?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A large number of studies have explored the effects of various nanoparticles (NPs) on different economically important plant species. In this study, yellow medick plants were grown for five weeks using hydroponics with the addition of Fe3O4 NPs at 1, 2 and 4 mg/L. Plant morphology, chlorophyll a, genotoxicity and expression of miR159c, one of the most important plant miRNA that is involved in plant response to fungal infections, were investigated. The results indicated that Fe3O4 NPs significantly increased plant root length (9%–32%), chlorophyll a fluorescence (1.94–2.8-fold), miRNA expression (0.31–0.42-fold), induced genotoxicity and reduced genome stability (12.5%–13.3%), compared to those of the control. The study demonstrated that Fe3O4 NPs simultaneously induce genome instability in yellow medick and increase expression of miR159c. Therefore, Fe3O4 NPs can be used to increase plant resistance to fungal diseases, such as powdery mildew.
1. Introduction
The growing population and subsequent increasing food requirements are important problems globally due to the resulting deterioration of the environment and decreasing crop productivity (Miao et al. Citation2015). Plants are incapable of changing location to avoid environmental changes; therefore, various abiotic stresses may influence plant growth, development, and productivity (Qu et al. Citation2016). These stresses can alter the morphological, physiological, molecular, and biochemical responses in plants (Song et al. Citation2012; Gehan et al. Citation2015). This occurs because, under abiotic stress conditions, plant metabolism is disturbed which leads to the reorganization of the metabolic network to retain its most important processes and allow it to acclimatize in a changed environment (Obata and Fernie Citation2012). Investigations have shown that hundreds of plant genes are regulated differently in response to abiotic stresses which are related to transcription factors, epigenetic modifications, plant hormones, noncoding RNAs, etc. (Wang et al. Citation2003; Miao et al. Citation2015; Laloum et al. Citation2018).
MicroRNAs (miRNAs), a class of endogenous single-stranded small noncoding RNAs, are found in all eukaryotic organisms (Li et al. Citation2016). They play a crucial role in the post-transcriptional regulation of gene expression by binding to complementary sites in their target mRNAs (Desvignes et al. Citation2015; Zhang et al. Citation2018). MiRNAs regulate all biological processes in both plants and animals, including responses to abiotic stresses (Barciszewska-Pacak et al. Citation2015; Budak and Akpinar Citation2015). Therefore, this field of science has been actively studied (Okamura et al. Citation2013; Liu et al. Citation2015; Gismondi et al. Citation2017; Kashani et al. Citation2019). MiR159 is an attractive miRNA with its role in several plant response mechanisms such as drought, hypoxia, fungal infection and nanoparticle (NP) stress (Kantar et al. Citation2011; Khraiwesh et al. Citation2012; Plaksenkova et al. Citation2019).
Also known as yellow medick, lucerna, or alfalfa, Medicago falcata L. (M. falcata) is a perennial leguminous plant grown and cultivated worldwide (Liu et al. Citation2015). M. falcata is an economically and ecologically important legume herbage. As a legume, M. falcata is capable of fixing atmospheric nitrogen through symbiotic relations with rhizobia (Graham and Vance Citation2003; Miao et al. Citation2015). This ability together with having multiple ploidy levels promotes a strong tolerance against several abiotic stressors such as drought, cold, soil infertility, salt, and heavy metals (Zhang et al. Citation2011; Liu et al. Citation2015). Moreover, as yellow medick belonging to the genus Medicago has the ability to remove heavy metals from soil through the hyperaccumulation of heavy metals in its tissue, it can grow well in polluted soil (Amer et al. Citation2013; Panchenko et al. Citation2017).
Nanoparticles have unique properties and, therefore, inorganic NPs can be extensively used in industrial, agricultural, and consumer products, and medical applications (Schaumann et al. Citation2015; McGillicuddy et al. Citation2017). This results in direct or indirect emission of NPs into the environment. Indirect emissions are possible via wastewater treatment plants, the application of biosolids, etc. (Bundschuh et al. Citation2018). The largest emission of NPs was estimated globally to be in soil and landfills followed by aquatic environments and the air (Keller et al. Citation2013). The presence of NPs in the environment raises question about environmental contamination by NPs and the risk of adversely effecting communities, ecosystems, and ecosystem functions (Bundschuh et al. Citation2018). Scientists around the world have investigated effects of various types of NPs on the environment, but the results are still not conclusive regarding the comparative usefulness and harm of NPs.
The aim of this study was to investigate Fe3O4 NP effects on M. falcata L., particularly its seedling morphology, chlorophyll fluorescence, genotoxicity, and miRNA.
2. Materials and methods
2.1. NPs used
A sample of 25 nm of Fe3O4 NPs was kindly provided by G. Libert’s Center of Innovative Microscopy, Daugavpils University.
