ABSTRACT
Citral is a mixture of neral and geranial, which are of great interest to the fragrance industry due to its lemon-scented aroma. A newly characterized nerol dehydrogenase of Persicaria minor (PmNeDH) from our recent findings has shown a capacity to convert citral from nerol. Differential gene expression analysis revealed that the expression level of PmNeDH was highly upregulated during early treatment of several stress-related phytohormones i.e. methyl jasmonate (MeJA), salicylic acid (SA) and abscisic acid (ABA). SA and ABA were shown to have a prolonged effect on PmNeDH expression level until second day of treatment. The findings were in agreement with the cis-regulatory elements predicted from the gene promoter. The phylogenetic relationship of PmNeDH with its homologs from the medium-chain dehydrogenases/reductases (MDR) superfamily was also mentioned. In this study, we proposed a possible biological function of PmNeDH gene in P. minor, which might play significant roles in plant defence mechanism.
Introduction
Citral (3,7-dimethyl-2,6-octadienal) is an isomeric mixture of two monoterpene aldehydes neral (cis) and geranial (trans) that has been widely used in the perfume and fragrance industry (Lalko and Api Citation2008; Chen and Viljoen Citation2010). Apart from its pleasant scent, citral renders many biological activities such as antioxidant, antimicrobial, antiviral and insect repellent activities (Maia and Moore Citation2011; Gilling et al. Citation2014). Naturally occurring citral in essential oils of several aromatic plants was also proposed to have allelopathic effects (Cheng and Cheng Citation2015), which can cause microtubules disruption (Chaimovitsh et al. Citation2010), reduction of intercellular communication and the growth of the root hairs (Grana et al. Citation2013). All of these bioactivity properties of citral can be essential for the resource competition and defence mechanism in plants.
Biosynthesis of citral has been well elucidated, which occurs in the first oxidative step of geraniol degradation pathway (Kanehisa and Goto Citation2000; Noge et al. Citation2008) (). Early research on the pathway was mainly based on the bioconversion products of microorganisms (Cantwell et al. Citation1978), while later attempts were focused on the characterization of the substrate specificity and kinetic properties of the natively purified and recombinant enzyme involved in citral production (Ikeda et al. Citation1991; Wolken and van der Werf Citation2001; Iijima et al. Citation2006; Noge et al. Citation2008; Hassan et al. Citation2012; Luddeke et al. Citation2012). Throughout the last decade, geraniol dehydrogenase (GeDH, EC 1.1.1.183) was the only enzyme known to produce citral via the oxidation of nerol and geraniol (Kanehisa and Goto Citation2000) (). However, there are very limited studies on the biological function and expression profile of the citral-producing enzyme under stress, particularly in plants. The GeDH from the astigmatide mite Carpoglyphus lactis was the only reported citral-producing enzyme with known biological functions, which is an important enzyme for the biosynthesis of alarm pheromone (Noge et al. Citation2008).
Figure 1. Simplified schematic diagram on geraniol degradation pathway.
Note: Thick gray lines represent the boundary of different pathway. Geraniol degradation pathway begins with the biosynthesis of geranyl-pyrophosphate (GPP) catalysed by geraniol synthase. It was then followed by a two-step oxidation reaction which oxidizes geraniol into geranial and geranial into geranic acid via GeDH and geranial dehydrogenase respectively. Subsequently, geranic acid is activated to the corresponding CoA ester trans-geranyl-CoA, which serves as a convergence point for both geraniol and citronellol pathway. Although geraniol was generally considered to be the major precursor for geraniol degradation pathway, nerol has also been found to act as the alternative precursor for geranial production through oxidation and isomerization process (Zhang et al. Citation2013).
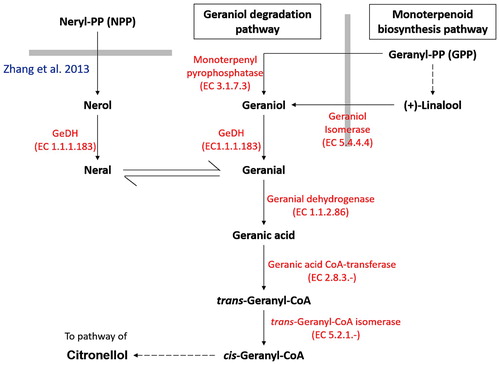
Recently, a newly identified gene from Persicaria minor, which encodes for the nerol dehydrogenase (PmNeDH), was isolated. Kinetic analyses using recombinant PmNeDH revealed that the protein can oxidize nerol with the highest affinity to produce citral (Tan et al. Citation2018). Meanwhile, geraniol has been found to be oxidized by recombinant PmNeDH at a much lower activity. In this work, hormonal regulations of PmNeDH gene expression in P. minor were investigated. In addition, in silico analysis of the promoter region of the PmNeDH gene was also presented in this paper to consolidate the relationship between the gene expression and the plant growth regulators.
