 2010 (Social Survey Research Information Co., Ltd), and treatment means were separated using Fisher's least significant difference (LSD) test. All analyses were conducted using a significance value of p ≤ 0.05.
3. Results
3.1. Suppression of Fusarium crown and root rot disease
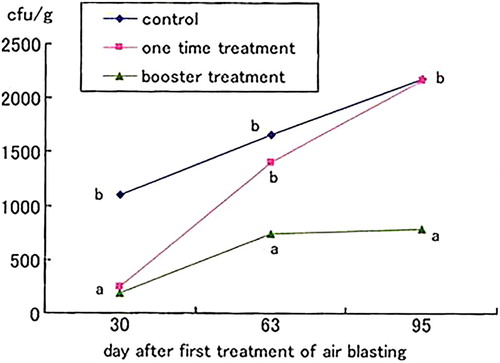
The first treatment group (group A) was subjected to blast processing using different wind speeds, i.e. 0–4 m/s for 30 min per day over a period of 5 days. The discoloration severity of the total root system was significantly lower at a wind velocity of 4 m/s compared with the other treatments (). In group B, the discoloration severity of the total root system was significantly reduced following the 30-min treatment compared with the control (). In group C, the discoloration severities of both the vascular tissue and the total root system were significantly suppressed following the single and booster treatments compared with the control (). Among all treatment groups, the most effective treatment was observed using a wind speed of 4 m/s for 30 min per day over a period of 5 days.
Figure 1. Effect of air blasting for one time and booster treatments on the discoloration severity of vascular tissue (a) and discoloration severity of total root system (b) of FORL in tomato. Assay was performed at 30, 63 and 95 days after first treatment of air blasting. Different letters indicate significant different according to Fisher's LSD test at 5%.
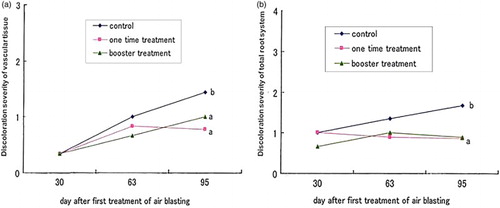
Table 2. Effect of air blasting with different wind speeds (0–4 m/s) on vascular discoloration and discoloration severity of total root system due to Fusarium crown and root rot caused by FORL.
Table 3. Effect of air blasting with long treatments (30–240 min) on vascular discoloration and discoloration severity of total root system due to Fusarium crown and root rot caused by FORL.
3.2. Monitoring of FORL in tomato roots
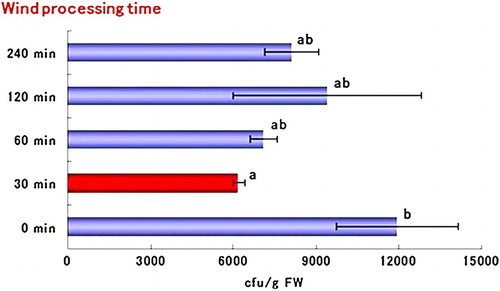
The pathogen population in tomato roots was significantly reduced in all treatments in group A compared with the control treatment. The most effective treatment was achieved using a wind speed of 4 m/s followed by 3 m/s (). In group B, a treatment time of 30 min or more reduced the pathogen population in the roots compared with the non-blowing (0-min treatment) control treatment. In particular, the 30-min treatment was the most effective and significantly reduced the pathogen population compared with the control (). In group C, the booster treatment significantly decreased the pathogen population in the roots compared with the single and control treatments ().
Figure 2. Effect of air blasting with different wind speeds (0–4 m/s) on population density of FORL in tomato root. Assay was performed at 25 days after inoculation of the pathogen. Bars labeled with the same letters are not significantly different according to Fisher's LSD test at 5%. Horizontal bars indicate the standard error.
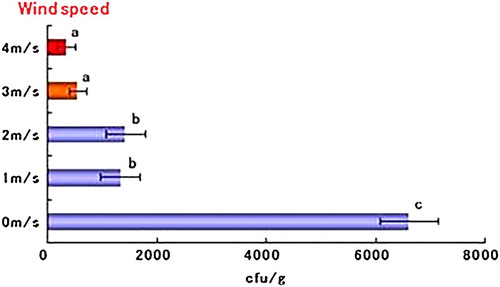
Figure 3. Effect of air blasting with long-time treatments (30–240 m/s) on population density of FORL in tomato root. Assay was performed at 25 days after inoculation of the pathogen. Bars labeled with the same letters are not significantly different according to Fisher's LSD test at 5%. Horizontal bars indicate the standard error.