2.2. Plant materials and growth conditions
Medicago falcata seeds were purchased. The seeds were rinsed with tap water and transferred to Petri plates at 24°C and kept in the dark for 2 d. The seedlings were transferred to a hydroponic solution with ½ MS under a 16/8 h day/night photoperiod at 23°C. For the treatment of NP stress, two-week-old seedlings were transferred to an aqueous hydroponic solution supplemented with 1, 2, or 4 mg/L of Fe3O4 NPs for five weeks. The plants were watered daily and fertilized with fresh ½ MS solution every day. For the analysis of plant morphology, chlorophyll a content, determination of genotoxicity and evaluation of miRNA level, fresh leaves were sampled.
2.3. Measurement of biomass, shoot and root length
The heights of the shoots and roots were measured, and then the medick plants were washed several times with flowing tap and deionized water. Next, the plants fresh weight was measured, and a fresh sample of leaves were used for chlorophyll a fluorescence detection.
2.4. Measurement of chlorophyll a fluorescence
A confocal laser scanning microscope (Nikon Eclipse Ti-E) was used for chlorophyll a fluorescence microscopy measurement. Fluorescence was excited at a 488 nm laser wavelength, and NP emission was detected using a spectral detector (523–743 nm) and analyzed at wavelengths 653.03–661.04 nm. All confocal system parameters were similar (laser: 19.8, HV: 175, and pinhole: 107.6) with the exception of focus which was customized for each leaf sample. Chlorophyll fluorescence was detected using NIS Elements (Nikon, Japan) microscope imaging software.
2.5. DNA isolation and randomly amplified polymorphic analysis
The genotoxic effects induced by Fe3O4 NPs were assessed using the randomly amplified polymorphic DNA (RAPD) technique. Genomic DNA was extracted from 200 samples of fresh rucola leaves (50 in each group). Extraction was done with slight modifications using the purification of total DNA from the plant tissue Mini Protocol (DNeasy Plant Mini Kit, Qiagen GmbH, Hilden, Germany). The quantity and quality of genomic DNA were assessed using a spectrophotometer (NanoDrop 1000, Thermo Scientific, Waltham, USA).
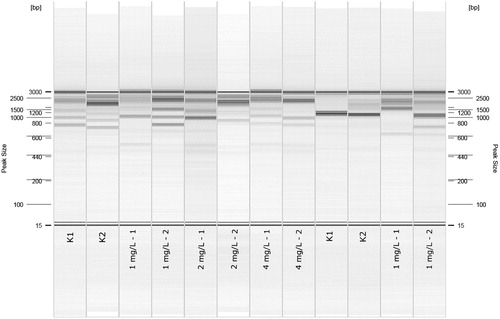
A total of 10 decamer primers, OPA-02, OPA-03, OPA-05, OPA-07, OPA-10, OPA-11, OPN-15, OPD-18, OPV-07, and CB-21, were selected for the RAPD analysis. The RAPD PCR program was set to initially denature at 94°C for 3 min, followed by a 35-cycle denaturation step at 94°C for 1 min, an annealing step at 37°C for 1 min 30 s, an extension of products at 72°C for 2 min and the final extension set at 72°C for 10 min using the Veriti 96-Well Thermal Cycler (Applied Biosystems, USA). The PCR reaction products were electrophoresed with a QIAxcel Advanced (Qiagen, Germany) instrument utilizing a QIAxcel DNA high-resolution kit according to the protocol (determination of DNA fragment sizes using the QIAxcel ScreenGel Software (Qiagen, Germany)). QX Size Marker 100 bp–2.5 kb and QX Alignment Marker 15 bp/3 kb (Qiagen, Germany) were used to determinate DNA fragment sizes. RAPD fragments were scored based on the presence or absence of band products for all tested primers. The whole RAPD-PCR procedure was repeated twice as quality control.
2.6. Detection of genotoxicity by estimation of genomic template stability
Genomic template stability (GTS) was calculated for each primer using the equation as reported by Salarizadeh and Kavousi (Citation2015):
where a is the average number of polymorphic bands found in each treated group and n is the number of total bands in the control samples. The polymorphic bands observed in the RAPD analysis were defined as the gain or loss of bands in comparison to those of the control profile.
2.7. Expression validation of microRNA using real-time qPCR
Small RNAs were extracted from the leaves of the five-week-old plants using a miRNeasy plant mini kit (Qiagen, Germany), and first-strand cDNA was synthesized with a miRCURY LNA RT Kit (Qiagen, Germany). A miRCURY SYBR Green polymerase chain reaction (PCR) Kit (Qiagen, Germany) was used to perform a miRNA qRT-PCR analysis according to the manufacturer’s protocol for quantitative, real-time PCR using individual miRCURY LNA and miRNA PCR assays. The spike-in UniSp6 RNA was used as an internal control. Mature miRNA specific PCR forward primers (sense DNA oligoidentical to the entire mature miRNA sequence) and the miRNA target-specific PCR primer lus-miR159c with locked nucleic acid were designed according to the miRNA sequence (Plaksenkova et al. Citation2019). 1-alpha (EF1α) was used as a reference gene for miRNAs expression data normalization (Cavaiuolo et al. Citation2017). The relative expression of the miRNAs in differently treated samples compared to that of the controls was calculated using the 2−ΔΔCt method (Livak and Schmittgen Citation2001).