Materials and methods
Plant materials
The plants of P. minor from Ulu Yam, Selangor, Malaysia which maintained in an experimental field of the Institute of Systems Biology, Universiti Kebangsaan Malaysia (UKM) were micropropagated in Murashige & Skoog (MS) agar medium. The in vitro plants were grown for 2 months before being harvested and frozen in liquid nitrogen for DNA and RNA extractions.
DNA and RNA extractions
Genomic DNA was extracted from the leaves of P. minor with the modified cetyltrimethylammonium bromide (CTAB) method (Doyle Citation1990). For DNA extraction, roughly 0.3 g of P. minor leaves were ground into fine powder in liquid nitrogen. The powder was mixed with 1 mL of CTAB extraction buffer (2% w/v CTAB, 100 mM Tris pH 8.0, 20 mM EDTA pH 8.0, 1.4 M NaCl) and incubated at 65°C for 45 min with a gentle inversion in 5 min intervals. The mixture was centrifuged with 13,000 rpm for 5 min at 4°C. The supernatant was transferred into a new 2 mL centrifuge tube and an equal volume of ice-cold chloroform: isoamyl (24:1) was added. The mixture was gently mixed for 1 min and centrifuged with 13,000 rpm for 10 min at 4°C. The aqueous phase was transferred into a new 2 mL centrifuge tube. A two-third volume of cold isopropanol was added. The mixture was gently inverted 20–30 times before being incubated overnight at −20°C. On the next day, the precipitated DNA sample was centrifuged with 13,000 rpm for 10 min at 4°C and the supernatant was discarded. The DNA pellet was washed with a washing buffer (70% ethanol, 10 mM ammonium acetate) to remove excessive salts. Again, the mixture was centrifuged with 13,000 rpm for 10 min at 4°C to discard the supernatant. The DNA obtained was dissolved in TE buffer and stored at −20°C.
Total RNA was isolated from 0.1 g of P. minor roots using PureLink™ Plant RNA Reagent (Ambion) according to the manufacturer’s protocols. The RNA sample was dissolved in diethylpyrocarbonate (DEPC)-treated water. The RNA was kept at −80°C until use. DNA and RNA samples were quantified using Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies, USA) and its integrity was assessed by 1.0% agarose gel electrophoresis.
Isolation of gene and promoter of PmNeDH
The isolation of PmNeDH gene was conducted using Pfu DNA polymerase (Thermo Scientific) with primer pair GeBspHI-f: 5′-GAT CAT GAT GGA AAT GGG CAA CGG AAC G-3′ and GeSalI-r: 5′-GAG TCG ACT TCA GAG CTT ATT AAA GAG TC-3′. The gene specific primers were designed based on the cDNA sequence of PmNeDH (GenBank accession no. JX185716). The PCR program began with early denaturation at 95°C for 1 min followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 63°C for 30 s and elongation at 72°C for 2 min. Final elongation was performed at 72°C for 10 min.
The promoter of PmNeDH was isolated using Clontech Universal GenomeWalker™ Kit as described by the manufacturer. A total of seven GenomeWalker libraries were generated using seven restriction enzymes as follows AluI, StuI, HpaI, SspI, PvuII, EcoRV and DraI. Each library was digested by a single restriction enzyme. The initial specific primer GSP1 (5′-CTG GAG AAA GTG AAA GGG GAG AGG TG-3′) was designed on the basis of the first exon of the previously isolated PmNeDH gene sequence, whereas a nested primer GSP2 (5′-TAC TGT GTG TTC TTC CGT TCC GTT GC-3′) was designed upstream of GSP1. Primary touchdown PCR using GSP1 and an adaptor primer AP1 (provided by the kit) was performed with early denaturation at 94°C for 3 min, followed by denaturation at 94°C for 25 s and annealing stage for 3 min where the annealing temperature gradually reduced at a rate of 1°C for every 2 cycling programs from 67°C to 64°C. The touchdown PCR was further amplified by 30 cycles at 94°C for 25 s and 62°C for 3 min, followed by a final extension at 62°C for 7 min.
Secondary PCR was conducted using nested primer GSP2 and an adaptor primer AP2 (provided by kit), where a 50 × diluted primary PCR product was used as a template. Amplification started with an early denaturation at 94°C for 3 min, 5 cycles of 94°C for 25 s and 68°C for 3 min, followed by 20 cycles at 94°C for 25 s and 64°C for 3 min and a final elongation at 64°C for 7 min. Gel electrophoresis was performed to analyse all PCR products on 1.5% agarose. The gene sequence including the promoter was deposited to NCBI with accession number MF098772.
Stress treatments for PmNeDH expression analysis via RT–PCR
The gene expression pattern of PmNeDH in response to phytohormones treatments were examined using the roots of P. minor. The phytohormones used in the experiments were MeJA (150 µM), SA (150 µM) and ABA (100 µM). In vitro cultures of P. minor were acclimatized in an MS liquid medium under 16 h/8 h day/night regime at 24 ± 2°C. The MS liquid medium was renewed after 24 h of acclimatization of the plantlets, where the phytohormones were added to start the treatment. MS liquid medium without phytohormone was used as the control. Roots of treated plantlets were harvested at 0, 3, 6, 12, 24 and 48 h after the treatment. The total RNA was isolated as mentioned above and treated with DNase from Turbo DNA-free Kit (Ambion). RevertAid cDNA Synthesis Kit (Thermo Scientific) was used to synthesize the first strand of cDNA from the extracted total RNA.
