Abstract
High temperature (HT) has become a global concern because it severely affects the growth and production of crops. Heat stress causes an abrupt increase in the expression of stress-associated proteins which provide tolerance by stimulating the defense response in plants. Heat-shock proteins (Hsps) and antioxidant enzymes are important in encountering heat stress in plants. The heat-shock response is characterized by repression of normal cellular protein synthesis and induction of Hsp synthesis. Under HT stress, upregulation of various enzymatic and nonenzymatic antioxidants, maintenance of cell membrane stability, production of various compatible solutes and hormonal changes occurs. Reactive oxygen species involving several pathways such as water–water cycle, Halliwell–Asada, glutathione peroxidase, Haber–Weiss and Fenton reactions helps in protecting plants against toxic radicals which otherwise could cause damage to lipophilic protein. Genetic approaches to elucidate and map genes or quantitative trait loci conferring thermotolerance will facilitate marker-assisted breeding for heat tolerance and also pave the way for characterizing genetic factors which could be useful for engineering plants with improved heat tolerance. This review discusses the protective mechanism of heat stress responses encompassing different pathways that provide tolerance during HT stress.
Abbreviations
| AA | = | ascorbic acid |
| APX | = | ascorbate peroxidase |
| AsA | = | ascorbate |
| DHAR | = | dehydroascorbate reductase |
| GPX | = | guaiacol peroxidase |
| GR | = | glutathione reductase |
| GSH | = | reduced glutathione |
| GSSG | = | oxidized glutathione |
| GST | = | glutathione S-transferase |
| •OH | = | hydroxyl radical |
| H2O2 | = | hydrogen peroxide |
| Hsfs | = | heat stress transcription factors |
| Hsps | = | heat-shock proteins |
| HT | = | high temperature |
| LOOH | = | lipid hydroperoxide |
| LPO | = | lipid peroxidation |
| MDA | = | malondialdehyde |
| MDHAR | = | monodehydroascorbate reductase |
| MTS | = | membrane thermal stability |
|
| = | superoxide radicals |
| ROS | = | reactive oxygen species |
Introduction
Global climate change particularly high temperature (HT) is predicted to increase by about 1–3°C by the mid-twenty-first century and by about 2–5°C by the late twenty-first century (Eitzinger et al. Citation2010, IPCC Citation2012). This trend is a major concern for crop production (Hatfield et al. Citation2011; Lobell et al. Citation2011) because it substantially affect plant growth and yield (Kurek et al. Citation2007; Ahmad & Prasad Citation2012). Therefore, selection of heat-tolerant lines and integrating biochemical pathways will help in understanding how crops respond to elevated temperature and how protection to HT can be improved by different mechanisms (Halford Citation2009). Emission of green-house gases such as carbon dioxide, methane and nitrous oxide from agricultural systems is one of the major concerns contributing to this global increase of temperature (Smith & Olesen Citation2010). Under HT conditions, plants accumulate different metabolites such as antioxidants, osmoprotectants, heat-shock proteins (Hsps) and metabolites from different pathways (Bokszczanin & Fragkostefanakis Citation2013). Reactive oxygen species (ROS) may damage cellular components and act as signaling molecules, leading to the expression of antioxidant enzymes, Hsps and a rebalancing of osmolyte concentrations that perturb cell-water balance (Bohnert et al. Citation2006).
Hsps play a role in stress signal transduction, protecting and repairing damaged proteins and membranes, protecting photosynthesis as well as regulating cellular redox state. Expression of various Hsps is known to be an adaptive strategy in heat tolerance. The heat-shock response is controlled at both the transcriptional and the translational level. A cis-acting DNA sequence, the heat-shock element (HSE), has been found to be necessary for heat-induced transcription (Nover & Baniwal Citation2006). The HSE has a common consensus sequence of –GAA—TTC –- and is found in multiple copies upstream of all HS genes. Khurana et al. (Citation2013) mentioned the importance of 5ʹ-UTR of sHsp26 promoter, thus emphasizing the probable role of imperfect CCAAT-box element or some novel cis-element with respect to heat stress. About 1.5 HSEs are required for heat-induced transcription. The induction of HS gene expression is mediated by the binding of a trans-acting transcriptional activator, the heat stress transcription factors (HSFs), to the HSE. Several studies revealed that while some HSFs are critical for thermotolerance, others play a less critical role (Hsp101, HSA32, HSFA1 and HSFA3), since knockout variants of these proved to have little impact on tolerance to heat (Larkindale & Vierling Citation2008; Schramm et al. Citation2008; Yoshida et al. Citation2011).
The protection mechanism of heat stress has been linked to increased thermotolerance of the photosynthetic apparatus (Hemantaranjan et al. Citation2014). The major sites of thermal damage are the oxygen-evolving complex (OEC) along with associated cofactors in photosystem II, carbon fixation by Rubisco and the ATP-generating system. HT stress also reduces the efficiency of electron transport and consequently leading to increased production of ROS in plant cells. Plants under HT stress usually accumulate more ROS in both chloroplasts and mitochondria, which can severely damage DNA and cause cell membrane lipid peroxidation (LPO). Thus, plant protection against HT is closely correlated to increased capacity of scavenging and detoxifying the ROS. Induction of thermotolerance may be ascribed to the maintenance of better membrane thermostability, and low level of ROS accumulation (Xu et al. Citation2006; Hameed et al. Citation2012) due to improved antioxidant capacity (Chakraborty & Pradhan Citation2011). Despite numerous studies, limited information is available on ROS production and dissipation in different cell organelles. Responses of plants to HT are mediated by inherent ability to survive and to acquire thermotolerance to lethal temperature. Several reports depict the genetic variability among crops due to the expression of stress-responsive genes (Farooq et al. Citation2011). To overcome stress, plants are equipped with different protective mechanisms including the maintenance of cell membrane stability, capturing the ROS, synthesis of antioxidants, accumulation and osmoregulation of osmoticum and upregulation of Hsp synthesis.
