ABSTRACT
Soil microbes play important roles in biochemical processes in the plant-soil-microbe ecosystem. However, the associations between soil microbes and herbal plants mediated by plant medical metabolites are poorly understood. We investigated the linkages of soil microbial biomass (SMB) and diversity based on an analysis of the phospholipid fatty acids and medical metabolites of Artemisia annua at 18 sites (54 plots) at altitudes ranging from 420 to 1420 m altitude in the Guizhou karst terrain of China. We found that the SMB and its diversity significantly linearly increased along the altitude gradient. The artemisinin concentration (0.54–20.82 g/kg) significantly linearly increased with increasing altitude. The artemisic acid concentration and total phenolics significantly linearly decreased with increasing altitude. SMB was significantly positively correlated to artemisinin and negatively correlated with total phenolics. Our results provide basic data regarding the linkages between soil microbes and A. annua medical metabolites, and provide an insight into their interactions.
Introduction
There is a need to better understand the associations among soil microbes and plants in the plant-soil-microbe ecosystem. Interactions between insects and plants in the animal-plant ecosystem are well documented (Ryan and Byrne Citation1988), and the interaction between soil microbes and plants in the microbe-plant ecosystem has also become a hot topic. Whether the association of soil microbes and herbal plants is mediated by the plant medical metabolites is a controversial and widely debated issue. Soil microbes play important roles in soil biochemical processes (Zak et al. Citation2003; Cregger et al. Citation2012) and are directly and/or indirectly affected by soil abiotic factors, and the diversity, biomass, and functional traits of plants (Frostegård et al. Citation1993; Sorensen et al. Citation2013; Chang et al. Citation2016; Zhao et al. Citation2016). As a key environmental factor in mountainous karst terrain, the altitude strongly affects soil microbial traits and the medical metabolites of herbal plants. Although the capacity of soil microbes to mediate soil biochemical processes, and the responses of plant medical metabolites to altitude is adequately documented (Falkowski et al. Citation2008; Zhang et al. Citation2009), few experimental and theoretical studies have investigated microbe-metabolite linkages in the herbal plant-soil-microbe ecosystem along an altitudinal gradient.
Many studies of the changes of microbial biomass, community composition, and diversity in soil along altitudinal gradients have been reported (Margesin et al. Citation2009; Chang et al. Citation2016). It has been reported that microbial biomass carbon (C) significantly increased with increasing altitude (from 600 to 1400 m) in a bamboo plantation (Chang et al. Citation2016). The population of Gram-negative bacteria and fungi was also found to increase along an altitude gradient (from 1500 to 2530 m) in pine soil (Margesin et al. Citation2009). Microbial diversity also shifts with altitude, with Bryant et al. (Citation2008) finding that bacterial diversity decreased with altitude in the mountains of the southwestern United States. Lin and Chiu (Citation2016) reported that bacterial diversity was greater at medium altitudes (1000 and 1200 m) than at lower and higher altitudes, although the opposite pattern was observed on Mount Halla in South Korea (Singh et al. Citation2014). Elevation changes in the Karst landforms of Guizhou Province in China are very common due to the wide range of altitudes from the eastern hilly region (≈ 200 m) to the western Yunnan-Guizhou Plateau (≈ 2900 m) (Yuan Citation1991). In the mountainous Karst terrain, the soils dominated by stone are thin and infertile (Zhang et al. Citation2014). It is of interest to determine if and how the soil microbes (including their biomass, community composition, and diversity) shift along the altitudinal gradient in this specific Karst landform.
The secondary metabolism of herbal plants is an important functional trait. Their medical metabolites, which have important physiological and ecological functions, have played a key role in the treatment of disease and maintenance of health for several thousand years (Liu et al. Citation2014). However, the accumulation of medical metabolites is affected by soil factors (such as soil nutrients), and climatic and geographic factors (such as elevation) (Zhang et al. Citation2009; Liu et al. Citation2014). Artemisia annua L. (Qinghao, in Chinese) belongs to the family Asteracea and genus Artemisia (Chinese Pharmacopoeia Commission Citation2015). It is distributed all over the world (mainly China and India) (Das Citation2012), but most adaptively originates in southwest China, including Guizhou, Chongqing, and Guangxi (Huang et al. Citation2010; Liu et al. Citation2013). Artemisia annua is a well-known herb that has low toxicity and a very efficient anti-malarial activity due to the presence of artemisinin (a medical metabolite of A. annua), the first-line drug recommended by the World Health Organization (WHO) (WHO Citation2010). Many of the activities of A. annua medical metabolites (including artemisic acid, artemisinin, and phenolics) are well known (Muzemil Citation2008; Ferreira et al. Citation2010; Huang et al. Citation2010; Das Citation2012; Lévesque and Peter Citation2012). The phenolics of A. annua (such as chrysosplenol-D and chrysosplenetin) have been confirmed to synergize the potential effects of artemisinin against malaria and cancer (Ferreira et al. Citation2010). The artemisinin content of A. annua plants ranges from 0.01% to 1.1% (Alam and Abdin Citation2011; Abdin and Alam Citation2015). The only way to obtain artemisinin is to isolate it from A. annua because its yield is improved through cell suspension culture, with in vitro culture, biosynthesis and total organic synthesis being complex and uneconomic (Delabays et al. Citation2001; Covello et al. Citation2007; Alam and Abdin Citation2011; Analogues et al. Citation2011; Lévesque and Peter Citation2012; Abdin and Alam Citation2015). However, the artemisinin yield from A. annua through conventional growing and breeding is affected by abiotic environmental factors, with several studies focused on evaluating the soil abiotic environment, and yield and quality of A. annua (including cultivation and improving growth conditions) (Aftab, Khan, Idrees, Ghauri, et al. Citation2010; Aftab, Khan, Idrees, Naeem, et al. Citation2010; Aftab et al. Citation2012; Luo et al. Citation2014). Investigations have also shown that the artemisinin content in A. annua leaves is significantly positively related to elevation (Zhang et al. Citation2009; Liu et al. Citation2014). The accumulation of artemisinin, artemisic acid, scopoletin, chrysoplenol-D, and chrysosplentin in A. annua along harvest time, temperature, humidity, and fertilization gradients have been investigated (Zhang et al. Citation2009; Yu Citation2010; Liu et al. Citation2014). However, whether or how the medical metabolites of A. annua change along altitudinal gradients in specific Karst landforms is unknown.