Reverse transcription PCR (RT–PCR) reactions were performed using MyTaq™ Red Mix (BIOLINE). The PmNeDH transcript was amplified with gene specific primers NeGSP-F (5′-CAC TTC GCC TGC CAA GAA GGA GGA C-3′) and NeGSP-R (5′-TCA AGG GAG GCA GAG GGT GAG AAG C-3′). RT–PCR was done under such cycling conditions: 1 min at 95°C, 30 cycles of denaturation at 95°C for 15 s, annealing at 55°C for 30 s and elongation at 72°C for 20 s. Final elongation was carried out for 5 min at 72°C. The Cyclophilin (CyP) gene was selected as the internal control, which was amplified under the same conditions as PmNeDH except that it was carried out for 26 cycles using the following primers: Cyc-F (5′-TCT ATG AAT TCA CCG TTT TGC TTC TGT T-3′) and Cyc-R (5′- TCC GCT TGT TGT TAA CGA TTA CAT ATC-3′).
Bioinformatic analysis
Comparative sequence analysis of PmNeDH was performed using NCBI BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Conserved domains were identified with the NCBI Batch CD-Search Tool (Marchler-Bauer et al. Citation2011). Alignment of multiple protein sequences was conducted with ClustalW2 using default parameters (Larkin et al. Citation2007). The promoter and the gene sequences of PmNeDH were assembled using BioEdit version 7.0.9.0 (Hall Citation1999). The Plant Promoter Analysis Navigator (PlantPAN 2.0) was used to search for transcription binding sites and important regulatory elements on the PmNeDH promoter sequence (Chow et al. Citation2016). A phylogenetic tree was drawn by the Neighbour-Joining method with 1000 replicates; bootstrap values for each node were calculated using MEGA 4 software (Tamura et al. Citation2007). All sequences used in the phylogenetic analysis and intron-exon comparison were retrieved from the NCBI GenBank database (http://www.ncbi.nlm.nih.gov/nucleotide/). For semi-quantification analysis of RT–PCR, the software ImageJ was used to quantify visual results (Schneider et al. Citation2012).
Results
Phylogenetic analysis
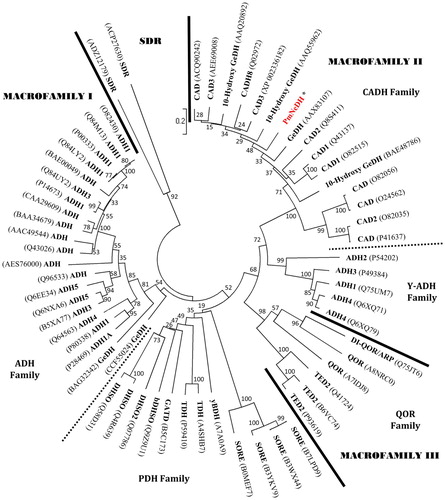
Phylogenetic relationships of PmNeDH and the protein members of the MDR superfamily were analyzed by the Neighbour-Joining method. Taxonomic categories of the proteins were assigned based on the classification of Riveros-Rosas et al. (Citation2003). A total of 59 nonredundant protein sequences (allelic forms excluded) of MDR superfamily were retrieved from GenBank nonredundant protein sequence database at the NCBI. Two sequences of short-chain dehydrogenase/reductase (SDR) from Oncidium hybrid cultivar and Riemerella anatipestifer were used as outgroup. Phylogenetic analysis was performed on the basis of 1000 replicates. The phylogenetic tree was clustered into three major macrofamilies and five monophyletic protein families (i.e. alcohol dehydrogenase family, ADH; yeast-ADH family, Y-ADH; cinnamyl alcohol dehydrogenase (CADH) family; polyol dehydrogenase family, PDH; quinone oxidoreductase family, QOR) (). Each monophyletic group was supported with high bootstrap value. The PmNeDH was located in the CADH family under macrofamily II, which is consistent with the prediction of the conserved domain search of NCBI. As expected, the PmNeDH and obGeDH (AAX83107) were located in the same family and closely related to other CADHs. However, the GeDH proteins of C. lactis (BAG32342) and Castellaniella defragrans (CCF55024) surprisingly more resembled the classical ADHs in the macrofamily I, even though they can use terpene alcohols as substrates such as geraniol. Similar results were obtained using the Minimum Evolution method.
Figure 2. The phylogenetic tree was constructed with the PmNeDH and other protein sequences that belong to the MDR superfamily by Neighbour-Joining method.
Note: The accession number of each sequence is shown in bracket. The scale of the distance is indicated. The values at the nodes represent the percentage of bootstrap confidence level using 1000 random bootstrap replicates. The tree is separated into three protein macrofamilies and one outgroup, SDR, by solid bars or main cluster of protein families by square dot bars.