2.8. Statistical analysis
The experiment had a completely randomized design with three replications. A one-way analysis of variance (ANOVA) was conducted to test differences in plant morphology. Tukey’s Multiple Comparison test was used at 0.05 p level to compare the means. The student t-test was used for the RAPD analysis. P values less than 0.05 were considered to be statistically significant.
3. Results and discussion
3.1. Measurements of biomass, shoot, and root length
The results showed that different Fe3O4 NPs concentrations influenced plant morphology. All tested NP concentrations affected both the sample length and number of leaves. NPs increased the yellow medick root length, with higher NP concentrations increasing root length. Furthermore, 2 and 4 mg/L NP concentrations increased root length significantly from 5 ± 0.74 cm (control) to 6.27 ± 1.51 cm (25%) and 6.6 ± 1.6 cm (32%), respectively (). There were no significant differences between plant shoot length in control samples and treated plants. Length varied from 3.25 cm for the control to 3.35 cm for the samples treated with 4 mg/L of NPs. The total sample length was most affected by the 4 mg/L NP concentration, and this concentration significantly affected the total length (9.95 ± 1.09 cm) in comparison to the control (8.25 ± 0.79 cm). The number of leaves was significantly affected by all tested NP concentrations. The control plant samples had approximately seven leaves per sample, while the plant samples grown with different NP concentrations ranged from 10 leaves (4 mg/L) to 11 leaves (1 mg/L). The weight of the plants was most affected by a NP concentration of 2 mg/L.
Table 1. Effect of various Fe3O4 NP concentrations on yellow medick morphological parameters.
These data are consistent with a recent study where the same NP concentrations were used to investigate the impact on rocket Eruca sativa morphology. The results were the same, and the length of the shoot and, root increased as the concentration increased. Nevertheless, the number of leaves were similar in all tested groups (Plaksenkova et al. Citation2019). Similarly, Elfeky et al. (Citation2013) presented results where iron oxide NPs were used as a growth factor for Ocimum basilicum L. The results showed that increasing concentrations of Fe3O4 NPs (1, 2 and 3 mg/L) also increased the total plant mass, root length, number of leaves, and weight. An enhancement in root elongation was also observed in ryegrass and pumpkin plants (Wang et al. Citation2011). Similar data were collected by Zahra et al. (Citation2015) where shoot and root elongation increased after exposure to Fe3O4 NPs. However, Lee et al. (Citation2010) declared the inhibition of root elongation in Arabidopsis under 400, 2000 and 4000 mg/L of Fe3O4 NPs (<50 nm). Also, Mushtaq (Citation2011) observed the same situation in cucumbers. A study was conducted by Ochoa et al. (Citation2017) where the addition of CuO NPs into soil were used as a growth factor for Pisum sativum L. plants. Plants were grown for 45 d with 50 mg/kg of soil and 100 mg/kg of NPs in the soil. As a result, both concentrations of NPs also increased the stem length, root length and number of leaves. Moreover, Salama et al. (Citation2019) showed that different concentrations (10, 20, 30 and 40 ppm) of zinc oxide NPs also affect dry bean (Phaseolus vulgaris) morphology. These NPs increased the bean shoot length, root length, and number of leaves. Results from similar research studies suggest that various concentrations of Fe3O4 and CuO NPs stimulate growth in plants of the Fabaceae family.
3.2. Measurement of chlorophyll a fluorescence
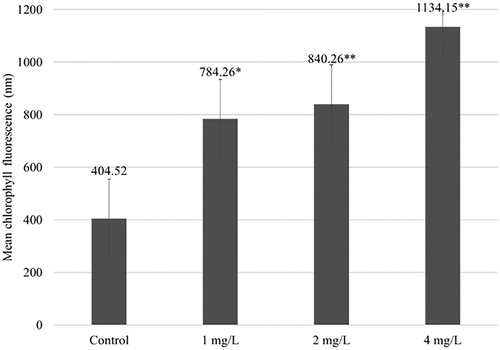
Iron oxide NPs increased the chlorophyll a level in all treated yellow medick plants. The mean value of chlorophyll fluorescence increased from 404.52 nm in the control samples to 784.26 nm (1.94-fold), 840.26 nm (2.08-fold), and 1134.15 nm (2.8-fold), for NP concentrations of 1, 2 mg/L, and 4 mg/L, respectively (). All tested concentrations significantly affected the medick plants: 1 mg/L at the P < 0.05 level, 2 mg/L at the P < 0.01 level, and 4 mg/L at the P < 0.01 level.