Various reports have identified abundant heat-tolerant genes, most of which are quantitative trait loci (QTL) (Rodriguez et al. Citation2005). However, these QTL are largely Hsf and Hsp genes which do not contribute to heat tolerance. A few of these QTLs have been associated with spikelet fertility. Thus, the use of classical and modern breeding protocols, identification of genetic diversity for HT tolerance, use of pre-sowing seed treatments and planting materials and development of plants with HT tolerance will be important (Mittler & Blumwald Citation2010). In rice, QTLs for heat tolerance at flowering stage have been mapped on almost all rice chromosomes, improving heat tolerance in rice varieties using the identified genetic resources (Ye et al. Citation2015).
Role of membranes in heat tolerance
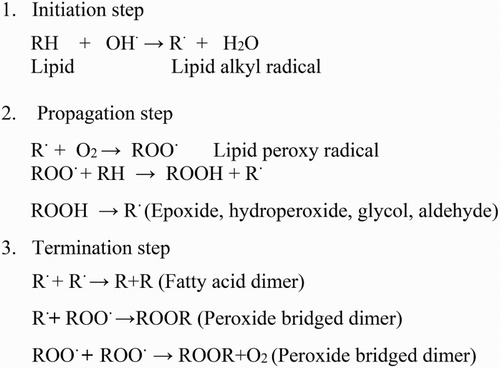
LPO is considered as one of the most damaging processes known to occur in every living organism. Modification in the membrane function under HT stress is mainly due to the alteration of membrane fluidity. Three commonly used parameters are related to membrane-based processes which include plasmalemma (cell membrane stability assay), photosynthetic membranes (chlorophyll fluorescence assay) and mitochondrial membranes (cell viability assay based on 2,3,5-triphenyl tetrazolium chloride, TTC reduction test). Membrane lipid saturation is considered an important element in HT tolerance. HT causes an increase in fluidity of membranes which can lead to disintegration of the lipid bilayer. Membrane damage is sometimes taken as a stress parameter to determine the level of lipid destruction. It has been recognized that LPO products are formed from polyunsaturated precursors that include small hydrocarbon fragments such as ketones, malondialdehyde (MDA) and compounds related to them (Garg & Manchanda Citation2009). MDA is a highly reactive three carbon dialdehydes produced as a byproduct of polyunsaturated fatty acid peroxidation and arachidonic acid metabolism. Some of these compounds react with thiobarbituric acid to form coloured products called thiobarbituric acid reactive substances (Hameed et al. Citation2012). LPO, in both cellular and organelle membranes, takes place when above-threshold ROS levels are reached, thereby affecting normal cellular functioning (Montillet et al. Citation2005). The mechanism of LPO involved three distinct stages: initiation, progression and termination steps (). The first step is initiated by the reaction of an activated free radical such as singlet oxygen (1O2, O2•−, or •OH) with a lipid substrate (LH) to produce extremely reactive carbon-centred lipid radical (•L). In the second step of LPO, molecular oxygen adds quickly to generate lipid peroxyl radical (LOO•). The LOO• eliminates a hydrogen atom from another lipid molecule (LH), generating lipid hydroperoxide (LOOH) and another extremely reactive carbon-centred radical (L•) which then elongates the chain reaction, and the third step involves termination of LPO that occurs through coupling of any two radicals to form non-radical products (). These products are stable but not able to propagate LPO reactions. Transition metal ions such as copper and iron are essential in LPO. Besides increasing the generation of initiating hydroxyl radicals (•OH), ferrous (Fe2+) and ferric (Fe3+) can catalyse the elongation of the LPO chain by degrading LOOH. The resulting alkoxyl (LO•) and peroxyl (LOO•) radicals are able to induce new radical chains by interacting with additional lipid molecules. The resulting LOOH can easily decompose into several reactive species including lipid alkoxyl radicals, aldehydes, alkanes, lipid epoxides and alcohols (Fam & Morrow Citation2003). A single initiation event thus has the potential to generate multiple peroxide molecules by a chain reaction.
The membrane integrity and functions are sensitive to HT as it alters the tertiary and quaternary structures of membrane proteins. Membranes are moving mosaics of proteins and lipids as lipids stagger between monolayers, diffuse within the plane of a monolayer and rotate about their own axes, with their acyl chains also rotating around carbon–carbon bonds. Since protein conformation changes with temperature, both temperature downshift and temperature upshift can lead to protein unfolding (Pastore et al. Citation2007). Membrane fluidity in temperature tolerance has been delineated by mutation analysis, transgenic and physiological studies. For example, a soybean mutant deficient in fatty acid unsaturation showed strong tolerance to HT (Pastore et al. Citation2007). Also, the thylakoid membranes of two Arabidopsis mutants deficient in fatty acid unsaturation (fad5 and fad6) showed increased stability to HT (Yamada et al. Citation2007) and increased lipid saturation in tobacco caused by silencing a ω-3 desaturase gene also rendered the plants more tolerant to HT (von Koskull-Doring et al. Citation2007). Wheat lines of high membrane thermostability tended to yield better than lines of low membrane thermal stability when grain filling occurred under hot conditions (Gupta et al. Citation2013). Genetic variation exists among genotypes for membrane thermostability which can be utilized in wheat breeding in heat-stressed environments.