3.3. Effects of root extracts on FORL germination and proliferation
The production of new FORL budding cells was markedly suppressed at wind speeds of 3 and 4 m/s compared with the control treatment (). Among all the treatments in group B, the 30-min treatment significantly suppressed the formation of new budding cells compared with the control (0-min treatment or non-blast treatment) (). This result is similar to that of the pathogen population in the roots. The production of new budding cells was significantly suppressed from 2 days after inoculation in all treatments compared with the control. This confirms the inhibition effect of tomato root extracts treated with blowing air on the formation of new budding cells.
Table 4. Effect of root extracts from tomato treated with air blasting with different speeds (0–4 m/s) on production of budding cell of FORL.
Table 5. Effect of root extracts from tomato treated with air blasting with long treatments (0–240 min) on production of budding cell of FORL.
3.4. Expression of defense-related genes in response to blast processing
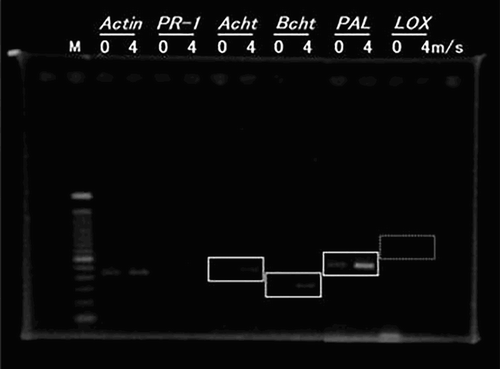
In tomato plants, blast processing at a wind speed of 4 m/s induced the expression of acidic chitinase. In addition, the expression of the gene encoding basic chitinase and phenylalanine ammonia-lyase was observed in response to blast processing at 4 m/s compared with the control treatment at 0 m/s ().
Figure 5. PR genes expression on tomato plants treated with air blasting. Host genes include PR-1, acidic and basic-chitinase (Acht and Bcht), phenylalanine ammonia-lyase (PAL) and lipoxygenase (LOX). Actin gene (house-keeping gene) was used as control. Digits indicate the wind speed: 0 = 0 m/s, 4 = 4 m/s.
4. Discussion
Fusarium crown and root rot disease, caused by FORL, results in severe symptoms on the infected plants (Muslim et al. Citation2003). Therefore, root discoloration was evaluated in this study. A fan-forced wind blowing at a speed of 4 m/s for 30 min per day over a period of 5 days effectively inhibited the discoloration severity of the total root system and suppressed the disease. Taguchi et al. (Citation2014) reported that the incidence of rice blast disease was suppressed by blast processing at a wind speed of 3–5 m/s in rice paddy fields. Cipollini (Citation1997) reported that the lesion area in kidney bean caused by anthracnose was reduced by blowing at a wind speed of 3 m/s, and the greatest suppressive effect was observed at a wind speed of 4 m/s. There are no reports of suppression of soil-borne diseases using the blasting process. In the current study, the pathogen population in the roots was inhibited by blowing; the most effective treatment was a wind speed of 4 m/s applied for 30 min per day. This treatment effectively inhibited the pathogen population in the roots and the formation of new FORL budding cells in the root extracts of blast-treated tomato plants. This result is closely related to the disease suppression effect of hypovirulent binucleate Rhizoctonia in the control of tomato crown and root rot disease. A high correlation between the pathogen population in the roots and disease severity has been reported (Muslim et al. Citation2003). The correlation between the inhibition of pathogen growth and disease suppression in the current study was consistent with the results reported by Muslim et al. (Citation2003) and strongly suggests that it is necessary to suppress the activity and growth of the pathogen in the roots to suppress the onset of disease. While there was no difference in the discoloration severity between the single and booster treatments, there was a significant difference in the pathogen population. Therefore, the booster treatment is important for pathogen control. In addition, as a mechanism of activity and growth inhibition of the pathogen, systemic resistance was induced in tomato through the stimulation provided by the blast processing. This is thought to be due to the accumulation of antibacterial substances, such as phytoalexin, in root tissues. The induced defense responses also include hypersensitive responses, the production of reactive oxygen species, pathogensis-related proteins and ion fluxes across the plasma membrane (Van Loon et al. Citation2006; Hassan et al. Citation2014; Elsharkawy et al. Citation2015). Cipollini (Citation1997) reported the induction of resistance in Phaseolus vulgaris through blast processing, which increased the activity of peroxidase (one of the enzymes involved in resistance) and the accumulation of lignin, which is involved in cell wall strengthening. Additionally, Knight et al. (Citation1992) reported an increase in Ca2+, which is a secondary messenger in signal transduction