Linkages between soil microbes and medical metabolites of herbal plants are a controversial and widely debated topic. It is generally accepted that interactions among insects and plants are mediated by plant metabolites (Lambers et al. Citation2009; Otienoburu et al. Citation2012); however, medical metabolites act as a ‘bridge’ linking soil microbes to herbal plants and play an important role in plant-soil-microbe interaction, although the processes involved are poorly understood. Where linkages between soil microbes and plant medical metabolites have been established, the relationships between certain properties of soil microbes (such as biomass, community composition, and diversity) and the properties of plant medical metabolites (such as their concentration and composition) should be investigated, with a linear change expected along specific gradients (e.g. altitudinal gradients). In this study, we selected A. annua in the Guizhou karst terrain in China, which is widely distributed along an altitudinal gradient. We investigated the linkages between soil microbes and plant metabolites in an area where limestone soil is the main base material (Yuan Citation1991). Artemisia annua is a well-known herb that contains important medical metabolites, and is adapted to grow in the thin and infertile stony soils of karst topography because of its ability to resist the infertile and rocky desert conditions (Luo et al. Citation2014; Zhang et al. Citation2014).
In this study, we hypothesized that there are interactions between soil microbes and medical metabolites from A. annua, which shifted along an altitude gradient in a karst landform. Specifically, we hypothesized that: (1) soil microbial biomass (SMB) and diversity changed along an altitudinal gradient; (2) the contents of medical metabolites shifted along the altitudinal gradient; and (3) soil microbes are related to some medical metabolites of A. annua. To test these hypotheses, we investigated SMB and soil diversity based on environmental factors, phospholipid fatty acids (PLFAs), and the concentrations of the main medical metabolites in A. annua at 18 sites (54 plots) ranging from 420 to 1420 m altitude in the karst topography of China. Prior to the study, we knew that SMB and diversity increased along the altitudinal gradient, the artemisinin content increased with altitude, artemisic acid and total phenolics decreased along the altitudinal gradient, and SMB was related to artemisinin and total phenolics.
This study provided basic data to assess the linkages between soil microbes and medical metabolites of A. annua, and also provided an insight into the interactions between soil microbes and medical metabolites of A. annua.
Materials and methods
Site description and sampling
Soil and leaf (including flower bud) samples of A. annua were collected from 18 sites (three plots per site, total of 54 plots) in Guizhou Province, China. The plot altitudes ranged from 420 to 1420 m (northern latitude (N) 25°20′–27°55′, east longitude (E) 106°28′–109°18′). All plot altitudes and geographical coordinates were recorded using GPS (eXplorist600, Magellan, East Mississauga, on Canada). Guizhou is located in a subtropical area, with a mild climate. All sampling sites were typical Karst landforms. Although there was adequate rainfall at all sites, rainwater could move into the ground through crevices and the surface soil could become parched between rains. The details of all sites are listed in . Soil and plant samples were collected in the bud stage of A. annua in the autumn of 2016. Soil material adhered to the root surface of nine A. annua plants in a plot were pooled and collected in a sterile plastic bag to comprise one soil sample, while the leaves of the same A. annua plants were considered to represent a plant sample. Three plots from each site were sampled, which constituted sample replication. The samples were placed in an ice box and rapidly transported to the laboratory. Each soil sample was subsequently divided into two portions, with one portion air dried and prepared for testing soil properties and the other placed into a freezer at 4°C for the later determination of soil microbial PLFAs. Each fresh leaf sample was placed in the freezer at 4 °C for the later determination of medical metabolite concentrations.
Table 1. Geographic information and stone content in soils for the A. annua 18 sites in Guizhou Karst topography of China (the code of sampling site was based on the elevation where the sampling site located in).
Analysis of soil parameters
The method described by Bao (Citation2000) was used the measure the soil parameters of organic matter (OM), available potassium (AK), available phosphorus (AP), available nitrogen (AN), total nitrogen (TN), total potassium (TK), total phosphorus (TP). Soil moisture (SM) was determined as the weight lost using an oven-drying method for 24 h at 105°C. The stone content of the soil expressed as the percentage of stones (≥2 mm) to the total soil sample for each plot. Soil pH was measured in a water and soil mixture (v/w ratio was 2.5:1) using a pH meter (Standard pH Meter PB-10, Sartorius, Göttingen, Germany).
Analysis of soil microbial PLFAs
PLFA extraction: The fresh soils (1.5 g dry weight equivalent) were extracted using a mixture of citric acid buffer, methanol, and trichloromethane, and then centrifuged and filtered. The extracted solutions were concentrated by blowing with a stream of nitrogen (N2). The extracts were then methylated with a mild alkaline solution (3 mL 0.1% KOH methanol solution and 2 mL hexane), and then dissolved in hexane containing methyl nonadecanoate (19:0) as an internal standard. The methylated solution was mixed into 2 mL water, centrifuged, layered, and finally the organic layer solutions were collected. The sample solutions were stored at 4°C prior to analysis by gas chromatography-mass spectrometry (GC-MS). The GC-MS conditions were as follows: GC-MS instrument, GCMS-QP2010 (Shimadzu, Kyoto, Japan); capillary column, HP-5 ms, 30 m × 0.25 mm × 0.25 μm; injector temperature, 250°C; column temperature program, 70°C (5 min), 70–190°C (20°C/min), 190°C (1 min), 190–200°C (5 °C/min), 200°C (2 min), 200–280°C (10°C/min), 280°C (8 min); carrier gas, helium (0.90 mL/min); split ratio, 10:1; ionization temperature, 230°C; ionization energy, 70 eV; scan time, 0.5 s; and mass range, 33–450 amu. Working solutions of the samples and PLFAs were obtained by diluting the sample solutions and mixing a PLFA standard (bacterial acid methyl ester mix, 47080-U, Sigma-Aldrich, St. Louis, MO, USA) further with an appropriate volume of hexane, respectively. The working solutions were analyzed by GC-MS. Finally, methyl nonadecanoate was used as a quantitative internal standard, and the peaks were identified by comparing the retention times of the samples with those of the standard. Microbial biomass was measured by the concentration of the total PLFAs. In brief, according to Buyer’s method (Buyer and Sasser Citation2012), 24 microbial PLFAs were evaluated to determine the biomass and structural diversity of soil microorganisms (Quideau et al. Citation2016; Zhao et al. Citation2016): 2-OH 10:0, 11:0, 12:0, 13:0, 2-OH 12:0, 3-OH 12:0, 14:0, i-15:0, α-15:0, 2-OH 14:0, i-16:0, 16:1ω7c, 16:0, i-17:0, cy17:0, 17:0, 2-OH 16:0, 18:2ω6,9, 18:1ω9c, 18:1ω9t, 10 Me18:0, cy19:0, and 20:0. The PLFAs were designated as X:YωZ, where X represents the number of carbon atoms, Y represents the number of unsaturated ethylene linkages, ω means a double bond, Z indicates the location of an ethylene linkage, and i, α, cy, Me, and c refer to isopropyl, anteisorpropyl, cyclopropyle, methyl branching, and cis-configuration, respectively (Frostegård et al. Citation1993).
