?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Drought is a major cause of the present decrease in crop yield and agricultural productivity around the globe. The disastrous effects of drought on plants call for a renewed concern on effective strategies to improve plant growth and yield under drought stress. This study was designed to evaluate the effectiveness of Arthrobacter arilaitensis and Streptomyces pseudovenezuelae to improve maize growth under drought stress at three soil moisture levels using two inoculation methods. Seven rhizosphere actinomycetes isolates were screened for the production of plant growth promoting (PGP) traits and it was observed that all isolates produced one or more PGP properties. The inoculated plants were not only protected from the deleterious effects of drought, but also showed significant increases in the physiological parameters measured. The findings of this study suggest that these isolates are important tools capable of being developed into bio-inoculants to effectively improve drought tolerance in plants.
Introduction
A major environmental problem facing most countries of the world today, regarding agricultural productivity and food availability, is drought. Drought has been a subject of concern as it has led to reduced plant growth and yield. It is therefore very important to seek means of reducing this menace, to increase food availability and sustain food security. At present, strategies like breeding and genetic modifications are being used to manage this problem (Langridge and Reynolds Citation2015; Maazou et al. Citation2016). Agricultural practices including soil amelioration and mulching have also been used (Jongdee et al. Citation2006). However, these strategies are not very efficient as they are not only time consuming but labor and cost intensive (Ashraf Citation2010; Eisenstein Citation2013). Often times, some desirable plants traits in the host plant gene pool can be unintentionally lost in the process of breeding (Philippot et al. Citation2013). Moreover, plant breeding transfers benefit to single host specie and not to other crop systems, as it is usually difficult to identify the genetic component responsible for this improvement (Coleman-Derr and Tringe Citation2014). The drawbacks mentioned above have made these technologies highly unreliable, leading to a quest for better and more efficient means to tackle this problem.
In recent times, the use of beneficial microbial species with plant growth promoting capabilities to relieve plants of the adverse effects of drought has become more relevant in agriculture (Babalola and Glick Citation2012). Bacteria are important soil components, able to form mutualistic and beneficial associations with most plants (Ndeddy Aka and Babalola Citation2016). Symbiotic bacteria are capable of conferring stress tolerance to a wide variety of plant hosts through phytohormonal modifications, production of exopolysaccharides, accumulation of osmolytes and acting as defense against reactive oxygen species (Zhang et al. Citation2008; Coleman-Derr and Tringe Citation2014). These bacteria are also able to synthesize antibiotic substances, fix atmospheric nitrogen, produce soluble iron compounds (siderophore), and solubilize inorganic phosphates (Babalola Citation2010; Adegboye and Babalola Citation2013). In addition, they serve as plant growth regulators by producing the phytohormones indole-acetic acid (IAA), 1-aminocyclopropane-1-carboxylic acid (ACC), cytokinins and gibberellins (GA) (Khantsi et al. Citation2013; Ndeddy Aka and Babalola Citation2017). These outstanding properties of the plant growth promoting bacteria (PGPB) facilitate the efficient stimulation of plant growth during unfavorable environmental conditions like drought (Yandigeri et al. Citation2012). Several studies have revealed the successful application of isolated PGPB on drought stress improvement in plants (Figueiredo et al. Citation2008; Yandigeri et al. Citation2012; Gusain et al. Citation2015). However, most of these studies have concentrated on certain groups of bacteria species, mostly Pseudomonas and Bacillus. The use of actinomycetes species to enhance stress tolerance in plants have received very little attention over the years. Actinomycetes, found mostly in soils, are widely known for their antibiotic and bioactive secondary metabolites production as well as their outstanding ability to survive in unfavorable environments (Adegboye and Babalola Citation2013; Adegboye and Babaloa Citation2015; Passari et al. Citation2015). Their ability to produce certain plant growth promoting properties has also been identified, but with little information on the extent of the properties produced (Ali et al. Citation2014; Sreevidya et al. Citation2016). Hence, this study was conducted to investigate the effects of actinomycetes inoculation on maize plant under well-watered, semi-watered and drought stressed conditions. It also examined the level of production of certain plant growth promoting (PGP) substances ACC, IAA, siderophore, phosphate and ammonia (NH3) by the drought tolerant actinomycetes.