Role of ROS in heat tolerance
At a cellular level, the generation and reactions of ROS singlet oxygen, superoxide radicals (), •OH and hydrogen peroxide (H2O2) are common events under heat stress (Almeselmani et al. Citation2006). Over production of ROS above a constitutive level is potentially harmful to all cellular compounds as it negatively influences cell metabolism (Esfandiari et al. Citation2007). To counteract the injurious effects of ROS, plants have evolved a complex antioxidative defense system that includes antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (GPX), ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR), glutathione reductase (GR) and glutathione S-transferase (GST) and nonenzymatic antioxidants such as flavanoids, anthocyanin, carotenoids and ascorbic acid (AA) (Suzuki et al. Citation2011). The enzyme SOD converts
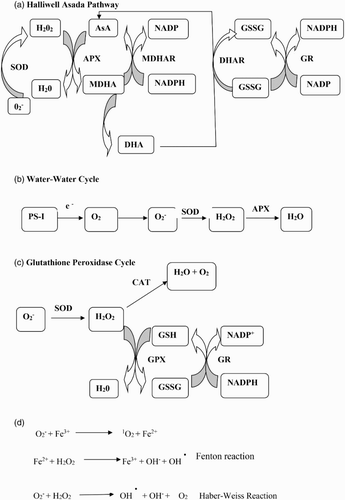
to H2O2, whereas CAT and peroxidases dismutate H2O2. Catalase eliminates H2O2 by breaking it down to H2O and O2 but peroxidases require reducing equivalents to scavenge H2O2. GPX requires a phenolic compound guaiacol as an electron donor to decompose H2O2, while APX uses a reduced form of ascorbate (AsA) to protect cells against damaging effects of H2O2 (Tripathy & Oelmüller Citation2012). The oxidized form of AsA produced by the action of APX is regenerated via the AsA–glutathione cycle or the Halliwell–Asada pathway (A) involving monodehydroascorbate reductase (MDHAR) and DHAR and finally the oxidized glutathione (GSSG) is reduced by GR using reducing power of NADPH. GSTs are a collection of multifunctional proteins that are found essentially in all organisms. AsA and reduced glutathione (GSH) are potent nonenzymatic antioxidant within cell. AsA scavenge most dangerous forms of ROS, that is, •OH,
, H2O2 through the action of APX while glutathione which participate in maintaining cellular AsA pool in reduced state through the Halliwell–Asada pathway (A) as well as serve as a major thiol disulfide redox buffer in plants. The water–water cycle or the Mehler-peroxidase reaction (B) involves the leakage of electrons from the photosynthetic electron transport chain to oxygen with the generation of superoxide which is further dismutated by SOD forming H2O2. ROS that escape this cycle undergo detoxification by SOD and the stromal AsA–glutathione cycle. GPX is also involved in H2O2 removal.
Activities of different antioxidant enzymes are temperature sensitive and activation occurs at different temperature ranges. Chakraborty and Pradhan (Citation2011) observed that CAT, APX and SOD showed an initial increase before declining at 50°C, whereas POX and GR activities declined at all temperatures ranging from 20°C to 50°C. In addition, total antioxidant activity was at a maximum of 35–40°C in the tolerant varieties and at 30°C in the susceptible ones. The activity of the enzymes GST, APX and CAT was more enhanced in the cultivar that showed better tolerance to heat stress and protection against ROS production (Suzuki & Mittler Citation2006; Goyal & Asthir Citation2010; Ahmad & Prasad Citation2012). The protection mechanism of heat stress in wheat varieties appeared to be correlated with the antioxidant level, though changes in activity were observed for different antioxidant enzymes.
Synchronized action of AA, α-tocopherol and glutathione results in detoxification of ROS and limit oxidative stress in plants (Hameed et al. Citation2012). AA is distributed in almost all the plant parts and is synthesized in the mitochondria and transported to other parts of the plant (Foyer Citation2015). AA is used as a substrate by APX to reduce H2O2 to H2O in the AsA–glutathione cycle and generate monodehydroascorbate, which further dissociate to AA and dehydroascorbate. Under abiotic stress conditions, the role of AA is diverse. α-Tocopherol along with other antioxidants scavenges lipid peroxyl radical. It acts as lipophilic antioxidant and interacts with polyunsaturated acyl groups of lipids and reduces the deleterious effects of ROS (Tripathy & Oelmüller Citation2012). α-Tocopherol stabilizes membrane and also acts as substance that modulates signal transduction. Glutathione are non-protein thiols that have a key role in H2O2 detoxification. It has been reported that the conversion ratio of reduced GSH to its oxidized form GSSG during the detoxification of H2O2 is the indicator of cellular redox balance via the glutathione peroxidase cycle (C) (Goyal & Asthir Citation2010). These events were widely reported in plants under various abiotic stresses. Glutathione and AA are now considered as an important component of redox signaling in plants (Suzuki et al. Citation2012).
One of the major lines of defense against ROS is SOD that converts superoxide to H2O2, whereas APX, GPX and CAT detoxify H2O2. The conversion of H2O2 to H2O by APX requires AsA and the reduced glutathione (GSH) regeneration system via the AsA–glutathione cycle. H2O2 is converted to H2O by oxidation of AsA to monodehydroascorbate (MDHA), which further dismutate to dehydroascorbate. Like APX, GPX uses GSH as a reducing agent to detoxify H2O2 to H2O (Wahid et al. Citation2007). The balance between SODs and the different H2O2-scavenging enzymes in cells is crucial in determining the steady-state level of and H2O2. This balance together with the sequestering of metal ions by ferritin and the metal-binding proteins prevents the formation of the highly toxic HO· radical via the metal-dependant Haber–Weiss reaction or the Fenton reaction. The reactions through which
, H2O2 and iron rapidly generate OH− is called the Haber–Weiss reaction, whereas the final step that involves the oxidation of Fe2+ by H2O2 is referred to as the Fenton reaction (D).