Analysis of leaf medical metabolites
The leaf medical metabolites of A. annua were extracted from approximately 0.5 g of freshly ground leaves from each sample, which was placed into a 40 mL storage bottle, with a spiral mouth and solid cover, and immediately mixed with 20 mL absolute ethanol (Jessing et al. Citation2009; Yu Citation2010). The bottle was then sealed and subjected to ultrasonic extraction for 2 h. The extracted solutions were centrifuged and filtered through a 0.45 µm membrane filter. The sample solutions were stored at 4°C until analyses. Stock solutions of artemisinin and artemisic acid were prepared. Briefly, artemisinin and artemisic acid were weighed (to 0.1 mg), and then dissolved in ethanol in measuring flasks (10 mL). The stock solutions were stored at 4°C. Artemisinin and artemisic acid were analyzed using GC-MS. The analysis conditions were as follows: GC-MS instrument, GCMS-QP2010; capillary column, Phase ZB, 30 m × 0.25 mm × 0.25 μm; injector temperature, 230°C; column temperature program, 50°C (1 min), 50–200°C (15°C/min), 200°C (5 min), 200–230°C (15°C/min), 230°C (10 min); carrier gas, helium (0.95 mL/min); split ratio, 10:1; ionization temperature, 230°C; ionization energy, 70 eV; scan pattern, SIM; and artemisinin and artemisic acid quantifier ions, 166 and 121. Working solutions of the samples, artemisinin, and artemisic acid were obtained by further diluting the sample solutions and the stock solution of artemisinin and artemisic acid with an appropriate volume of ethanol. The working solutions were analyzed by GC-MS. The artemisinin and artemisic acid concentrations in the samples were calculated based on the peak areas of the artemisinin and artemisic acid quantifier ions.
Scopoletin, chrysosplenol-D, and chrysosplenetin were analyzed using high performance liquid chromatography (HPLC) (Yu Citation2010). A stock solution of scopoletin, chrysosplenol-D, and chrysosplenetin was prepared. Briefly, scopoletin, chrysosplenol-D, and chrysosplenetin were weighed (to 0.1 mg) and then dissolved in ethanol in 10 mL measuring flasks. The stock solution was stored at 4°C. The analysis conditions were as follows: HPLC instrument, LC-20AT (Shimadzu) and analytical chromatographic columns, reversed-phase shim-pack CLC-ODS in the column oven (150 mm × 6.0 mm × 5 μm, No. 61626630). The linear gradient elution was conducted as follows: eluent A (acetonitrile: methanol = 11:5 (v/v)) and eluent B (0.1% formic acid (v/v)); 5% A (0–5 min); 5%–16% A (5–8 min); 16%–24% A (8–30 min); 24%–32% A (30–47 min); 32%–64% A (47–68 min); 64% A (68–75 min); 64%–100% A (75–78 min); 100% A (78–88 min); 100%–5% A (88–89 min); and 5% A (89–95 min). The flow rate program was conducted as follows: 1.4 mL/min (0–5 min); 1.4–0.6 mL/min (5–10 min); 0.6–0.8 mL/min (10–47 min); 0.6–1.4 mL/min (47–50 min); and 1.4 mL/min (50–95 min). The detection wavelength was 345 nm and the column temperature was 40°C. Working solutions of the samples, scopoletin, chrysosplenol-D, and chrysosplenetin were obtained by further diluting the sample solutions and scopoletin, chrysosplenol-D and chrysosplenetin stock solutions with appropriate ethanol. The working solutions were analyzed by HPLC. The scopoletin, chrysosplenol-D, and chrysosplenetin concentrations in the samples were calculated based on the peak areas of scopoletin, chrysosplenol-D, and chrysosplenetin.
Total phenolics were measured using the Folin–Ciocalteu colorimetric method with a 721-UV spectrophotometer (Kamath et al. Citation2015). In brief, the sample solutions (1.0 mL) were added to test tubes and Folin-Ciocalteu’s reagent (1.5 mL) and 7.5% sodium carbonate (1.0 mL) were added. The tubes were mixed and then left to stand for 30 min. Absorption at 765 nm was determined by a 721-UV spectrophotometer. The total phenolics were expressed as gallic acid equivalents in g / kg dry leaves of A. annua.
Statistical analysis
Microsoft Office Excel 2010 was used to analyze the basic data for every plot, with every parameter measured in three replicate samples. Results were expressed on a dry weight basis. The SMB was equivalent to the total PLFAs. Diversity indices (Shannon, Evenness, and Simpson) regarding the soil microbial community composition (SMCC), a canonical correspondence analysis (CCA) (F = 0.681, P < 0.05), and Mantel tests were conducted in Rv. 3.1.2 with the Vegan package. Shannon = −ΣPilnPi, Pi is the ratio of Ni to the total PLFA content of each plot, Ni is the PLFA content in a plot, Evenness = H/lnS, S represents the number (24) of PLFAs, H represents the value of the Shannon index, and Simpson = 1 – ΣPi2. The CCA and Mentel tests conducted using Rv. 3.1.2 were used to estimate the linkages of soil microbial PLFA composition and environmental factors (Lipson et al. Citation2000; Zhao et al. Citation2016), and a CCA diagram was constructed using Sigmaplot 12.5 according to the vectors of the CCA and Mantel tests. An analysis of variance (ANOVA) for the pairwise t-tests and correlation coefficients of all variables was conducted with SPSS (PASW statistic 18). Each of the cumulative variables (SBM, soil diversity indexes, main medical metabolites) was subjected to an analysis of its relationship with elevation to determine if altitude was correlated with these variables (nutrients and mainly medical metabolites). Linear regression analyses were conducted using Microsoft Office Excel 2010.