Figure 1. Mean chlorophyll a fluorescence in yellow medick leaves after a 5 week exposure with different concentrations of Fe3O4 NPs. The mean is averaged from three replicates and error bars correspond to standard derivation of mean. *Indicates significant difference from control (P < 0.05); **indicates significant difference from control (P < 0.01).
For the same experiment with Eruca sativa, the NPs increased the chlorophyll a level compared to that of the control group; however, an increasing NP concentration decreased the amount of chlorophyll a (Plaksenkova et al. Citation2019). Tombuloglu et al. (Citation2019) declared that concentrations 125, 250, 500, and 1000 mg/L of Fe3O4 NPs (∼13 nm) increased chlorophyll a and b levels in barley through the dramatic upregulation of photosystem marker genes. Therefore, the increased level of chlorophyll a promoted the growth of the M. falcata. However, according to Sharma and Uttam (Citation2017), copper oxide NPs also affect the wavelength of absorption of chlorophyll a in wheat plants. In the case of increasing copper oxide NP concentrations, the fluorescence level decreases.
3.3. RAPD analysis
The RAPD technique has been effectively utilized to detect genotoxic effects in several plants induced by various NPs (Remédios et al. Citation2012; Mattiello et al. Citation2015; Ghosh et al. Citation2019). This sensitive method is capable of detecting variations in plant genome profiles (Salama et al. Citation2019).
Ten decamer primers were used to study the genotoxic effects of Fe3O4 NPs for both control and NP treated samples. All utilized primers generated a stable RAPD banding pattern. The genomic changes were noted as (a) appeared or (b) disappeared bands in treated plant DNA as compared to those of the control. The appearance of new patterns can be explained by changes in genomic DNA template stability due to mutations, large deletions, homologous recombination, or changes in priming sites leading to new annealing events (AlQuraidi et al. Citation2019). Nevertheless, the absence of normal DNA bands can be characterized as DNA disintegration or rearrangement (Venkatachalam et al. Citation2017). Band changes were detected in all experimental groups using the primers (). The number of total bands varied from 7 (OPA-07) to 47 (OPA-11). The largest number of polymorphic bands (n = 29) showed OPA-11 in plants treated with 4 mg/L of NPs. Nevertheless, the lowest number of polymorphic bands (n = 1) showed OPA-07 in plants treated with 1 mg/L of NPs. Overall, 79 new bands appeared in treated plants for NP concentrations of 1 and 2 mg/L and 67 new bands in plants treated with 4 mg/L of NPs. However, 72 (1 mg/L), 71 (2 mg/L) and 74 (4 mg/L) disappeared bands were detected compared to the case with the control samples. The example of electropherogram is presented in .
Table 2. Results of RAPD analysis: the primers used, number of polymorphic bands in plants treated with 1, 2 mg/L and 4 mg/L of Fe3O4 NPs, total number of bands for each primer and average number of polymorphic bands for every plant group.
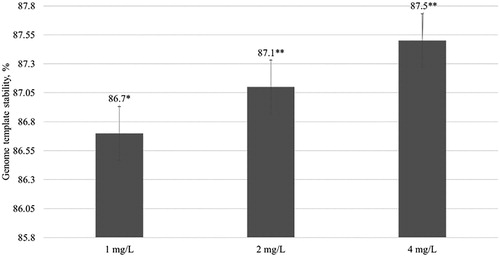
Overall, the RAPD results demonstrated that Fe3O4 NPs significantly changed the genome of the yellow medick plants. Additionally, the GTS for all treated plants was calculated (). The GTS for untreated (control) seedlings were fixed as 100%. There was a significant (P < 0.01) decrease in the GTS of all treated plants; however, genome stability increased by 13.3% in plants treated with 1 mg/L of NPs and by 12.5% in plants exposed to 4 mg/L of NPs. This indicates that the largest genome changes were induced by the lowest NP concentrations. It can be concluded from the results that the Fe3O4 NPs significantly reduce the stability of the yellow alfalfa genome.
Figure 3. Genome template stability (%) in plants after 5 week exposure with different concentrations of Fe3O4 NPs. The mean is averaged from three replicates and error bars correspond to standard derivation of mean. *Indicates significant difference from control (P < 0.05); **indicates significant difference from control (P < 0.01).