Role of Hsps in heat tolerance
Hsps, are known as stress-induced proteins or stress proteins (Gupta et al. Citation2010). All stresses induce gene expression and synthesis of Hsps in cells. However, stressing agents lead to an immediate block of every important metabolic process, including DNA replication, transcription, mRNA export and translation, until the cells recover (Biamonti & Caceres Citation2009).
Stress lead to a production of a group of proteins called Hsps or stress-induced proteins. These are further grouped in plants into five classes based on their molecular masses: (1) Hsp100, (2) Hsp90, (3) Hsp70, (4) Hsp60 and (5) small heat-shock proteins (sHsps). Plants, in general, have around 20 sHsps and there might be 40 kinds of these sHsps in one plant species. The diversification of these proteins reflects an adaptation to tolerance to heat stress. Transcription of Hsp genes is mainly controlled by regulatory proteins called Hsfslocated in the cytoplasm in an inactive state. Plants show at least 21 Hsfs with each one having its role in regulation, but they also cooperate in all phases of periodical heat stress responses (triggering, maintenance and recovery). Major Hsps have some kind of related roles in solving the problem of misfolding and aggregation, as well as their role as chaperones. Plants are characterized by a large number of transcriptional factors (Nover & Baniwal Citation2006). Three classes of Hsf exist according to the structural differences in their aggregation in triples, that is, oligomerization domains as Plant HsfA such as HsfA1 and HsfA2 in Lycopersicon esculentum, Plant HsfB such as HsfB1 in L. esculentum, Plant HsfC (Tripp et al. Citation2009).
Each factor has its role in the regulatory network in plants. However, all cooperate in regulating many functions and different stages of response to periodical heat stress (triggering, maintenance and recovery). This role is demonstrated in the tomato system where HsfA1a is the master regulator that is responsible for the induced-stress gene expression including the synthesis of both HsfB1 and HsfA2 as these factors are found after the induction by heat treatment. These three factors are necessary for plant acquisition of heat tolerance. Hence, there is an acquired thermotolerance phenomenon which is supported by a study on Arabidopsis thaliana that indicated the participation of HsfA2 (Charng et al. Citation2007). Furthermore, HsfA2 was finely regulated with Hsp17-CII during anther development of a heat-tolerant tomato genotype and was further induced under both short and prolonged heat stress conditions (Giorno et al. Citation2010). The results indicate that HsfA2 may be directly involved in the activation of protection mechanisms in the tomato anther during heat stress and, thereby, may contribute to tomato fruit set under adverse temperatures.
The protective mechanism of pathways leading to the expression of genes to synthesize Hsps is composed of sensing temperature that is connected to the signal transfer to Hsfs where the activation of gene expression occurs by binding to the HSE in DNA (Larkindale et al. Citation2005). HSE is a specific recognition sequence located in the region of gene activator in DNA. HSE was defined as alternating units of 5′-nGAAn-3′ and efficient binding requires at least three units. In the absence of stressing factors, Hsfs are present in the cytoplasm as single and free as there is no binding activity with DNA, but when stress starts the factors aggregate in triplet and accumulate in the nucleus. The binding of Hsfs to DNA in tomato seedlings Solanum lycopersicum was promoted by salicylic acid that did not promote the transcription of Hsp70 mRNA or the expression of Hsfs such as hsfA2 and hsfB1. This could indicate that salicylic acid has a role in modulating the Hsf for binding (Snyman & Cronje Citation2008).
The general role of Hsps is to act as molecular chaperones regulating the folding and accumulation of proteins as well as localization and degradation in all plants and animal species (Lindquist & Crig Citation1988; Panaretou & Zhai Citation2008; Hu et al. Citation2009; Gupta et al. Citation2010). These proteins, as chaperones, prevent the irreversible aggregation of other proteins and participate in refolding proteins during heat stress conditions (Tripp et al. Citation2009; Morrow & Tanguay Citation2012). Each group of these Hsps has a unique mechanism and a defined role to play. Denaturation of proteins and the processing of newly synthesized proteins during stress are assumed to be a result of the pool of free chaperones (Timperio et al. Citation2008). They exist as inactive proteins mostly found in the cytoplasm. Stress causes activation and oligomerization and, eventually, recompartmentation to the nucleus, where it binds to its target sequences (HSE) present in the promoter of hs genes.
Over expression of HSP101 from Arabidopsis in rice plants results in a significant improvement of growth performance during recovery from heat stress (Liu et al. Citation2011). Induction of many heat-inducible genes is attributed to the conserved HSE in the promoter. HSE consists of alternating units of pentameric nucleotides (50-nGAAn-30) that serve as the binding site for Hsf. Efficient Hsf binding requires at least three alternating units (50-nGAAnnTTCnnGAAn-30). In spite of their conserved transactivation function upon heat shock, HSFs show differences in induction threshold and regulation of the heat response, which could provide diverse induction profiles for target genes under various stress conditions, Arabidopsis has 21 HSF genes belonging to three major classes: HsfA, HsfB and HsfC based on structural differences. HsfAs appear to be the major factor(s) responsible for heat-induced activation of heat-shock genes. HsfBs apparently lack the heat-inducible transactivation function in spite of having normal DNA binding function, and might act as co-activators of transcription with HsfAs. In spite of extensive studies on HSFs, no immediate upstream factors to HSF in heat signal transduction have been identified. Several HSFgenes are heat inducible, indicating the presence of transcriptional activators for HSF genes. Whether they are HSFs themselves or other novel transcriptional factors awaits further investigation.