Results
Geochemical properties and relationships of soil properties with altitude
The major geographic properties and soil stone contents of the 18 sampling sites are listed in , which shows that the soil physicochemical characteristics were quite variable at the different sites (). The soil pH varied from 5.29 to 8.24 and was not markedly correlated with altitude (P > 0.05) ( and ). The mean concentrations of soil OM, AN, AK, and AP varied from 9.05 to 57.71 g/kg, 4.91 to 39.24 mg/kg, 45.41 to 181.57 mg/kg, and 15.80 to 161.77 mg/kg, respectively (), and there were significant positive correlations of soil AN and AP with altitude (P < 0.01), but soil OM and AK were not significantly related to altitude (P > 0.05) (). The ranges of mean soil TN, TP, TK, and SM were from 0.2 to 3.45 g/kg, 0.33 to 1.31 g/kg, 6.72 to 36.83 g/kg, and 5.85 to 21.57% (), and there were significant negative correlations of soil TN, TP, and TK with altitude (P < 0.05 or P < 0.01), but SM was not significantly correlated with altitude (P > 0.05) ().
Table 2. Soil environmental factors analyzed by ANOVA among 18 A. annuna soil sites. OM, organical matter; AK, available potassium; AP, avaible phosphorus; AN, available nitrogen; TN, total nitrogen; TK, total potassium; TP, total phosphorus; SM, soil moisture. Data present the mean value and standard error, and different lowcase letter within the same column means significant diferent.
Table 3. Pearson correlation coefficients (r-value) of soil microbial variables and environmental factors from two-way ANOVAs.
Shifts in SBM and soil microbial diversity along the altitudinal gradient
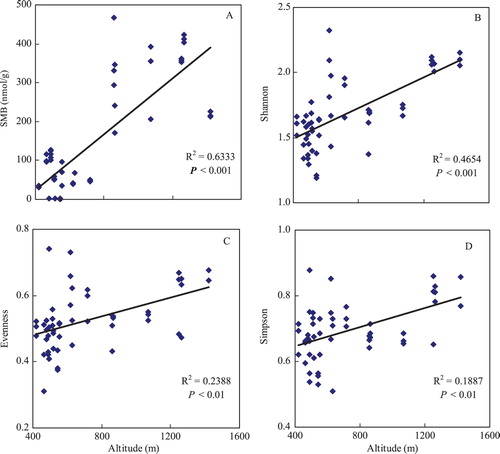
SMB varied from 1.02 to 467.35 nmol/g and linearly increased with increasing altitude (P < 0.001; (A)). Moreover, the mean SMB of the sites at altitudes above 800 m (320.58 nmol/g) was significantly higher than that at altitudes below 800 m (53.68 nmol/g) ((A)). Altitude also significantly affected soil microbial diversity, with significant positive correlations between altitude and the three diversity indexes (Shannon, P < .001, (B); Evenness, P < 0.01, (C); Simpson, P < 0.01, (D)). The mean diversity values of the sites at altitudes above 800 m were higher (1.87, 0.57, and 0.74, respectively) than those of sites below 800 m (1.59, 0.50, and 0.67, respectively) ((B–D)).
Correlations of soil microbial variables with other environmental factors
Soil microorganisms were also affected by various other environmental factors. Positive correlations between SMB and soil OM, AN, AP, and SM were found, but they were not significant, while SMB was significantly positively correlated to soil AK (). There were significant negative relationships between SMB and soil stone content, TN, TP, and TK (). The SMCC was also very different among the sites. The CCA results showed that the variation of soil PLFA data was 24.47% and 10.89% explained by the CCA1 and CCA2 axes, respectively. The SMCC (as indicated by PLFA content) in Luodian (site numbers 2, 4, and 5) and Jianhe (site numbers 3, 7, and 8) was separated from the other locations along CCA1 (). The Mantel test results also indicated that SMCC was significantly related to altitude (R = 0.334, P = 0.001, ). Soil pH and TP were extremely significantly correlated with the SMCC (R = 0.144, 0.347, respectively, and P = 0.001), while soil AN and TK were also significantly correlated with the SMCC (R = 0.063, 0.095, and P = 0.044, P = 0.009, respectively) ().
Figure 2. Canonical correspondence analysis (CCA) of soil microbial community composition (based on soil microbial PLFAs) with environmental factors among 18 A. annua soil sites (54 plots). 1–18 numbers represent sampling sites; OM, organic matter; pH, power of hydrogen; AN, available nitrogen; AK, available potassium; AP, available phosphorus; TN, total nitrogen; TP, phosphorus; TK, total potassium; SM, soil moisture.; Stone, percentage of stone (≥2 mm) in soil.
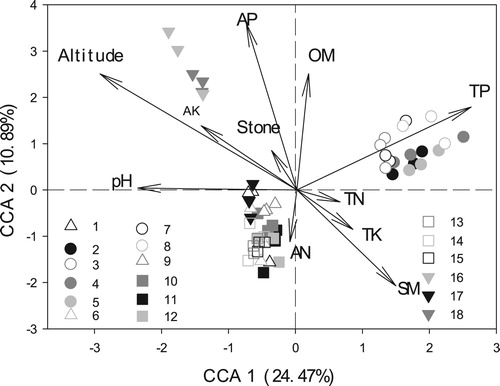
Table 4. Mantel tests of Microbial community compositions (SMCCs, based on PLFAs) with environmental properties among 54 A. annua soil plots. OM, organical matter; AK, available potassium; AP, avaible phosphorus; AN, available nitrogen; TN, total nitrogen; TK, total potassium; TP, total phosphorus; SM, soil moisture. Bold type number means significanct relationship.