Exon-Intron structure of PmNeDH gene
The intron pattern of PmNeDH and several plant ADH genes were presented in Figure S3. All selected genes were retrieved from the GenBank database at the NCBI, where information of their introns and exons were provided. Introns of each gene were numbered according to their equivalent positions in the standard plant ADH pattern of nine introns (Strommer Citation2011). Pinus banksiana ADH gene (U48376) has the highest number of introns among the selected genes, where all nine introns were retained (Figure S3). However, the ADH2 gene of Hordeum vulgare (barley) appeared to lack intron IX. The selected CADH and mannitol alcohol dehydrogenase (MAD) genes generally showed a lack of intron III, IV, V, VII and IX, except that the intron IV was found in the CADH gene of A. thaliana (NC_003075). The PmNeDH gene only comprised of three introns and four exons, which had the least number of introns compared to other selected genes. Intron I, II and VIII were retained in the PmNeDH gene similar to other selected CADH genes, but intron VI was not found in the PmNeDH gene sequence.
In silico promoter analysis
The promoter of PmNeDH with the length of 1169 bp was successfully isolated using genome walking PCR based technique (Figure S1). The putative transcription start site (TSS), which is marked as +1 was found 49 nucleotides upstream of the start codon. TATA box was predicted 44 nucleotides upstream of TSS. Computational analysis of promoter with PlanCARE program for the prediction of cis-acting elements revealed that the majority of the motifs were involved in the plant developmental process and defence mechanism (Figure S2). To gain insight into the possible role of this gene in the response to P. minor towards external stimuli, stress-related motifs as predicted on the promoter sequence are listed in .
Table 1. Cis-acting elements related to stresses and stress-related phytohormones predicted on positive strand promoter of PmNeDH gene.
Expression analysis of PmNeDH during phytohormone treatments
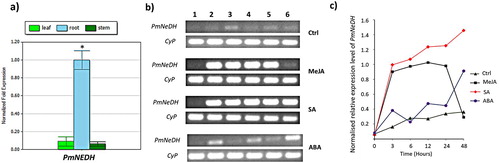
The expression patterns of PmNeDH gene in P. minor over the course of treatments were measured using RT–PCR. The roots of the treated plantlets were used for the expression analysis as the basal expression of PmNeDH was highest in roots compared to leaves and stems as confirmed by quantitative real-time PCR ((a)). In general, the expression of PmNeDH was positively regulated by all the treatments ((a & b)). The transcript level of PmNeDH significantly increased towards MeJA and SA as early as 3 h of treatment. PmNeDH gene expression peaked at 12 h of MeJA treatment and gradually dropped afterwards, where the PmNeDH expression was restored back to its basal level during prolonged treatment up to 48 h. Unlike MeJA treatment, PmNeDH was found not only highly responsive towards early treatment of SA, but also consecutively expressed with an increasing level until 48 h of observation period. Conversely, PmNeDH was shown to be weakly induced by ABA with an up–down sequential fluctuation but an overall incrementing trend.
Figure 3. The relative expression level of PmNeDH gene. (a) Expression analysis of PmNeDH in different tissues of P. minor via qRT-PCR. P. minor plants used in this experiment were obtained from experimental field (3°16′14.63″”N, 101°41′11.32″”E). The transcript levels of PmNeDH are presented relative to the amount of β-actin and Tubulin. The data are shown as the mean ± SE (n = 3), in arbitrary units. *, P < 0.05 represents the significant difference as analyzed by Tukey’s test. (b) The RT-PCR products for the PmNeDH and the housekeeping gene CyP on 1.5% of agarose gel. The period of treatment was numbered on top, where 1: 0 h; 2: 3 h; 3: 6 h; 4:12 h; 5: 24 h; 6: 48 h. (c) Semi-quantitative analysis of PmNeDH gene expression after the treatment of phytohormones.
Discussion
Previously, the recombinant PmNeDH was structurally and kinetically characterized, which showed that it was dimeric in nature and had a higher affinity towards nerol (cis-configured 3,7-dimethyl-2,6-octadien-1-ol) than geraniol (trans) (Tan et al. Citation2018). Our findings on the spatial expression of PmNeDH in different tissues of P. minor obtained from the experimental plot revealed that the transcript level of PmNeDH was highest in roots than other aboveground tissues such as leaves and stems ((a)). This might be due to the more direct and larger surface area of contact between the root system with soil-borne pathogens and insect community in its immediate vicinity. Root exudation, including citral, to the densely populated rhizosphere was reported to be crucial for the survival of plants by exerting its negative allelopathic effect against other competitors (Muzell Trezzi et al. Citation2016) or under challenges from bacteria, fungi and insects in soil (Walker et al. Citation2003).