Developing grains contain Hsp100 and relatively tolerant cultivar maintained a higher catalytic efficiency of soluble starch synthase at elevated temperature and had a higher content of Hsp100 (Sumesh et al. Citation2008). However, there was no increase in grain Hsp100 content with an increase in temperature and it was constitutively present in higher amount in the tolerant type. The relatively tolerant cultivars showed a higher content of Hsp18 compared with susceptible types under heat stress. The HT induction of Hsp18 was, therefore, also revealed from the observed higher relative expression of Hsp18 at 20 days after anthesis. Low molecular weight Hsps represent a set of homologous proteins in the range of 15–30 kDa (Hemantaranjan et al. Citation2014).
Role of various protectant in heat tolerance
In recent decades, exogenous application of protectant, such as osmoprotectants, phytohormones, signaling molecules and trace elements, have shown a beneficial effect on plants grown under heat tolerance as these protectants has growth-promoting and antioxidant capacity (Hasanuzzaman et al. Citation2011). The accumulation of osmolytes such as proline, glycine betaine and trehalose is a well-known adaptive mechanism in plants against abiotic stress conditions including heat tolerance. Since heat-sensitive plants apparently lack the ability to accumulate these substances, heat tolerance in such plants can be improved by exogenous application of osmoprotectants (Rasheed et al. Citation2011). Proline and glycine betaine application considerably reduced the H2O2 production, improved the accumulation of soluble sugars and protected the developing tissues from heat stress effects. However, Pro was more effective than glycine betaine in that study. Exogenous proline and glycine betaine application also improved the K+ and Ca2+ contents, and increased the concentrations of free proline, glycine betaine and soluble sugars which rendered the buds more tolerant to heat tolerance. Identically, exogenous applications of several phytohormones were found to be effective in mitigating heat stress in plants. Chhabra et al. (Citation2009) studied the phytohormones-induced amelioration of heat tolerance stress in Brassica juncea and found that soaking seeds in 100 μM indole acetic acid (IAA), 100 μM GA, 50 and 100 μM Kinetin and 0.5 and 1 μM abscisic acid (ABA) were effective for mitigating the effect of heat stress (47 ± 0.5°C). The significant observation was that both growth-promoting and growth-retarding hormones were effective in mitigation of heat stress effects. The role of growth-promoting hormone in the mitigation of heat stress was at a concentration which was otherwise lethal or toxic to its growth seedling stage.
Tocopherol plays an important role in signal transduction pathways and in the gene expression regulation in different processes such as plant defense and export of photoassimilates (Falk & Munne-Bosch Citation2010). It acts as a key lipid soluble redox buffer and an important scavenger of singlet oxygen species as it scavenges other ROS (Foyer Citation2015). The role of tocopherol is important under the conditions of severe stress only (Bosch Citation2005). The antioxidant activity of tocopherol depends on its ability of donation of its phenolic hydrogen to free radicals. α-Tocopherol has the highest antioxidant activity of all the tocopherol types, δ-tocopherol has the lowest and the β- and γ-tocopherols have the intermediate activity (Kapoor et al. Citation2015). The amount of tocopherol is tightly controlled in the photosynthetic membranes to properly regulate the membrane stability. The role of tocopherol in preventing LPO has been noticed in many reports. Lipid peroxyl radicals, which are involved in the propagation of LPO, are scavenged by tocopherol.
L-AsA may also act as an alternative electron donor of PSII; in those cases, the electron transfer is inhibited due to the inactivation of OEC (Gururani et al. Citation2012). Heat-induced inactivation of PSII was strongly influenced by the AsA content of leaves (Tóth et al. Citation2011). This experiment proved experimentally the physiological role of AsA as an alternative PSII electron donor in heat-stressed leaves with inactive OEC. This result suggests that the role of AsA as an alternative PSII electron donor is to decelerate the processes of photoinactivation and minimize the ROS activity in the photosynthetic thylakoid membranes, and thus minimize the damage to the entire photosynthetic apparatus (Venkatesh & Park Citation2014).
Future pioneering studies in model plants can pave the way to identify the key regulators as target for gene manipulation of stress tolerance in crop plants. It has also been envisaged that metabolic fingerprinting can be used as a breeding tool for development of plants with the best potential to tolerate abiotic stresses.
Genetic engineering and molecular markers for heat stress tolerance
Genetic improvement leads to the development of cultivars that can tolerate environmental stresses and thus improves the economic yield. It involves incorporation of individual gene of interest into the recipient genotypes that helps in improving heat tolerance (Barnabas et al. Citation2008). Protein synthesis elongation factor in chloroplast (Ef-Tu) has been related to heat tolerance in several crops. Modifications in cultural practices, such as planting time, soil and irrigation management, and plant density, can minimize stress effects. Further progress in breeding for stress tolerance depends on physiological mechanisms and genetic basis of heat tolerance is scarce, though the use of molecular marker technology and genetic transformations has resulted in the development of plants with improved heat tolerance (Vinocur & Altman Citation2005). The genome plasticity in plants such as directed mutation and epigenetic such as methylation, chromatin remodeling and histone acetylation changes allow long-term adaptation to environmental changes, which are necessary for the long-term survival of genotypes. The use of biotechnological approaches reduces the loss caused by HT. The genomic information of maize, rice and sorghum can be exploited to improve heat tolerance in other crops.