Changes in the main medical metabolites along the altitudinal gradient
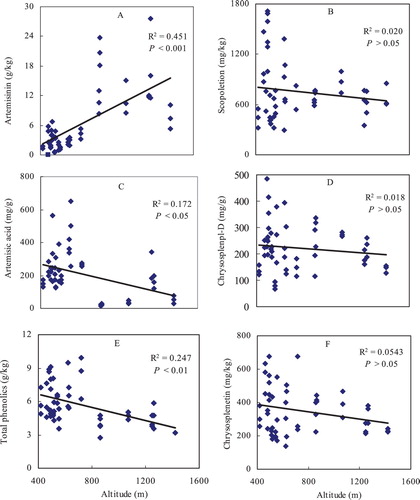
Artemisinin and artemisic acid are terpene compounds in A. annua leaves and their concentrations across the 54 plots ranged from 0.54 to 20.82 g/kg and 20.00 to 648.25 mg/kg, respectively ((A and C)). The mean artemisinin concentration at sites with an altitude above 800 m (13.43 g/kg) were significantly higher than those at sites below 800 m (2.79 g/kg). Moreover, across all 54 plots, the artemisinin concentrations of A. annua leaves increased linearly with altitude (P < 0.001, (A)), but the artemisic acid concentrations decreased significantly with altitude (P < 0.05, (C)). In contrast to artemisinin, total phenolics decreased linearly with altitude, ranging from 9.47 to 2.99 g/kg (P < 0.01, (E)). However, scopoletin ((B)), chrysosplenol-D ((D)), and chrysosplenetin ((F)) concentrations did not shift significantly with altitude.
Response of the main medical metabolites to other environmentally biotic and abiotic factors
In addition to altitude, the concentrations of the main medical metabolites from A. annua leaves were correlated with some of the other soil biotic and abiotic factors. Among the biotic factors, the artemisinin concentration significantly increased with increasing SMB (P < 0.001), while total phenolics significantly decreased with an increase in SMB (P < 0.01) (). Soil pH was found to have a significant negative correlation with artemisic acid (P < 0.05), scopoletin (P < 0.001), chrysosplenol-D (P < 0.05), chrysosplenetin (P < 0.001), and total phenolics (P < 0.05) (). There were significant negative correlations between soil TN, TP, and TK and atermisinin (P < 0.01 or P < 0.001), but the opposite was true for total phenolics (P < 0.001, P < 0.05, and P < 0.01, respectively) (). Soil TP was significantly positively correlated to artemisic acid, scopoletin, and chrysosplenetin (P < 0.05) ().
Table 5. Pearson correlation coefficients (r-value) of plant mainly medical metabolites and soil biotic factors of A. annua.
Table 6. Pearson correlation coefficients (r-value) of plant mainly medical metabolites of leaves and soil abiotic factors from A. annua.
Discussion
Environmental and plant factors affecting SMCC and SMB have previously been identified (Chang et al. Citation2016) and the structure of soil microbes has been reported to vary along elevation gradients (Nottingham et al. Citation2015; Yasir et al. Citation2015; Lin and Chiu Citation2016). On the basis of data from 16S tRNA sequences, elevation has been shown to affect soil bacterial community diversity (Lin and Chiu Citation2016). A PLFA analysis is a useful technique in studies of SMCC and SMB (Farrell et al. Citation2010; Frostegård et al. Citation2011). A clear increase in soil total PLFA content with increasing altitude has been reported (Farrell et al. Citation2010; Chang et al. Citation2016).
In this study, SMB and soil microbial diversity indexes (Shannon, Evenness, and Simpson) at A. auuna sampling sites were significantly positively related to altitude over the range of 420–1420 m ((A–D)), which agreed with the similar results reported for a bamboo forest across an elevation gradient of 600–1400 m (Chang et al. Citation2016; Lin and Chiu Citation2016). The diversity was very different from the β diversity reported in a woody forest, which was negatively correlated with elevation (Kraft et al. Citation2008). This is likely because various environmental factors affect soil microorganisms in addition to elevation, including vegetation, temperature, SM, and nutrients (Lipson et al. Citation2000; Cong et al. Citation2015; Zhao et al. Citation2016). There were significant positive correlations between AP, and the Evenness, Simpson indexes (P < 0.05), whereas there were significant negative relationships between these parameters and SM (P < 0.05 and P < 0.01, respectively). SMB was positively correlated with AN, AP, and AK (AN and AP P > 0.05; AK P < 0.05, respectively), but negatively correlated with TN, TP and TK (P < 0.001) (). Similarly, altitude also affected the soil nutrients available to A. annua, with significant positive correlations observed for soil AN and AP with altitude (P < 0.01, respectively), while soil TN, TP, and TK were significantly negatively correlated with altitude (). These results are inconsistent with those of previous studies in a subtropical woody forest (He et al. Citation2016) and moso bamboo forest (Chang et al. Citation2016), This may be because A. annua absorbs large amounts of nutrients from the soil during its short growth cycle as an annual herbaceous plant, with an annual growth cycle from April to September in the Guizhou mountainous karst terrain (Chinese Pharmacopoeia Commission Citation2015). In contrast, woody and bamboo forests are perennial and regularly absorb lesser amounts of soil nutrients.
Earlier studies conducted in India and China have attempted to enhance the artemisinin concentration through culturing root hairs, breeding technology, plant cell culture, or the biosynthesis pathway using its regulatory enzymes (Nair and Basile Citation1993; Bharel et al. Citation1998; MaujiRam et al. Citation2010). An improvement in artemisinin yield through chemical synthesis and the genetic engineering of artemisinin has been reported (Liu et al. Citation2011; Alam et al. Citation2014). However, these methods have proven to be commercially unfeasible (Zhu and Cook Citation2012). The conventional growing and breeding of A. annua remains an important source of artemisinin production. However, in addition to the improved varieties, the artemisinin contents in A. annua plants can be affected by many factors, both biotic and abiotic.