Compared to the same study with Eruca sativa with the same concentrations of Fe3O4 NPs, the GTS decreased with an increase in the concentration of NPs from 93.9% (1 mg/L) to 87.9% (4 mg/L) (Plaksenkova et al. Citation2019). The present experiment showed that these NPs have a stronger effect on yellow medick compared to that on Eruca sativa. In contrast, barley with concentrations of 125, 250, 500, and 1000 mg/L of Fe3O4 NPs (∼13 nm) did not show any toxic effects (Tombuloglu et al. Citation2019). Similarly, Wang et al. (Citation2011) demonstrated that 25 nm large Fe3O4 NPs significantly induce oxidative stress in ryegrass and pumpkin plants grown in hydroponics. A previous study revealed that 0.5, 1, and 1.5 mg/L of Fe3O4 NPs (25 nm) induced genotoxicity in a flax callus culture. Nanoparticle genotoxicity in plants could be caused by direct NPs intercalation in DNA strand or indirect by releasing free ions which can cause DNA damage (Kruszewski et al. Citation2011). For example, Cu NPs (Zhang et al. Citation2017) or Au NPs (Baetsen-Young et al. Citation2018) can intercalate into the DNA strand. It is not yet known whether Fe3O4 NPs are able to intercalating between DNS strands, but we suppose that released Fe2+ or Fe3+ ions (Mahdavi et al. Citation2013) could directly affect DNA.
Data from RAPD analysis suggest that M. falcata is sensitive to the effects of Fe3O4 NPs based on the number of band alterations in the NP samples compared with the control.
3.4. microRNA analysis
The Rotor Gene Q Series Software was used to analysis the miR159c expression level. Each sample group had different results. According to the Gurjar et al. (Citation2016), miRNA expression can be measured by the logarithmic formula Log2(treatment/control).
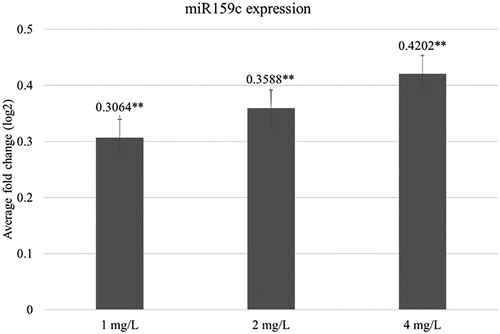
The results showed that all NP concentrations used in the study slightly increased the specific amount of miRNA in M. falcata L. plants. With an increase in NP concentration, the miRNA expression level also increased: 0.31-fold at 1 mg/L of NPs, 0.36-fold at 2 mg/L of NPs and 0.42-fold at 4 mg/L of NPs (). This indicates that 25 nm Fe3O4 NPs can increase the expression of miR159c after five weeks of exposure. Interestingly, data from the same study conducted on rockets showed diverse results, where increasing NP concentrations decreased the miR159c expression level (Plaksenkova et al. Citation2019). This suggests that miR159c in different plant species responded to Fe3O4 treatment in different ways. One potential reason is that plant species may respond differently to abiotic stress, including with miRNAs. According to previous studies, miR159 is important not only for plant growth but also for environmental stress responses, including that of fungal infection such as powdery mildew in wheat (Khraiwesh et al. Citation2012; Venkat Rajam Citation2012). Unfortunately, data on the miR159 role in yellow medick is unknown; however, the obtained results and data of independent studies suggest that this approach may enhance some plants resistance to serious fungal diseases, such as powdery mildew.
Figure 4. Average fold change of the miR159c expression level in plants after 5 week exposure with different concentrations of Fe3O4 NPs. The mean is averaged from three replicates and error bars correspond to standard derivation of mean. *Indicates significant difference from control (P < 0.05); **indicates significant difference from control (P < 0.01).
The findings of this study demonstrate that the exposure of M. falcata plants grown on hydroponics to 25 nm Fe3O4 NPs affects their morphology, chlorophyll a level, specific miRNA expression level, and induces genotoxic effects. The only difference between the treated and control plants was the presence or absence of Fe3O4 NPs, which supports the theory that the changes in plants were caused by this effect of the NPs. Furthermore, Zhu et al. (Citation2008) confirmed that 20 nm Fe3O4 NPs can penetrate into pumpkin cells and translocate and accumulate in the plant tissues. Moreover, it has been proven that 25 nm Fe3O4 NPs can penetrate flax callus culture cells (Kokina et al. Citation2017). As our best knowledge, there has not yet been studied the impact of Fe3O4 NPs on plant miR159c which is one of the most important plant miRNA that is involved in plant response to fungal pathogen. Obtained results could be used in future to develop new technology for successful increasing of plant resistance against fungal pathogen.
4. Conclusion
According to previous studies, Fe3O4 NPs positively enhance rocket, basil, ryegrass, and pumpkin root elongation. The same results were obtained in this study for yellow medick. Many investigations also confirmed an increase in chlorophyll a level in several plants after being exposed to different sizes of Fe3O4 NPs. Similarly, this study demonstrated a significant enhancement of the chlorophyll a level. The NPs did not frequently induce genotoxic effects in plants, but the RAPD analysis of this study confirmed the genotoxic effect of Fe3O4 NPs which induced genomic DNA modifications in M. falcata. One important finding of this study was the increase in miR159c expression which may indicate that these NPs can be used for increasing plant resistance against fungal pathogens.