Many citral production-related genes in plants have been reported in term of their kinetic properties (Potty and Bruemmer Citation1970; Singh Sangwan et al. Citation1993; Sekiwa-Iijima et al. Citation2001; Iijima et al. Citation2006), but their biological function remains largely unexplored. Exogenous application of phytohormone on plants can be an effective approach to mimic and investigate plant responses towards biotic and abiotic stresses. Several indicative regulatory motifs identified from the promoter sequence of PmNeDH, for example MYB, MYC, WRKY and ERF/AP2 (), provide clues to postulate the possible biological function of PmNeDH. Transcription factors for the mentioned regulatory motifs are unexceptionally implicated in the phytohormone-mediated signaling of plant defence mechanism (Gutterson and Reuber Citation2004; Bari and Jones Citation2009; Seo and Park Citation2010; Kazan and Manners Citation2013), or to some extent, involved in the abiotic stress such as MYB (Ambawat et al. Citation2013). MeJA and SA are important phytohormones in regulating the expression of defence-related genes against the invasion of pathogens and pests which involved systemic acquired resistance (SAR) (Bari and Jones Citation2009; Deng et al. Citation2013; Zhu et al. Citation2014), while ABA is an essential endogenous messenger for plants to respond to abiotic stress resulting from drought, wounding, cold and salt (Tuteja Citation2007; Sah et al. Citation2016). Three of these phytohormones were therefore selected for PmNeDH expression analysis.
Our expression analyses showed that SA and MeJA were effective elicitors that have activated the expression of PmNeDH. On the other hand, poor response of PmNeDH towards ABA treatment implied that the role of PmNeDH in abiotic stress tolerance was relatively minor. ABA signals mediated by MYB96 transcription factor in Arabidopsis was nonetheless reported to promote the biosynthesis of SA which in turn enhanced the pathogen resistance of the plant. Thus, the MYB96 was enrolled in the cross-talking between the ABA-SA signaling (Seo and Park Citation2010). The observed expression pattern of PmNeDH in response to SA, MeJA and ABA is consistent with that of another MDR gene member i.e. AdZADH2 from Arachis diogoi. It has been reported that the transcript level of AdZADH2 rapidly accumulates within 3 h of SA and MeJA treatment, which is earlier than that observed under ABA treatment (Kumar et al. Citation2016). The same study also revealed that the transcript level of AdZADH2 under SA and MeJA treatment were relatively higher than that under ABA treatment at all time points. The highly similar expression pattern of PmNeDH and AdZADH2 under SA and MeJA treatment indicated that similar regulatory interaction networks may exist in plant defence signalling pathway. From the study of Schenk et al. (Citation2000), they showed 55 genes were co-induced by SA and MeJA and this contradict the theory of antagonistic effect between SA and MeJA.
PmNeDH is a member of the MDR superfamily, which is a large group of oxidoreductases with over 15000 members from all types of organisms (Consortium Citation2007). Many studies have been conducted since the last decade to classify the members of the MDR superfamily into different families on the basis of their phylogenetic relationship, functional and sequence similarities (Nordling et al. Citation2002; Riveros-Rosas et al. Citation2003; Hedlund et al. Citation2010). In this study, PmNeDH was classified into CADH family under macrofamily II. According to Riveros-Rosas et al. (Citation2003), CADH family consists of NADP(H)-dependent Zn-type oxidoreductases, which are functionally related to lignin biosynthesis, defence activities and wound response. The CADH genes from the tea plant (Cammellia sinensis) classified under CADH family were suggested to play a role in defence against pathogens and herbivorous insects (Deng et al. Citation2013). This classification was consistent with the proposed biological function of PmNeDH involved in defence mechanism of P. minor as well as its catalytic property that used NADP+ for its alcohol oxidation reactions (Tan et al. Citation2018). Unlike PmNeDH, NAD+ was the sole coenzyme for the oxidation activities of GeDHs from C. defragrans and C. lactis (Noge et al. Citation2008; Luddeke et al. Citation2012). Apart from the coenzyme requirement, sequence similarity of PmNeDH was higher to members in CADH family (∼70%) than the GeDHs from C. defragrans and C. lactis in ADH family (∼20%). Low sequence conservation suggests that PmNeDH and GeDH from plants are likely to evolve convergently and independently from the bacterial and animal GeDHs, which eventually gain the similar functionality of oxidizing the 3,7-dimethyl-2,6-octadien-1-ol.
The specific nerol-oxidizing activity of PmNeDH as a result of its highest affinity towards nerol was different from the previously reported P. minor GeDH isoenzymes (PmGeDH), which have the highest preference to use geraniol as substrate and produce geranial as its product (Hassan et al. Citation2012). Due to their highly related role in P. minor, PmNeDH can be regarded as a paralogous protein of PmGeDHs that probably arose from a gene duplication event but eventually received a new function that was specific to nerol. Multiple sequence alignment revealed that PmNeDH has the lowest intron number among the compared ADHs from plants (Figure S3). According to Strommer (Citation2011), the ancestral number of introns in plant ADH genes is presumed to be nine where the intron positions in ADH loci are generally conserved. The PmNeDH only retained three out of nine introns in its gene sequence. The loss of individual introns is thought to be caused by a double-strand break repair (Hu Citation2006). Since reinsertion of the introns at the same sequence position where they were lost is nearly impossible, the genes that retain a fuller complement of introns are considered resemble more the ancestral structure. Hence, we propose that the PmNeDH gene in P. minor may acquire its nerol-specific activity in a more recent evolutionary process.