pathways of induced resistance in the plant cytosol in response to blast processing. Tomato leaves, petioles and stems undergo a physical stimulus contact and friction during the blowing process. The tomato petioles and leaves were shaken fairly vigorously at a wind speed 4 m/s. While this was not fatally damaging to the plant as a whole, there may have been severe injuries at the cellular level. It is believed that wound-induced systemic resistance (WSR) is induced in tomato as a result of this injury response, which subsequently inhibits pathogen growth. Expression of the genes that encode phenylalanine ammonia-lyase, an enzyme involved in phytoalexin synthesis, and basic chitinase of the basic pathogenesis-related (PR) protein was observed during blast processing at 4 m/s. Basic PR proteins are induced by jasmonic acid (JA) (Elsharkawy et al. Citation2012a, Citation2012b, Citation2013). The presence of JA, which is a signal substance of WSR, proves that WSR is involved in disease suppression resulting from blast processing (Elsharkawy et al. Citation2012a). However, expression of the lipoxygenase gene was not observed because lipoxygenase is an enzyme that acts in the early stage of JA synthesis. Lipoxygenase expression had already ceased when sampling was conducted 24 h after the blowing process. Therefore, monitoring the expression of lipoxygenase immediately after wind treatment is important in future studies. These results suggest that the synthesis of JA by blast processing, the synthetic induction of phytoalexins and the induction of the basic PR protein group (basic chitinase) that depends on JA could be considered as the mechanisms of the formation of a series of WSR and the expression of disease resistance. In addition, the expression of genes encoding acidic chitinase as an acidic PR protein was also observed. The salycilic acid (SA) pathway, which induces disease resistance, functions at the same time as JA pathway (Elsharkawy et al. Citation2012a, Citation2012b, Citation2013). It has been demonstrated that a network of interconnected signal transduction pathways in which SA, JA and ethylene play central roles regulates plant defense responses (Robert-Seilaniantz et al. Citation2007). These signaling pathways do not function independently but influence each other through a complex network of synergistic and antagonistic interactions (Koornneef & Pieterse Citation2008). However, to clarify this simultaneous activation of induced resistance, there is a need to examine the gene expression of other acidic PR proteins induced by SA. In conclusion, the results show that blast processing can effectively control FORL. To the best of our knowledge, this is the first report of the control of a soil-borne disease using blast processing. Induced disease resistance in plants using fan-forced wind without the use of chemicals or organic substances can be implemented as a management method with low environmental load.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Armstrong GM, Armstrong JK. 1981. Formae speciales and races of Fusarium oxysporum causing wilt disease. In: Nelson PE, Toussoun TA, Cook RJ, editors. Fusarium: disease, biology and taxonomy. University Park: The Pennsylvania State University; p. 392–399.
- Benhamou N, Charest PM, Jarvis WR. 1989. Biology and host parasite relations of Fusarium oxysporum f. sp. radicis-lycopersici. In: Tjamos EC, Beckman CH, editors. Vascular wilt disease of plants: basic studies and control. NATO ASI Ser Ser H Cell Biol Vol H28. Berlin: Springer Verlag; p. 95–105.
- Biddington NL. 1986. The effects of mechanically-induced stress in plants- a review. J Plant Growth Regul. 4:103–123.
- Dekker J. 1979. Acquired resistance to fungicides. Annu Rev Phytopathol. 14:405–428.
- Cipollini DF. 1997. Wind-induced mechanical stimulation increases pest resistance in common bean. Oecologia. 111:84–90.
- Cipollini DF. 1998. The induction of soluble peroxidase activity in bean leaves by wind-induced mechanical perturbation. Am J Bot. 85:1586–1591.
- Elsharkawy MM, Shimizu M, Takahashi H, Hyakumachi M. 2012a. Induction of systemic resistance against Cucumber mosaic virus by Penicillium simplicissimum GP17-2 in arabidopsis and tobacco. Plant Pathol. 61:964–976.
- Elsharkawy MM, Shimizu M, Takahashi H, Hyakumachi M. 2012b. The plant growth-promoting fungus Fusarium equiseti and the arbuscular mycorrhizal fungus Glomus mosseae induce systemic resistance against Cucumber mosaic virus in cucumber plants. Plant Soil. 361:397–409.
- Elsharkawy MM, Shimizu M, Takahashi H, Ozaki K, Hyakumachi M. 2013. Induction of systemic resistance against Cucumber mosaic virus in Arabidopsis thaliana by Trichoderma asperellum SKT-1. Plant Pathol J. 29:193–200.