Application of QTL mapping has led to the genetic relationship among tolerance to various stresses. Molecular marker technology has identified and characterized QTL with significant effects on stress protection during different stages of plant development in order to find genetic relationships among different stresses (Fooland Citation2005). In Arabidopsis, four genomic loci (QTLs) deterring its capacity to acquire thermotolerance were identified. The use of restriction fragment length polymorphism revealed mapping of 11 QTLs for pollen germination and pollen tube growth under heat stress in maize. QTL mapping studies for heat tolerance have been conducted on various rice populations at flowering stages. However, confirmation and fine mapping of the identified QTLs for heat tolerance have not been reported yet (Ye et al. Citation2012). Multiple loci for heat tolerance have been identified in wheat (Paliwal et al. Citation2012) and maize (Bai Citation2011). A study on Arabidopsis mutants sensitive to heat also revealed QTLs involved in acquiring thermotolerance (Hong et al. Citation2003). A major QTL for HT germination and an additional QTL having smaller effects were identified as well in a genetic analysis of lettuce seed thermo-inhibition (Argyris et al. Citation2008). The markers linked to these QTLs could be used to improve heat tolerance in available germplasm. At present, HT tolerance QTL identification is performed using different traits, such as the thousand-grain weight, grain-filling duration, canopy temperature detection, yield (Pinto et al. Citation2010) or senescence-related traits (Vijayalakshmi et al. Citation2010). Heat tolerance in rice at the flowering stage is controlled by several QTLs with small effects and stronger heat tolerance could be attained through pyramiding validated heat tolerance QTLs. QTL qHTSF4.1 was consistently detected across different genetic backgrounds and could be an important source for enhancing heat tolerance in rice at the flowering stage (Ye et al. Citation2015). In wheat, two QTLs were identified that controlled grain-filling duration, a trait thought to be correlated with heat tolerance (Collins et al. Citation2008). Studies investigating multiple parameters related to heat tolerance in wheat provided evidence for genetic variability and multiple tolerance mechanisms (Dhanda & Munjal Citation2006; Nicholas et al. Citation2008). Polymorphic SNP markers in these QTL regions can be used for future fine mapping and developing SNP chips for marker-assisted breeding.
Conclusion
An understanding of the nature of the heat-shock signaling cascades as well as the specific genes expressed in response to HT will be valuable for developing stress-tolerant plants. However, detailed mechanisms of thermotolerance remains indefinable that needs appropriate research. Genetic improvement of crop tolerance to heat by altering sensing, signaling or regulatory pathways will help in identifying targets for modification that do not disrupt other vital processes. Though significant advances have been achieved to understand the role of ROS in plants, it is still not clear how ROS plays a pivotal role in stress regulation and metabolism. Metabolic engineering of plants to synthesize compatible compounds may be an alternative way of developing thermotolerance in important crop plants
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Ahmad P, Prasad MNV. 2012. Environmental adaptations and stress tolerance of plants in the era of climate change. New York, NY: Springer; p. 297–324.
- Almeselmani M, Deshmukh PS, Sairam RK, Kushwaha SR, Singh TP. 2006. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 171:382–388.
- Argyris J, Dahal P, Hayashi E, Still DW, Bradford KJ. 2008. Genetic variation for lettuce seed thermoinhibition is associated with temperature-sensitive expression of abscisic acid, gibberellin, and ethylene biosynthesis, metabolism, and response genes. Plant Physiol. 148:926–947.
- Bai J. 2011. Genetic variation of heat tolerance and correlation with other agronomic traits in a maize (Zea mays L.) recombinant inbred line population. Available from: http://hdl.handle.net/2346/13572
- Barnabás B, Jäger K, Fehér A. 2008. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 31:11–38.
- Biamonti G, Caceres JF. 2009. Cellular stress and RNA splicing. Trends Biochem Sci. 34:146–153.
- Bohnert HJ, Gong QQ, Li PH, Ma SS. 2006. Unraveling abiotic stress tolerance mechanisms – getting genomics going. Curr Opin Plant Biol. 9:180–188.
- Bokszczanin KL, Fragkostefanakis S. 2013. Perspectives on deciphering mechanisms underlying plant heat stress response and thermotolerance. Front Plant Sci. 4:315–335. doi:10.3389/fpls.2013.00315
- Bosch SM. 2005. The role of α-tocopherol in plant stress tolerance. J Plant Physiol. 162:743–748.
- Chakraborty U, Pradhan D. 2011. High temperature-induced oxidative stress in Lens culinaris, role of antioxidants and amelioration of stress by chemical pre-treatments. J Plant Interact. 6:43–52.
- Charng YY, Liu HC, Liu NY, Chi WT, Wang CN, Chang SH, Wang TT. 2007. A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol. 143:251–262.
- Chhabra ML, Dhawan A, Sangwan N, Dhawan K, Singh D. 2009. Phytohormones induced amelioration of high temperature stress in Brassica juncea (L.) Czern & Coss. Proceedings of 16th Australian Research Assembly on Brassicas, September 10–14; Ballarat, Australia.
- Collins NC, Tardieu F, Tuberosa R. 2008. Quantitative trait loci and crop performance under abiotic stress: where do we stand?. Plant Physiol. 147:469–486.
- Dhanda SS, Munjal R. 2006. Inheritance of cellular thermotolerance in bread wheat. Plant Breed. 125:557–564.
- Eitzinger J, Orlandini S, Stefanski R, Naylor REL. 2010. Climate change and agriculture: introductory editorial. J Agric Sci. 148:499–500.
- Esfandiari E, Shekari F, Shekari F, Esfandiar M. 2007. The effect of salt stress on antioxidant enzymes activity and lipid peroxidation on the wheat seedlings. Not Bot Hort Agrobot Cluj. 35:48–56.
- Falk J, Munne-Bosch S. 2010. Tocochromanol functions in plants: antioxidation and beyond. J Exp Bot. 61:1549–1566.