In essence, the positive response of PmNeDH towards biotic stress signaling suggests its involvement in the defence mechanism of P. minor. The prediction was in agreement with the defence-related motifs found in the promoter sequence of PmNeDH. However, the expression level of defence-related transcription factors in association with the defence performance of the treated plants merits further analysis to strengthen the presumption of PmNeDH biological function. In this study, we provide new insight into the possible biological function of the citral-producing genes and their relationships with other homologs from MDR superfamily.
Acknowledgement
This project was financially supported by grants DIP-2016-016 from the Universiti Kebangsaan Malaysia awarded to Zamri Zainal. We also would like to take this opportunity to thanks to the Ministry of Education Malaysia (MOE) for awarding the MyPhD scholarship under the program of MyBrain15 to one of the authors (Dr. Tan Cheng Seng).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Cheng Seng Tan
Cheng Seng Tan was a postdoctoral researcher at the Faculty of Science and Technology, Universiti Kebangsaan Malaysia (UKM). His research was focused mainly on the understanding of substrate specificity of nerol dehydrogenase, a broad substrate range oxidoreductase from plants, via structural-guided mutagenesis studies. He is currently engaging in the development of agarwood inducer by integrating the existing knowledge of secondary metabolic pathways involved in the agarwood resin biosynthesis.
Nur-Athirah Abd-Hamid
Nur-Athirah Abd-Hamid is a PhD student of Institute of Systems Biology at Universiti Kebangsaan Malaysia (UKM). Previously, she focused on the molecular characterization of ADH gene in plant. Currently, she is active in the F-box protein study in plant which involved protein-protein interaction.
Jin Kiat Chew
Jin Kiat Chew (PhD) was a postdoctoral researcher at Universiti Kebangsaan Malaysia (UKM) in Institute of Systems Biology. His research was mainly focused on understanding the transcriptional regulatory mechanism involved in the modulation of plant secondary metabolism as well as plant response mechanism against environmental stresses. Currently, he is actively engaged in the research of crop improvement through genetic engineering.
Maizom Hassan
Maizom Hassan (PhD) is a research fellow in Institute of Systems Biology at Universiti Kebangsaan Malaysia (UKM). Her research interests are enzymology and proteomics and integrated pest management, which focuses on developing novel and potential bio-insecticides against several Lepidoptera pests, including Plutella xylostella (diamondback moth), Metisa plana (bagworm), Conopomorpha cramerella (Snellen) (cocoa pod borer) and Chrysodeixis sp.
Ismanizan Ismail
Ismanizan Ismail (PhD) is a Professor of Plant Cell Biotechnology at the Centre for Biotechnology and Functional Food, Faculty of Science and Technology, Universiti Kebangsaan Malaysia. He obtained his PhD degree from the University of Edinburgh in 1997, working on the glyoxylate cycle in plant cells. He has vast experiences working plant cells especially involving techniques in plant molecular cell biotechnology and tissue culture systems. His research interests focus on the molecular event in plant stresses and plant secondary metabolism using model plant system. He is now working on the involvement of the non-coding genome sequence or particularly the miRNA in controlling the gene involves in plant stresses. The regulatory roles of miRNA in plant stresses has aspired him to explore further miRNA-transcription factor co-expression networking in order to understand the plant metabolite biosynthesis and for possible manipulation.
Chyan Leong Ng
Chyan Leong Ng (PhD) is a research fellow in Institute of Systems Biology at Universiti Kebangsaan Malaysia. His research interests focus on the structural and functional studies of biologically important macromolecules and pathways with a particular interest on secondary metabolites biosynthesis, microbial pathogenesis and human disease-associated proteins.
Zamri Zainal
Zamri Zainal (PhD) is a Professor of Centre for Biotechnology and Functional Food, Faculty of Science and Technology, Universiti Kebangsaan Malaysia. He is also appointed as a principle research fellow at Institute of System Biology, UKM. His research interests are on the understanding plant responses towards stresses and genetic enhancement of crops through the application of latest tools and techniques of molecular genetics.
References
- Ambawat S, Sharma P, Yadav NR, Yadav RC. 2013. MYB transcription factor genes as regulators for plant responses: an overview. Physiol Mol Biol Plants. 19:307–321. doi: 10.1007/s12298-013-0179-1
- Bari R, Jones JD. 2009. Role of plant hormones in plant defence responses. Plant Mol Biol. 69:473–488. doi: 10.1007/s11103-008-9435-0
- Cantwell SG, Lau EP, Watt DS, Fall RR. 1978. Biodegradation of acyclic isoprenoids by Pseudomonas species. J Bacteriol. 135:324–333.