Acknowledgements
Authors are thankful to the G. Libert’s Center of Innovative Microscopy, Daugavpils University, and especially Dr. Phys. Vjačeslavs Gerbreders for kindly providing the nanoparticles.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Inese Kokina
Dr. Inese Kokina is a professor and head of Department of Biotechnology at the Daugavpils University, Latvia. Her current fields of scientific activity include plant biotechnology, nanobiotechnology, genetics of plant immunity, plant tissue cultures and their use in genetics and selection. Her work focuses on investigations of nanoparticle impact on plant growth, development, DNA, miRNA. In addition, professor is a Latvian Council of Science expert in biology - sub-branch of genetics.
Ilona Plaksenkova
Ilona Plaksenkova is a doctoral candidate in biology and scientific assistant in Laboratory of Genomics and Bitechnology at the Daugavpils University. Her work focuses on investigations of nanoparticle impact on plant growth, development, genetic changes.
Marija Jermaļonoka
Marija Jermaļonoka is a doctoral candidate in biology and scientific assistant in Laboratory of Genomics and Bitechnology at the Daugavpils University. Her wor focuses on investigations of nanoparticle impact on plant tissue culture growth, development, DNA, miRNA.
Anastasija Petrova
Anastasija Petrova is a MSc student and lab assistant in Laboratory of Genomics and Biotechnology at the Daugavpils University. Her work focuses on investigations of nanoparticle impact of plant morphology, miRNA.
References
- AlQuraidi AO, Mosa KA, Ramamoorthy K. 2019. Phytotoxic and genotoxic effects of copper nanoparticles in coriander (Coriandrum sativum—apiaceae). Plants. 8(1):19. doi:10.3390/plants8010019.
- Amer N, Chami ZA, Bitar LA, et al. 2013. Evaluation of Atriplex halimus, Medicago lupulina and Portulaca oleracea for phytoremediation of Ni, Pb, and Zn. Int J Phytoremediation. 15(5):498–512. doi:10.1080/15226514.2012.716102.
- Baetsen-Young AM, Vasher M, Matta LL, et al. 2018. Direct colorimetric detection of unamplified pathogen DNA by dextrin-capped gold nanoparticles. Biosens Bioelectron. 101:29–36. doi: 10.1016/j.bios.2017.10.011
- Barciszewska-Pacak M, Milanowska K, Knop K, et al. 2015. Arabidopsis microRNA expression regulation in a wide range of abiotic stress responses. Front Plant Sci. 6:410. doi: 10.3389/fpls.2015.00410.
- Budak H, Akpinar BA. 2015. Plant miRNAs: biogenesis, organization and origins. Funct Integr Genomics. 15(5):523–531. doi 10.1007/s10142-015-0451-2.
- Bundschuh M, Filser J, Lüderwald S, et al. 2018. Nanoparticles in the environment: where do we come from, where do we go to? Environ Sci Eur. 30(1):6. doi:10.1186/s12302-018-0132-6.
- Cavaiuolo M, Cocetta G, Spadafora ND, et al. 2017. Gene expression analysis of rocket salad under pre-harvest and postharvest stresses: a transcriptomic resource for Diplotaxis tenuifolia. PloS one. 12(5):e0178119. doi:10.1371/journal.pone.0178119.
- Desvignes T, Batzel P, Berezikov E, et al. 2015. miRNA nomenclature: a view incorporating genetic origins, biosynthetic pathways, and sequence variants. Trends Genet. 31(11):613–626. doi:10.1016/j.tig.2015.09.002.
- Elfeky S, Mohhamed M, Khater M, et al. 2013. Effect of magnetite nano – fertilizer on growth and yield of Ocimum basilicum L. Int J Indig Med Plants. 46(Issue 3):1286–1293.
- Gehan MA, Greenham K, Mockler TC, McClung C R. 2015. Transcriptional networks – crops, clocks, and abiotic stress. Curr Opin Plant Biol. 4:39–46. doi:10.1016/j.pbi.2015.01.004.
- Ghosh M, Ghosh I, Godderis L, et al. 2019. Genotoxicity of engineered nanoparticles in higher plants. Mutat Res/Genet Toxicol Environ Mutagen. 842:132–145. doi:10.1016/j.mrgentox.2019.01.002.
- Gismondi A, Di Marco G, Canini A. 2017. Detection of plant microRNAs in honey. PLOS ONE. 12(2):e0172981. doi:10.1371/journal.pone.0172981.