In this study, the leaf concentration of artemisinin, the most effective plant component for treating malaria, linearly and significantly increased with elevation ((A)), with the exception that leaf artemisinin concentrations at 1420 m were lower than at 1264 m, which was consistent with the results of two previous studies in Guangxi and Chongqing, China (Zhang et al. Citation2009; Liu et al. Citation2014). Artemisinin is one of the sesquitepene endoperoxide lactones, which are all derived from the same precursor, farnesyl diphosphate biosynthesized by the catalysis of sesquiterpene synthase (SS). Farnesyl diphosphate is converted by amorpha-4,11-diene synthase (ADS) into amorpha-4,11-diene, with several oxidation and reduction steps controlled by enzymes, and finally amorpha-4,11-diene is converted into artemisinin (Bouwmeester et al. Citation1999; Chang et al. Citation2000). Therefore, SS and ADS are important synthases and rate-limiting enzymes in the biosynthesis of artemisinin. Their activity can be induced and accelerated by a decrease in atmospheric density, changes in the solar spectra, and the increasing intensity of short wavelength light with an increase in altitude (Brown Citation2010; Xie et al. Citation2012). In contrast, the total phenolic content decreased significantly with an increase in altitude (P < 0.01, (E)).
In addition to elevation, other environmental biotic and abiotic factors were also related to levels of the main medical metabolites in A. annua ( and ), with SMB markedly and positively correlated with artemisinin (P < 0.001). Compared to the other metabolites, the artemisinin content in A. annua leaves was high at 2% (w/w), and there were hardly any other plants present in areas where A. annua plants were clustered due to its strong allelopathy (Shen Citation2006). It is possible that some artemisinin was leached from the leaves of A. annua by rainfall and became a carbon source for the growth of soil microorganisms, improving the growth of soil microbes. Soil TN, TP, and TK also significantly negatively impacted the artemisinin concentration (TN and TP, P < 0.01; TK, P < 0.001) (). In contrast to artemisinin, total phenolics were markedly and negatively correlated with SMB (P < 0.01) (), negatively correlated with pH (P < 0.05), OM, AK, AP, and AN (P > 0.05), and significantly and positively correlated with TN, TP, and TK (TN, P < 0.001; TP, P < 0.05; TK, P < 0.01) (), which suggests that A. annua could accelerate the biosynthesis of phenolics for improving health and fertility. Soil pH also negatively and significantly impacted the artemisic acid, scopoletin, chrysosplenol-D, and chrysosplenetin concentrations (P < 0.05 or P < 0.001), which suggested that increasing the concentrations of artemisic acid and these phenolics would decrease soil pH, likely because some of these components can be leached from the A. annua leaves by rainfall; therefore, increasing the concentration of soil hydrogen ions [H+].
Conclusion
The results of the study indicate that SMB and diversity, and the artemisinin, artemisic acid, and total phenolic concentrations of A. annua leaves vary along an altitude gradient. The correlation of artemisinin concentrations and total phenolic content of A. annua leaves was correlated with SMB, suggesting a linkage between soil microbes and medical metabolites in A. annua leaves. Our results provide basic data to address the critical questions regarding the linkages between soil microbes and medical metabolites in A. annua leaves.
Acknowledgements
We thank Song Zhang at Guizhou Normal University for assistance in soil determination and the Institute for Environmental Genomics of Oklahoma University for references and statistic assistance.
Data availability statement
All data that support the findings of this study are available from the corresponding author, ZHAN-NAN YANG (email: [email protected]), upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Shi-qiong Luo http://orcid.org/0000-0001-7253-6160
Cheng Zhao http://orcid.org/0000-0001-6707-8353
Zhan-nan Yang http://orcid.org/0000-0002-2983-7432
Juan Hu http://orcid.org/0000-0001-8099-7477
Sheng-juan Di http://orcid.org/0000-0002-5542-9184
Additional information
Funding
Notes on contributors
Shi-qiong Luo
Shi-qiong Luo, a professor of agricultural microbiology.
Cheng Zhao
Cheng Zhao, a master graduate student.
Zhan-nan Yang
Zhan-nan Yang, a professor of botany.
Juan Hu
Juan Hu, a master graduate student.
Sheng-juan Di
Sheng-juan Di, a master graduate student.
References
- Abdin MZ, Alam P. 2015. Genetic engineering of artemisinin biosynthesis: prospects to improve its production. Acta Phsiol Plant. 37:33. doi:10.1007/s11738-015-1771-5.
- Aftab T, Khan MMA, Idrees M, Ghauri N, Hashmi N, Moinuddin AS. 2010. Effect of salt stress on growth, membrane damage, antioxidant metabolism and artemisinin accumulation in Artemisia annua L. Plant Stress. 4:36–43.
- Aftab T, Khan MMA, Idrees M, Naeem M, Ram M. 2010. Boron induced oxidative stress, antioxidant defense response and changes in artemisinin content in Artemisia annua L. J Agron Crop Sci. 196:423–430. doi: 10.1111/j.1439-037X.2010.00427.x
- Aftab T, Khan MM, Naeem M, Idrees M, Moinuddin AS, Teixeira da Silva JA, Ram M. 2012. Exogenous nitric oxide donor protects Artemisia annua from oxidative stress generated by boron and aluminium toxicity. Ecotoxicol Environ Safe. 80:60–68. doi: 10.1016/j.ecoenv.2012.02.007
- Alam P, Abdin MZ. 2011. Over-expression of HMG-CoA reductase and amorpha-4, 11-diene synthese genes in Artemisia annua L. and its influence on artemisinin content. Cell Rep. 10:1919–1928. doi: 10.1007/s00299-011-1099-6
- Alam, P, Kamaluddin, Khan MA, Mohammad A, Khan R, Abdin MZ. 2014. Enhanced artemisinin accumulation and metabolic profiling of transgenic Artemisia annua L. plants over-expressing by rate limiting enzymes from isoprenoid pathway. J Plant Inter. doi:10.1080/17429145.2014.893030.
- Analogues HO, Hiruma Y, Yamaqishi H, Ishiyama A, Otoquro K, Yamada H, Ōmura S. 2011. Generation of anti-trypanosomal agents through concise synthesis and structural diversification of sesquiterpene analogues. J Am Chem Soc. 133:7096–7105. doi: 10.1021/ja200374q
- Bao SD. 2000. Soil and agricultural chemistry analysis. Beijing: Agriculture Publication; p. 33–100.