- Fam SS, Morrow JD. 2003. The isoprostanes: unique products of arachidonic acid oxidation-a review. Curr Med Chem. 10:1723–1740.
- Farooq M, Bramley H, Palta JA, Siddique KHM. 2011. Heat stress in wheat during reproductive and grain-filling phases. Crit Rev Plant Sci. 30:491–507.
- Fooland MR. 2005. Breeding for abiotic stress tolerances in tomato. In: Ashraf M, Harris PJC, editors. Abiotic stresses: plant resistance through breeding and molecular approaches. New York, NY: The Haworth Press; p. 613–684.
- Foyer CH. 2015. Redox homeostasis: opening up ascorbate transport. Nat Plants 1:1401. doi:10.1038/nplants.2014.12
- Garg N, Manchanda G. 2009. ROS generation in plants: boon or bane? Plant Biosyst. 143:81–96.
- Giorno F, Wolters-Arts, M, Grillo S, Scharf K, Vriezen WH, Mariani C. 2010. Developmental and heat stress-regulated expression of HsfA2 and small heat shock proteins in tomato anthers. J Exp Bot. 61:453–462.
- Goyal M, Asthir B. 2010. Polyamine catabolism influences antioxidative defense mechanism in shoots and roots of five wheat genotypes under high temperature stress. Plant Growth Regul. 60:13–25.
- Gupta NK, Agarwal S, Agarwal VP, Nathawat NS, Gupta S, Singh G. 2013. Effect of short-term heat stress on growth, physiology and antioxidative defence system in wheat seedlings. Acta Physiol Plant. 35:1837–1842.
- Gupta SC, Sharma A, Mishra M, Mishra R, Chowdhuri DK. 2010. Heat shock proteins in toxicology: how close and how far? Life Sci. 86:377–384.
- Gururani MA, Upadhyaya CP, Strasser RJ, Woong YJ, Park SW. 2012. Physiological and biochemical responses of transgenic potato plants with altered expression of PSII manganese stabilizing protein. Plant Physiol Biochem. 58:182–194.
- Halford NG. 2009. New insights on the effects of heat stress on crops. J Exp Bot. 60:4215–4216.
- Hameed A, Goher M, Iqbal N. 2012. Heat stress-induced cell death, changes in antioxidants, lipid peroxidation and protease activity in wheat leaves. J Plant Growth Regul. 31:283–291.
- Hasanuzzaman M, Hossain MA, Fujita M. 2011. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol Rep. 5:353–365.
- Hatfield JL, Boote KJ, Kimball BA, Ziska LH, Izaurralde RC, Ort D. 2011. Climate impacts on agriculture: implications for crop production. Agron J. 103:351–370.
- Hemantaranjan A, Nishant Bhanu A, Singh MN, Yadav DK, Patel PK. 2014. Heat stress responses and thermotolerance. Adv Plant Agric Res. 1:1–10.
- Hong SW, Lee U, Vierling E. 2003. Arabidopsis hot mutants define multiple functions required for acclimation to high temperatures. Plant Physiol. 132:757–767. doi:10.1104/pp.102.017145
- Hu W, Hu G, Han B. 2009. Genome-wide survey and expression profiling of heat shock proteins and heat shock factors revealed overlapped and stress specific response under abiotic stresses in rice. Plant Sci. 176:583–590.
- IPCC. 2012. Managing the risks of extreme events and disasters to advance climate change adaptation. A special report of working groups I and II of the intergovernmental panel on climate change. In: Field CB, Barros V, Stocker TF, Qin D, Dokken DJ, Ebi KLMD, editors. Cambridge: Cambridge University Press; p. 1–582.
- Kapoor D, Sharma R, Handa N, Kaur H, Rattan A, Yadav P, Gautam V, Kaur R, Bhardwaj R. 2015. Redox homeostasis in plants under abiotic stress: role of electron carriers, energy metabolism mediators and proteinaceous thiols. Front Environ Sci. 3: (col. 13). doi:10.3389/fenvs.2015.00013
- Khurana N, Chauhan H, Khurana P. 2013. Wheat chloroplast targeted sHSP26 promoter confers heat and abiotic stress inducible expression in transgenic Arabidopsis plants. PLoS ONE. 8(1):e54418. doi:10.1371/journal.pone.0054418
- von Koskull-Doring P, Scharf KD, Nover L. 2007. The diversity of plant heat stress transcription factors. Trends Plant Sci. 12:452–457.
- Kurek I, Chang TK, Bertain SM, Madrigal A, Liu L, Lassner MW, Zhu G. 2007. Enhanced thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. Plant Cell. 19:3230–3241.
- Larkindale J, Hall JD, Knight MR, Vierling E. 2005. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 138:882–897.
- Larkindale J, Vierling E. 2008. Core genome responses involved in acclimation to high temperature. Plant Physiol. 146:748–761.
- Lindquist S, Crig EA. 1988. The heat-shock proteins. Annu Rev Genet. 22:631–677.
- Liu HC, Liao HY, Charng YY. 2011. The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ. 34:738–751.
- Lobell DB, Schlenker W, Costa- Roberts J. 2011. Climate trends and global crop production since 1980. Science. 333:616–620.
- Mittler R, Blumwald E. 2010. Genetic engineering for modern agriculture: challenges and perspectives. Annu Rev Plant Biol. 61:443–462.
- Montillet JL, Chamnongpol S, Rustérucci C, Dat J, van de Cotte B, Agnel JP, Battesti C, Inzé D, Van Breusegem F, Triantaphylides C. 2005. Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol. 138:1516–1526.