- Chaimovitsh D, Abu-Abied M, Belausov E, Rubin B, Dudai N, Sadot E. 2010. Microtubules are an intracellular target of the plant terpene citral. Plant J. 61:399–408. doi: 10.1111/j.1365-313X.2009.04063.x
- Chen W, Viljoen AM. 2010. Geraniol — A review of a commercially important fragrance material. S Afr J Bot. 76:643–651. doi: 10.1016/j.sajb.2010.05.008
- Cheng F, Cheng Z. 2015. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front Plant Sci. 6:1020.
- Chow C-N, Zheng H-Q, Wu N-Y, Chien C-H, Huang H-D, Lee T-Y, Chiang-Hsieh Y-F, Hou P-F, Yang T-Y, Chang W-C. 2016. PlantPAN 2.0: an update of plant promoter analysis navigator for reconstructing transcriptional regulatory networks in plants. Nucleic Acids Res. 44:D1154–D1160. doi: 10.1093/nar/gkv1035
- Consortium TU. 2007. The universal protein resource (UniProt). Nucleic Acids Res. 36:D190–D195. doi: 10.1093/nar/gkm895
- Deng WW, Zhang M, Wu JQ, Jiang ZZ, Tang L, Li YY, Wei CL, Jiang CJ, Wan XC. 2013. Molecular cloning, functional analysis of three cinnamyl alcohol dehydrogenase (CAD) genes in the leaves of tea plant, Camellia sinensis. J Plant Physiol. 170:272–282. doi: 10.1016/j.jplph.2012.10.010
- Doyle JJ. 1990. Isolation of plant DNA from fresh tissue. Focus. 12:13–15.
- Gilling DH, Kitajima M, Torrey JR, Bright KR, Schaffner DW. 2014. Mechanisms of antiviral action of plant antimicrobials against murine norovirus. Appl Environ Microbiol. 80:4898–4910. doi: 10.1128/AEM.00402-14
- Grana E, Sotelo T, Diaz-Tielas C, Araniti F, Krasuska U, Bogatek R, Reigosa MJ, Sanchez-Moreiras AM. 2013. Citral induces auxin and ethylene-mediated malformations and arrests cell division in Arabidopsis thaliana roots. J Chem Ecol. 39:271–282. doi: 10.1007/s10886-013-0250-y
- Gutterson N, Reuber TL. 2004. Regulation of disease resistance pathways by AP2/ERF transcription factors. Curr Opin Plant Biol. 7:465–471. doi: 10.1016/j.pbi.2004.04.007
- Hall TA. {BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT}. In ‘Nucleic acids symposium series’, 1999, pp. 95–98.
- Hassan M, Maarof ND, Ali ZM, Noor NM, Othman R, Mori N. 2012. Monoterpene alcohol metabolism: identification, purification, and characterization of two geraniol dehydrogenase isoenzymes from Polygonum minus leaves. Biosci Biotechnol Biochem. 76:1463–1470. doi: 10.1271/bbb.120137
- Hedlund J, Jörnvall H, Persson B. 2010. Subdivision of the MDR superfamily of medium-chain dehydrogenases/reductases through iterative hidden Markov model refinement. BMC Bioinformatics. 11:534–534. doi: 10.1186/1471-2105-11-534
- Hu K. 2006. Intron exclusion and the mystery of intron loss. FEBS Lett. 580:6361–6365. doi: 10.1016/j.febslet.2006.10.048
- Iijima Y, Wang G, Fridman E, Pichersky E. 2006. Analysis of the enzymatic formation of citral in the glands of sweet basil. Arch Biochem Biophys. 448:141–149. doi: 10.1016/j.abb.2005.07.026
- Ikeda H, Esaki N, Nakai S, Hashimoto K, Uesato S, Soda K, Fujita T. 1991. Acyclic monoterpene primary alcohol:NADP+ oxidoreductase of Rauwolfia serpentina cells: the key enzyme in biosynthesis of monoterpene alcohols. J Biochem. 109:341–347.