- Bharel S, Gulati A, Abdin MZ, Srivastava PS, Vishwakarma RA, Jain SK. 1998. Enzymatic synthesis of artemisinin from natural and synthetic precursors. J Nat Prod. 61:633–636. doi: 10.1021/np970024s
- Bouwmeester HJ, Wallaart TE, Janssen MHA, Loo BV, Jansen BJM, Posthumus MA, Schmidt CO, Kraker J-WD, König WA, Franssen MCR. 1999. Amorpha-4,11-diene synthase catalyze the first probable step in artemisinin biosynthesis. Phytochemistry. 52:843–854. doi: 10.1016/S0031-9422(99)00206-X
- Brown GD. 2010. The biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L. (Qinghao). Molecules. 15:7603–7698. doi: 10.3390/molecules15117603
- Bryant JA, Lamanna C, Morlon H, Kerkhoff AJ, Enquist B, Green JL. 2008. Microbes on mountainsides: contrasting elevational patterns of bacterial and plant diversity. Proc Natl Acad Sci USA. 105:11505–11511. doi: 10.1073/pnas.0801920105
- Buyer JS, Sasser M. 2012. High throughput phospholipid fatty acid analysis of soils. Appl Soil Ecol. 61:127–130. doi: 10.1016/j.apsoil.2012.06.005
- Chang E-H, Chen T-H, Tian G, Chiu C-Y. 2016. The effect of altitudinal gradient on soil microbial community activity and structure in moso bamboo plantations. Appl Soil Ecol. 98:213–220. doi: 10.1016/j.apsoil.2015.10.018
- Chang YJ, Song S-H, Park S-H, Kim SU. 2000. Amorpha-4,11-diene synthase of Artemisia annua: cDNA isolation and bacterial expression of a terpene synthase involved in artemisinin biosynthesis. Arch Biochem Biophys. 383:178–184. doi: 10.1006/abbi.2000.2061
- Chinese Pharmacopoeia Commission. 2015. Pharmacopoeia of China. Beijing: China Medical Science and Technology Press; p. 184.
- Cong J, Yang Y, Liu X, Lu H, Liu X, Zhou J, Li D, Yin H, Ding Y, Zhang Y. 2015. Analyses of soil microbial community compositions and functional genes reveal potential consequences of natural forest succession. Sci Rep-UK. 5:100007..
- Covello PS, Teoh KH, Polichuk DR, Reed DR, Nowak G. 2007. Functional genomics and the biosynthesis of artemisinin. Phytochemistry. 68(14):1864–1871. doi: 10.1016/j.phytochem.2007.02.016
- Cregger MA, Schadt CW, McDowell NG, Pockman WT, Classen AT. 2012. Response of the soil microbial community to changes in precipitation in a semiarid ecosystem. Appl Environ Microbiol. 78:8587–8594. doi: 10.1128/AEM.02050-12
- Das S. 2012. Artemisia annua (Qinghao): a pharmacological review. Int J Pharm Sci Res. 3:4573–4577.
- Delabays N, Simonnet X, Gaudin M. 2001. The genetics of artemisinin content in Artemisia annua L. and the breeding of high yielding cultivars. Curr Med Chem. 8(15):1795–1801. doi: 10.2174/0929867013371635
- Falkowski PG, Fenche T, Delong EF. 2008. The microbial engines that drive earth’s biogeochemical cycles. Science. 320:1034–1039. doi: 10.1126/science.1153213
- Farrell M, Griffith GW, Hobbs PJ, Perkins WT, Jones DL. 2010. Microbial diversity and activity are increased by compost amendment of metal-contaminated soil. FEMS Microbiol Ecol. 71:94–105. doi: 10.1111/j.1574-6941.2009.00793.x
- Ferreira JFS, Luthria DL, Sasaki T, Heyerick A. 2010. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules. 15:3135–3170. doi: 10.3390/molecules15053135
- Frostegård A, Tunlid A, Baath E. 1993. Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl Environ Microbiol. 59:3605–3617.
- Frostegård Å, Tunlid A, Erland B. 2011. Use and misuse of PLFA measurements in soils. Soil Biol Biochem. 43:1621–1625. doi: 10.1016/j.soilbio.2010.11.021
- He X, Hou E, Liu Y, Wen D. 2016. Altitudinal patterns and controls of plant and soil nutrient concentrations and stoichiometry in subtropical China. Sci Rep-UK. 6:24261. doi:10.1038/srep24261.
- Huang L, Xie C, Duan B, Chen S. 2010. Mapping the potential distribution of high artemisinin-yielding Artemisia annua L. (Qinghao) in China with a geographic information system. Chin Med. 5:18. doi:10.1186/1749- 8546-5-18 doi: 10.1186/1749-8546-5-18
- Jessing KK, Cedergreen N, Jensen J, Hansen HCB. 2009. Degradation and ecotoxicity of the biomedical drug artemisinin in soil. Environ Toxicol Chem. 28:701–710. doi: 10.1897/08-153R.1
- Kamath SD, Arunkumar D, Avinash NG, Samshuddin S. 2015. Determination of total phenolic content and total antioxidant activity in locally consumed food stuffs in Moodbidri, Karnataka, India. Adv Appl Sci Res. 6:99–102.