- Graham PH, Vance CP. 2003. Legumes: importance and constraints to greater use. Plant Physiol. 131(3):872–877. doi/10.1104/pp.017004.
- Gurjar A, Panwar A, Gupta R, Mantri S. 2016. PmiRExAt: plant miRNA expression atlas database and web applications. Database. 2016:1–10. Article ID baw060. doi: 10.1093/database/baw060
- Kantar M, Lucas SJ, Budak H. 2011. miRNA expression patterns of Triticum dicoccoides in response to shock drought stress. Planta. 233(3):471–484. doi:10.1007/s00425-010-1309-4.
- Kashani B, Bidgoli MH, Motahari SA, et al. 2019. You are what you eat: sequence analysis reveals how plant microRNAs may regulate the human genome. Comput Biol Med. 106:106–113. doi:10.1016/j.compbiomed.2019.01.020.
- Keller AA, McFerran S, Lazareva A, Suh S. 2013. Global life cycle releases of engineered nanomaterials. J Nanopart Res. 15(6):1692. doi 10.1007/s11051-013-1692-4.
- Khraiwesh B, Zhu JK, Zhu J. 2012. Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants. Biochim Biophys Acta (BBA)-Gene Regul Mech. 1819(2):137–148. doi:10.1016/j.bbagrm.2011.05.001.
- Kokina I, Mickeviča I, Jahundoviča I, et al. 2017. Plant explants grown on medium supplemented with Fe3O4 nanoparticles have a significant increase in embryogenesis. J Nanomater. doi:10.1155/2017/4587147.
- Kruszewski M, Brzoska K, Brunborg G, et al. 2011. Toxicity of silver nanomaterials in higher eukaryotes. Adv Mol Toxicol. 5:179–218. doi: 10.1016/B978-0-444-53864-2.00005-0
- Laloum T, Martín G, Duque P. 2018. Alternative splicing control of abiotic stress responses. Trends Plant Sci. 23(2):140–150. doi:10.1016/j.tplants.2017.09.019.
- Lee CW, Mahendra S, Zodrow K, et al. 2010. Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ Toxicol Chem: Int J. 29(3):669–675. doi: 10.1002/etc.58.
- Li H, Wang Y, Wang Z, et al. 2016. Microarray and genetic analysis reveals that csa-miR159b plays a critical role in abscisic acid-mediated heat tolerance in grafted cucumber plants. Plant Cell Environ. 39(8):1790–1804. doi: 10.1111/pce.12745.
- Liu M, Wang TZ, Zhang WH. 2015. Sodium extrusion associated with enhanced expression of SOS1 underlies different salt tolerance between Medicago falcata and Medicago truncatula seedlings. Environ Exp Bot. 110:46–55. doi:10.1016/j.envexpbot.2014.09.005.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 25(4):402–408. doi:10.1006/meth.2001.1262.
- Mahdavi M, Namvar F, Ahmad M, Mohamad R. 2013. Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules. 18(5):5954–5964. doi: 10.3390/molecules18055954
- Mattiello A, Filippi A, Pošćić F, et al. 2015. Evidence of phytotoxicity and genotoxicity in Hordeum vulgare L. exposed to CeO2 and TiO2 nanoparticles. Front Plant Sci. 6:1043. doi: 10.3389/fpls.2015.01043.
- McGillicuddy E, Murray I, Kavanagh S, et al. 2017. Silver nanoparticles in the environment: sources, detection and ecotoxicology. Sci Total Environ. 575:231–246. doi:10.1016/j.scitotenv.2016.10.041.
- Miao Z, Xu W, Li D, et al. 2015. De novo transcriptome analysis of Medicago falcata reveals novel insights about the mechanisms underlying abiotic stress responsive pathway. BMC Genomics. 16:818. doi 10.1186/s12864-015-2019-x.
- Mushtaq YK. 2011. Effect of nanoscale Fe3O4, TiO2 and carbon particles on cucumber seed germination. J Environ Sci Health. 46(14):1732–1735. Part A. doi:10.1080/10934529.2011.633403.
- Obata T, Fernie AR. 2012. The use of metabolomics to dissect plant responses to abiotic stresses. Cell Mol Life Sci. 69(19):3225–3243. doi:10.1007/s00018-012-1091-5.
- Ochoa L, Medina-Velo I, Barrios A, et al. 2017. Modulation of CuO nanoparticles toxicity to green pea (Pisum sativum Fabaceae) by the phytohormone indole-3-acetic acid. Sci Total Environ. 589:513–524. doi: 10.1016/j.scitotenv.2017.04.063
- Okamura K, Ladewig E, Zhou L, Lai EC. 2013. Functional small RNAs are generated from select miRNA hairpin loops in flies and mammals. Genes Dev. 27(7):778–792. doi:10.1101/gad.211698.112.