- Kanehisa M, Goto S. 2000. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30. doi: 10.1093/nar/28.1.27
- Kazan K, Manners JM. 2013. MYC2: the master in action. Mol Plant. 6:686–703. doi: 10.1093/mp/sss128
- Kumar D, Rampuria S, Singh NK, Kirti PB. 2016. A novel zinc-binding alcohol dehydrogenase 2 from Arachis diogoi, expressed in resistance responses against late leaf spot pathogen, induces cell death when transexpressed in tobacco. FEBS Open Bio. 6:200–210. doi: 10.1002/2211-5463.12040
- Lalko J, Api AM. 2008. Citral: identifying a threshold for induction of dermal sensitization. Regul Toxicol Pharmacol. 52:62–73. doi: 10.1016/j.yrtph.2008.01.006
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. 2007. Clustal w and Clustal X version 2.0. Bioinformatics. 23:2947–2948. doi: 10.1093/bioinformatics/btm404
- Luddeke F, Wulfing A, Timke M, Germer F, Weber J, Dikfidan A, Rahnfeld T, Linder D, Meyerdierks A, Harder J. 2012. Geraniol and geranial dehydrogenases induced in anaerobic monoterpene degradation by Castellaniella defragrans. Appl Environ Microbiol. 78:2128–2136. doi: 10.1128/AEM.07226-11
- Maia MF, Moore SJ. 2011. Plant-based insect repellents: a review of their efficacy, development and testing. Malar J. 10:S11–S11. doi: 10.1186/1475-2875-10-S1-S11
- Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, et al. 2011. CDD: a conserved domain database for the functional annotation of proteins. Nucleic Acids Res. 39:D225–D229. doi: 10.1093/nar/gkq1189
- Muzell Trezzi M, Vidal RA, Balbinot Junior AA, von Hertwig Bittencourt H, da Silva Souza Filho AP. 2016. Allelopathy: driving mechanisms governing its activity in agriculture. J Plant Interact. 11:53–60. doi: 10.1080/17429145.2016.1159342
- Noge K, Kato M, Mori N, Kataoka M, Tanaka C, Yamasue Y, Nishida R, Kuwahara Y. 2008. Geraniol dehydrogenase, the key enzyme in biosynthesis of the alarm pheromone, from the astigmatid mite Carpoglyphus lactis (Acari: Carpoglyphidae). Febs j. 275:2807–2817. doi: 10.1111/j.1742-4658.2008.06421.x
- Nordling E, Jornvall H, Persson B. 2002. Medium-chain dehydrogenases/reductases (MDR). Family characterizations including genome comparisons and active site modeling. Eur J Biochem. 269:4267–4276. doi: 10.1046/j.1432-1033.2002.03114.x
- Potty VH, Bruemmer JH. 1970. Oxidation of geraniol by an enzyme system from orange. Phytochemistry. 9:1003–1007. doi: 10.1016/S0031-9422(00)85218-8
- Riveros-Rosas H, Julian-Sanchez A, Villalobos-Molina R, Pardo JP, Pina E. 2003. Diversity, taxonomy and evolution of medium-chain dehydrogenase/reductase superfamily. Eur J Biochem. 270:3309–3334. doi: 10.1046/j.1432-1033.2003.03704.x
- Sah SK, Reddy KR, Li J. 2016. Abscisic acid and abiotic stress tolerance in crop plants. Front Plant Sci. 7:571. doi: 10.3389/fpls.2016.00571
- Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, Manners JM. 2000. Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci U S A. 97:11655–11660. doi: 10.1073/pnas.97.21.11655
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 9:671–675. doi: 10.1038/nmeth.2089
- Sekiwa-Iijima Y, Aizawa Y, Kubota K. 2001. Geraniol dehydrogenase activity related to aroma formation in ginger (Zingiber officinale Roscoe). J Agric Food Chem. 49:5902–5906. doi: 10.1021/jf0102970
- Seo PJ, Park CM. 2010. MYB96-mediated abscisic acid signals induce pathogen resistance response by promoting salicylic acid biosynthesis in Arabidopsis. New Phytol. 186:471–483. doi: 10.1111/j.1469-8137.2010.03183.x
- Singh Sangwan R, Singh-Sangwan N, Luthra R. 1993. Metabolism of acyclic monoterpenes: partial purification and properties of geraniol dehydrogenase from Lemongrass (Cymbopogon flexuosus Stapf.) leaves. J Plant Physiol. 142:129–134. doi: 10.1016/S0176-1617(11)80952-1
- Strommer J. 2011. The plant ADH gene family. Plant J. 66:128–142. doi: 10.1111/j.1365-313X.2010.04458.x
- Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: molecular evolutionary Genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 24:1596–1599. doi: 10.1093/molbev/msm092
- Tan CS, Hassan M, Mohamed Hussein ZA, Ismail I, Ho KL, Ng CL, Zainal Z. 2018. Structural and kinetic studies of a novel nerol dehydrogenase from Persicaria minor, a nerol-specific enzyme for citral biosynthesis. Plant Physiol Biochem. 123:359–368. doi: 10.1016/j.plaphy.2017.12.033
- Tuteja N. 2007. Abscisic acid and abiotic stress signaling. Plant Signal Behav. 2:135–138. doi: 10.4161/psb.2.3.4156
- Walker TS, Bais HP, Grotewold E, Vivanco JM. 2003. Root exudation and rhizosphere biology. Plant Physiol. 132:44–51. doi: 10.1104/pp.102.019661
- Wolken WA, van der Werf MJ. 2001. Geraniol biotransformation-pathway in spores of Penicillium digitatum. Appl Microbiol Biotechnol. 57:731–737. doi: 10.1007/s002530100821
- Zhang M, Liu J, Li K, Yu D, Liu J-H. 2013. Identification and characterization of a novel monoterpene synthase from soybean restricted to neryl diphosphate precursor. PLoS One. 8:e75972. doi: 10.1371/journal.pone.0075972
- Zhu F, Xi DH, Yuan S, Xu F, Zhang DW, Lin HH. 2014. Salicylic acid and jasmonic acid are essential for systemic resistance against tobacco mosaic virus in Nicotiana benthamiana. Mol Plant Microbe Interact. 27:567–577. doi: 10.1094/MPMI-11-13-0349-R