- Kraft NJB, Valencia R, Ackerly DD. 2008. Functional traits and niche-based tree community assembly in an Amazonian forest. Science. 322:580–582. doi: 10.1126/science.1160662
- Lambers H, Mougel C, Jaillard B, Hinsinger P. 2009. Plant-microbe-soil interactions in the rhizosphere: an evolutionary perspective. Plant Soil. 321:83–115. doi: 10.1007/s11104-009-0042-x
- Lévesque F, Peter HS. 2012. Continuous-flow synthesis of the anti-malaria drug artemisinin. Angew Chem Int Edit. 51:1706–1709. doi: 10.1002/anie.201107446
- Lin Y-T, Chiu C-Y. 2016. Elevation gradient of soil bacterial communities in bamboo plantations. Bot Stud. 57:8–13. doi: 10.1186/s40529-016-0123-0
- Lipson DA, Schmidt SK, Monson RK. 2000. Carbon availability and temperature control the post-snowmelt decline in alpine soil microbial biomass. Soil Biol Biochem. 32:441–448. doi: 10.1016/S0038-0717(99)00068-1
- Liu H, Tian X, Zhang Y, Wang C, Jiang H. 2013. The discovery of Artemisia annua L. in the Shengjindian cemetery, Xinjiang, China and its implications for early uses of traditional Chinese herbal medicine qinghao. J Ethnopharmacol. 146:278–286. doi:10.1016/ j.jep.2012.12.044 doi: 10.1016/j.jep.2012.12.044
- Liu B, Wang H, Du Z, Li G, Ye H. 2011. Metabolic engineering of artemisinin biosynthesis in Artemisia annua L. Plant Cell Rep. 30(5):689–694. doi: 10.1007/s00299-010-0967-9
- Liu P, Yang F, Hao HP, Xu Y, Shi XW, Zhang DD, Zheng HC, Wen QY, Li WW, Ji H, et al. 2014. Bioactive equivalence of combinatorial components identified in screening of an herbal medicine. Pharmac Res. 31:1788–1800. doi: 10.1007/s11095-013-1283-1
- Luo S, Huang J, Yuan L. 2014. Nutrients and microorganisms in soils with wild Artemisia annua L. Acta Pedol Sin Chin. 51:200–211.
- Margesin R, Jud M, Tscherko D, Schinner F. 2009. Microbial communities and activities in alpine and subalpine soils. FEMS Microbiol Ecol. 67:208–218. doi: 10.1111/j.1574-6941.2008.00620.x
- MaujiRam KM, Jha P, Khan S, Kiran U, Ahmad MM, Javed S, Abdin MZ. 2010. HMG-CoA reductase limits artemisinin biosynthesis and accumulation in Artemisia annua L. plants. Acta Physiol Plant. 32:859–866. doi: 10.1007/s11738-010-0470-5
- Muzemil A. 2008. Determination of artemisinin and essential oil contents of Artemisia annua L. grown in Ethiopia and In vivo antimalarial activity of its crude extracts against Plasmodium berghei in mice. School of Pharmacy, Addis Ababa University. doi:10.13140/2.1.5172.2569.
- Nair MSR, Basile DV. 1993. Bioconversion of arteannuin B to artemisinin. J Nat Prod. 56:1559–1156. doi: 10.1021/np50099a015
- Nottingham AT, Turner BL, Whitaker J, Ostle NJ, Mcnamara N, Bardgett R, Salinas N, Meir P. 2015. Soil microbial nutrient constraints along a tropical forest elevation gradient: a belowground test of a biogeochemical paradigm. Biogeosciences. 12:6071–6083. doi: 10.5194/bg-12-6071-2015
- Otienoburu PE, Ebrahimi B, Phelan PL, Foster WA. 2012. Analysis and optimization of a synthetic milkweed floral attractant for mosquitoes. J Chem Ecol. 38:873–881. doi: 10.1007/s10886-012-0150-6
- Quideau SA, Mclntosh ACS, Norris CE, Lloret E, Swallow MJB, Hannam K. 2016. Extraction and analysis of microbial phospholipid fatty acids in soils. J Visual Exp. 114:1–9.
- Ryan MF, Byrne O. 1988. Plant-insect coevolution and inhibition of acetylcholinesterase. J ChemEcol. 14:1965–1975.
- Shen H. 2006. Release route and allelopathic mechanism for allelochemicals of Artemisia annua L. Lanzhou: Ganshu Agricultural University.
- Singh D, Larisa L-C, Kim W-S, Kerfahi D, Chun J-H, Adams JM. 2014. Strong elevational trends in soil bacterial community comsition on Mt. Halla, South Korea. Soil Biol Biochem. 68:140–149. doi: 10.1016/j.soilbio.2013.09.027
- Sorensen PO, Germino MJ, Feris KP. 2013. Microbial community responses to 17 years of altered precipitation are seasonally dependent and coupled to co-varying effects of water content on vegetation and soil C. Soil Biol Biochem. 64:155–163. doi: 10.1016/j.soilbio.2013.04.014
- World Health Organization. 2010. World malaria report 2010. Geneva: WHO, 28.
- Xie G-W, Fan Y, Li X-M. 2012. Effect of elevation and preservation on artemisinin of Chongqing Artemisia annua L. J Sousthwest Chin Nor Univ, (Nat Sci Edit). 37:83–87.
- Yasir M, Azhar EI, Khan I, BiBi F, Baabdullah R, Al-Zahrani IA, Al-Ghamdi AK. 2015. Composition of soil microbiome along elevation gradients in southwestern highlands of Saudi Arabia. BMC Microbiol. 15:65–73. doi: 10.1186/s12866-015-0398-4
- Yu Z. 2010. Study on the metabolic accumulation and correlation of anti-malaria-related compounds in Artemisia annua L. (In Chinese). Chongqing: Chongqing University.
- Yuan DX. 1991. Karst of China. Beijing, People’s Republic of China: Geological Publishing House.
- Zak DR, Holmes WE, White DC, Peacock AD, Tilman D. 2003. Plant diversity, soil microbial communities, and ecosystem function: are there any links? Ecology. 84:2042–2050. doi: 10.1890/02-0433
- Zhang Z, Hu B, Hu G. 2014. Spatial heterogeneity of soil chemical properties in a subtropical karst forest, Southwest China. Sci World J. 2014: 473651. doi:10.1155/2014/473651.
- Zhang X-B, Wang L-H, Guo L-P, Wei X, Huang L-Q, Liang L-K, Sun Y-Z, Lü J-R. 2009. Analysis of the effect of topographical conditions on the artemisinin content in sweet wormwood herb in Guangxi, China. Acta Ecol Sin. 29:688–691.
- Zhao C, Miao Y, Yu C, Zhu L, Wang F, Jiang L, Hui D, Wan S. 2016. Soil microbial community composition and respiration along an experimental precipitation gradient in a semiarid steppe. Sci Rep-UK. 6:24317. doi:10.1038/srep24317.
- Zhu C, Cook SP. 2012. A concise synthesis of (+)-artemisinin. J Am Chem Soc. 134:13577–13579. doi: 10.1021/ja3061479