- Morrow G, Tanguay RM. 2012. Small heat shock protein expression and functions during development. Int J Biochem Cell Biol. 44:1613–1621.
- Nicholas CC, Francxois T, Roberto T. 2008. Quantitative trait loci and crop performance under abiotic stress: Where do we stand?. Plant Physiol. 147:469–486.
- Nover L, Baniwal SK. 2006. Multiplicity of heat stress transcription factors controlling the complex heat stress response of plants. International Symposium on Environmental Factors, Cellular Stress and Evolution, Varanasi, India, October 13–15, p. 15.
- Paliwal R, Röder MS, Kumar U, Srivastava J, Joshi AK. 2012. QTL mapping of terminal heat tolerance in hexaploid wheat (T. aestivum L.). Theor Appl Genet. 125:561–575. doi:10.1007/s00122–012-1853-3
- Panaretou B, Zhai C. 2008. The heat shock proteins: their roles as multi-component machines for protein folding. Fungal Biol Rev. 22:110–119.
- Pastore A, Martin SR, Politou A, Kondapalli KC, Stemmler T. 2007. Unbiased cold denaturation: low- and high-temperature unfolding of yeast frataxin under physiological conditions. J Am Chem Soc. 129:5374–5375.
- Pinto RS, Reynolds MP, Mathews KL, McIntyre CL, Olivares- Villegas, JJ, Chapman SC. 2010. Heat and drought adaptive QTL in a wheat population designed to minimize econ found in gagronomic effects. Theor Appl Genet. 121:1001–1021. doi:10.1007/s00122-010-1351-4
- Rasheed R, Wahid A, Farooq M, Hussain I, Basra SMA. 2011. Role of proline and glycine betaine pretreatments in improving heat tolerance of sprouting sugarcane (Saccharum sp.) buds. Plant Growth Regul. 65:35–45.
- Rodriguez M, Canales E, Borras-Hidalgo O. 2005. Molecular aspects of abiotic stress in plants. Biotechnol Appl. 22:1–10.
- Schramm F, Larkindale J, Kiehlmann E, Ganguli A, Englich G, Vierling E. 2008. A cascade of transcription factor DREB2A and heat stress transcription factor HsfA3 regulates the heat stress response of Arabidopsis. Plant J. 53:264–274.
- Smith P, Olesen JE. 2010. Synergies between the mitigation of, and adaptation to, climate change in agriculture. J Agric Sci, Cambridge. 148:543–552.
- Snyman M, Cronje MJ. 2008. Modulation of heat shock factors accompanies salicylic acid-mediated potentiation of Hsp70 in tomato seedlings. J Exp Bot. 59:2125–2132.
- Sumesh KV, Sharma-Natu P, Ghildiyal MC. 2008. Starch synthase activity and heat shock protein in relation to thermal tolerance of developing wheat grains. Biol Plant. 52:749–753.
- Suzuki N, Koussevitzky S, Mittler R, Miller G. 2012. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 35:259–270.
- Suzuki N, Miller G, Morales J, Shulaev V, Torres MA. 2011. Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol. 14:691–699.
- Suzuki N, Mittler R. 2006. Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol Plant. 126:45–51.
- Timperio AM, Egid, MG, Zolla L. 2008. Proteomics applied on plant abiotic stresses: role of heat shock proteins (HSP). J Proteomics. 71:391–411.
- Tóth SZ, Nagy V, Puthur JT, Kovács L, Garab G. 2011. The physiological role of ascorbate as photosystem II electron donor: protection against photoinactivation in heat–stressed leaves. Plant Physiol. 156:382–392.
- Tripathy BC, Oelmüller R. 2012. Reactive oxygen species generation and signaling in plants. Plant Signal Behav. 7:1621–1633.
- Tripp J, Mishra SK, Scharf K-D. 2009. Functional dissection of the cytosolic chaperone network in tomato mesophyll protoplasts. Plant Cell Environ. 32:123–133.
- Venkatesh J, Park SW. 2014. Role of L-ascorbate in alleviating abiotic stresses in crop plants. Bot Stud. 55:38–57.
- Vijayalakshmi K, Fritz AK, Paulsen GM, Bai G, Pandravada S, Gill BS. 2010. Modeling and mapping QTL for senescence- related traits in winter wheat under high temperature. Mol Breed. 26:163–175. doi:10.1007/s11032-009-9366-8
- Vinocur B, Altman A. 2005. Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol. 16:123–132.
- Wahid A, Gelani S, Ashraf M, Foolad MR. 2007. Heat tolerance in plants: an overview. Environ Exp Bot. 61:199–223.
- Xu S, Li J, Zhang X, Wei H, Cui L. 2006. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ Exp Bot. 56:274–285.
- Yamada K, Fukao Y, Hayashi M, Fukazawa M, Suzuki I. 2007. Cytosolic HSP90 regulated the heat shock response that is responsible for heat acclimation in Arabidopsis thaliana. J Biol Chem. 282:37794–37804.
- Ye C, Argayoso MA, Redoña ED, Sierra SN, Laza MA, Dilla CJ. 2012. Mapping QTL for heat tolerance at flowering stage in rice using SNP markers. Plant Breed. 131:33–41.
- Ye C, Tenorio FA, Argayoso MA, Laza MA, Koh HJ, Redoña ED, Jagadish KSV, Gregorio GB. 2015. Identifying and confirming quantitative trait loci associated with heat tolerance at flowering stage in different rice populations. BMC Genetics. 16:1–51.
- Yoshida T, Ohama N, Nakajima J, Kidokoro S, Mizoi J, Nakashima K. 2011. Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shock-responsive gene expression. Mol Genet Genomics. 286:321–332.