- Elsharkawy MM, Shivanna MB, Meera MS, Hyakumachi M. 2015. Mechanism of induced systemic resistance against anthracnose disease by plant growth promoting fungi. Acta Agric Scand Sec B Soil Plant Sci. 65:287–299.
- Fazio G, Stevens MR, Scott JW. 1999. Identification of RAPD markers linked to Fusarium crown and root rot resistance (Frl) in tomato. Euphytica. 105:205–210.
- Hahlbrook K, Scheel D. 1989. Physiology and molecular biology of phenylpropanoid metabolism. Annu Rev Plant Physiol. Plant Mol Biol. 40:347–369.
- Hassan N, Elsharkawy MM, Shivanna MB, Meera MS, Hyakumachi M. 2014. Elevated expression of hydrolases, oxidase, and lyase in susceptible and resistant cucumber cultivars systemically induced with plant growth-promoting fungi against anthracnose. Acta Agric Scand Sec B Soil Plant Sci. 64:155–164.
- Horinouchi H, Katsuyama N, Taguchi Y, Hyakumachi M. 2008. Control of Fusarium crown and root rot of tomato in a soil system by combination of a plant growth-promoting fungus, Fusarium equiseti, and biodegradable pots. Crop Prot. 27:859–864.
- Horinouchi H, Muslim A, Suzuki T, Hyakumachi M. 2007. Fusarium equiseti GF191 as an effective biocontrol agent against Fusarium crown and root rot of tomato in rock wool systems. Crop Prot. 26:1514–1523.
- Hrazdina G, Parsons GF. 1982. Induction of flavonoid synthesizing enzymes by light in etiolated pea (Pisum sativum cv. Midfreeze) seedlings. Plant Physiol. 70:506–510.
- Hyakumachi M, Nishimura M, Arakawa T, Asano T, Yoshida S, Tsushima S, Takahashi H. 2013. Bacillus thuringiensis suppresses bacterial wilt disease caused by Ralstonia solanacearum with systemic induction of defense-related gene expression in tomato. Microbes Environ. 28:128–134.
- Jarvis WR, Shoemaker RA. 1978. Toxonomic status of Fusarium oxysporum causing foot and root rot of tomato. Phytopathology. 68:1679–1680.
- Knight ME, Halpin C, Schuch W. 1992. Identification and characterization of cDNA clones encoding cinnamyl alcohol dehydrogenase from tobacco. Plant Mol Biol. 19:793–801.
- Komada H. 1975. Development of a selective medium for quantitative isolation of Fusarium oxysporum from natural soil. Rev Plant Prot Res. (Tokyo). 8:114–125.
- Koornneef A, Pieterse CMJ. 2008. Cross talk in defense signaling. Plant Physiol. 146:839–844.
- Meadows R. 2013. NEWS OVERVIEW: researchers develop alternatives to methyl bromide fumigation. Calif Agr. 67:125–127.
- Muslim A, Horinouchi H, Hyakumachi M. 2003. Control of Fusarium crown and root rot of tomato with hypovirulent binucleate Rhizoctonia in soil and rock wool systems. Plant Dis. 87:739–747.
- Robert-Seilaniantz A, Navarro L, Bari R, Jones JD. 2007. Pathological hormone imbalances. Curr. Opin. Plant Biol. 10:372–379.
- Rowe RC, Farley JD. 1977. New greenhouse tomato disease can be controlled. Ohio Rep. 62:41–43.
- Steinkellner S, Mammerler R, Vierheilig H. 2005. Microconidia germination of the tomato pathogen Fusarium oxysporum in the presence of root exudates. J Plant Interac. 1:23–30.
- Taguchi Y, Elsharkawy MM, Hassan N, Hyakumachi M. 2014. A novel method for controlling rice blast disease using fan-forced wind on paddy fields. Crop Protect. 63:68–75.
- Taguchi Y, Elsharkawy MM, Hyakumachi M. 2015. Effect of artificially-generated wind on removing guttation and dew droplets from rice leaf surface for controlling rice blast disease. Afr J Biotechnol. 14:1039–1047.
- Todd GW, Chadwick DL, Tsai SD. 1972. Effect of wind on plant respiration. Physiol Plant. 27:342–346.
- Vanitha SC, Umesha S. 2011. Pseudomonas fluorescens mediated systemic resistance in tomato is driven through an elevated synthesis of defense enzymes. Biol. Plantarum. 55:317–322.
- Van Loon LC, Rep M, Pieterse CMJ. 2006. Significance of inducible defence-related proteins in infected plants. Annu. Rev Phytopath. 44:135–162.