- Panchenko L, Muratova A, Turkovskaya O. 2017. Comparison of the phytoremediation potentials of Medicago falcata L. and Medicago sativa L. in aged oil-sludge-contaminated soil. Environ Sci Pollut Res. 24(3):3117–3130. doi 10.1007/s11356-016-8025-y.
- Plaksenkova I, Jermaļonoka M, Bankovska L, et al. 2019. Effects of Fe3O4 nanoparticle stress on the growth and development of rocket Eruca sativa. J Nanoparticles. 2019:1–10.
- Qu Y, Duan M, Zhang Z, et al. 2016. Overexpression of the Medicago falcata NAC transcription factor MfNAC3 enhances cold tolerance in Medicago truncatula. Environ Exp Bot. 129:67–76. doi:10.1016/j.envexpbot.2015.12.012.
- Remédios C, Rosário F, Bastos V. 2012. Environmental nanoparticles interactions with plants: morphological, physiological, and genotoxic aspects. J Bot. 2012:8. Article ID 751686.
- Salama D, Osman S, Abd El-Aziz M, et al. 2019. Effect of zinc oxide nanoparticles on the growth, genomic DNA, production and the quality of common dry bean (Phaseolus vulgaris). Biocatal Agric Biotechnol. 18:1–11. doi: 10.1016/j.bcab.2018.10.019
- Salarizadeh S, Kavousi HR. 2015. Application of random amplified polymorphic DNA (RAPD) to detect the genotoxic effect of cadmium on tow Iranian ecotypes of cumin (Cuminum cyminum). J Cell Mol Res. 7(1):38–46. doi: 10.22067/jcmr.v7i1.42174.
- Schaumann GE, Philippe A, Bundschuh M, et al. 2015. Understanding the fate and biological effects of Ag-and TiO2-nanoparticles in the environment: the quest for advanced analytics and interdisciplinary concepts. Sci Total Environ. 535:3–19. doi:10.1016/j.scitotenv.2014.10.035.
- Sharma S, Uttam KN. 2017. Rapid analyses of stress of copper oxide nanoparticles on wheat plants at an early stage by laser induced fluorescence and attenuated total reflectance Fourier transform infrared spectroscopy. Vib Spectrosc. 92:135–150. doi: 10.1016/j.vibspec.2017.06.004
- Song S, Chen Y, Zhao M, Zhang WH. 2012. A novel Medicago truncatula HD-Zip gene, MtHB2, is involved in abiotic stress responses. Environ Exp Bot. 80:1–9. doi:10.1016/j.envexpbot.2012.02.001.
- Tombuloglu H, Slimani Y, Tombuloglu G, et al. 2019. Uptake and translocation of magnetite (Fe3O4) nanoparticles and its impact on photosynthetic genes in barley (Hordeum vulgare L.). Chemosphere. 226:110–122. doi:10.1016/j.chemosphere.2019.03.075.
- Venkatachalam P, Jayaraj M, Manikandan R, et al. 2017. Zinc oxide nanoparticles (ZnONPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: a physiochemical analysis. Plant Physiol Biochem. 110:59–69. doi: 10.1016/j.plaphy.2016.08.022
- Venkat Rajam M. 2012. Micro RNA interference: a new platform for crop protection. Cell Dev Biol. 1:e115. doi: 10.4172/2168-9296.1000e115.
- Wang H, Kou X, Pei Z, et al. 2011. Physiological effects of magnetite (Fe3O4) nanoparticles on perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) plants. Nanotoxicology. 5(1):30–42. doi: 10.3109/17435390.2010.489206.
- Wang W, Vinocur B, Altman A. 2003. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 218(1):1–14. doi 10.1007/s00425-003-1105-5.
- Zahra Z, Arshad M, Rafique R, et al. 2015. Metallic nanoparticle (TiO2 and Fe3O4) application modifies rhizosphere phosphorus availability and uptake by Lactuca sativa. J Agric Food Chem. 63(31):6876–6882. doi:10.1021/acs.jafc.5b01611.
- Zhang Y, Yun Z, Gong L, et al. 2018. Comparison of miRNA evolution and function in plants and animals. Microrna. 7(1):4–10. doi:10.2174/2211536607666180126163031.
- Zhang J, Zhang W, Guo J, et al. 2017. Electrochemical detection of C-reactive protein using copper nanoparticles and hybridization chain reaction amplifying signal. Anal Biochem. 539:1–7. doi: 10.1016/j.ab.2017.09.017
- Zhang LL, Zhao MG, Tian QY, Zhang WH. 2011. Comparative studies on tolerance of Medicago truncatula and Medicago falcata to freezing. Planta. 234(3):445–457. doi:10.1007/s00425-011-1416-x.
- Zhu H, Han J, Xiao JQ, Jin Y. 2008. Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J Environ Monit. 10(6):713–717. doi: 10.1039/b805998e.