Materials and methods
Isolation and selection of drought tolerant bacteria
In a previous study (Chukwuneme Citation2018), seven drought tolerant bacteria were isolated from two maize plantations. These bacterial isolates were identified and deposited in the GenBank with the following names and accession numbers: Streptomyces werraensis MG547867, Streptomyces luteogriseus MG669347, Streptomyces indiaensis MG547868, Arthrobacter arilaitensis MG547869, Streptomyces pseudovenezuelae MG547870, Microbacterium oxydans MG640368, and Streptomyces sp. MG640369. The isolates were selected for this study based on their ability to withstand drought stress by growing at 20% concentrations of PEG 8000. In the present study, these bacteria were qualitatively and quantitatively screened for the presence of plant growth promoting traits. Due to their higher tolerance of higher concentrations of PEG 8000, S. pseudovenezuelae MG547870 and A. arilaitensis MG547869 were chosen for greenhouse studies, to assess the effect of their individual and combined inoculation on the growth of maize plants under drought stress.
Qualitative and quantitative assessment of plant growth promoting properties of bacterial isolates
Ammonia production
The production of ammonia by actinomycetes isolates was tested according to the protocol of Islam et al. (Citation2009). 10 µl (0.2 OD) of freshly prepared actinomycetes cultures were inoculated into test tubes containing 10 ml of peptone water. The inoculated test tubes were incubated at 25°C for 7days after which 1 ml of Nessler's reagent was added to each test tube, and any color changes were observed. A change in the color of the media to yellow or brown specifies a positive result for ammonia production. The experiment was done in triplicate.
Phosphate solubilization activity
To evaluate the ability of actinomycetes isolates to solubilize phosphate, the method of Islam et al. (Citation2009) was used. 10 µl of freshly prepared culture was spot inoculated on Pikovskaya’s agar plates containing 2% tri-calcium phosphate. Inoculated plates were incubated at 37°C for 7 days, plates were observed for the appearance of a clear zone around the actinomycetes colonies.
Hydrogen cyanide activity
Hydrogen cyanide activity was determined according to the protocol of Bakker and Schippers (Citation1987). Bacterial cultures were separately streaked on Luria Bertani (LB) agar amended with 0.4% (w/v) of glycine. A Whatman no. 1 filter paper soaked in 0.5% (w/v) picric acid in 2% (w/v) sodium carbonate was placed on the lid of the Petri dish. Thereafter, plates were properly sealed with parafilm and incubated for seven days. The change in color of the filter paper from yellow to deep orange when observed with the eyes indicates a positive result.
Indole-3-acetic acid production
The method of Ndeddy Aka and Babalola (Citation2016) was used for qualitative determination of indole-3-acetic acid production by actinomycetes isolates. Freshly prepared bacterial cultures (20 µl) were inoculated in LB broth (20 ml) amended with 5 mmol L-tryptophan (Merck, SA) and incubated at 25°C for 7 days. After incubation, 1 ml of bacterial culture was transferred into sterile Eppendorf tubes and centrifuged at 5000 g for 15 min. The supernatant was collected in a 15 ml centrifuge tube. Subsequently, 2 ml of the supernatant and 2–3 drops of orthophosphoric acid was added to 4 ml of Salkowsky reagent (50 ml of 35% perchloric acid in 1 ml of 0.5 M FeCl3). The contents in the tubes were incubated at room temperature under dark conditions for 20 min, the development of a pink color indicated IAA production. The absorbance of the pink color was read using a UV spectrophotometer (ThermoSpectronic, Merck chemical, SA) at 530 nm.
The amount of IAA produced by each bacterial isolate was determined by the generation of a standard curve. Standards were made in LB broth at 0,5,10, 20, 50 and 100 µg/l including a control consisting of LB broth only, 4 ml of Salkowsky reagent was added to 2 ml of each standard and incubated at room temperature for 20 min. Absorbance was read at 530 nm using a UV spectrophotometer (ThermoSpectronic, Merck chemical, SA).
Siderophore production
The production of siderophore by bacterial isolates was assayed according to a modified protocol described by Schwyn and Neilands (Citation1987) using an indicator dye, chrome azurol S (CAS from Merck, SA). Briefly, 60.5 mg of CAS was dissolved in 50 ml of distilled water and mixed with 10 ml of iron (III) solution (1 mM FeCl3. 6H20 in 10 mMHCl). The mixture was slowly added while constantly stirring with a magnetic stirrer to 72.9 mg of hexadecyltrimethylammonium (HDTMA, Merck, SA) bromide dissolved in 40 ml distilled water and then autoclaved at 121°C for 15 min. The final mixture (100 ml) was added while stirring to 900 ml of sterilized LB broth adjusted to pH 6.8 and poured into Petri plates. Upon solidification, freshly prepared bacterial cultures were spot inoculated on the Petri plates and incubated at 25°C for 7 days. A yellowish – orange halo around the bacterial colonies was considered a positive result for siderophore production.
The amount of siderophore produced by each bacterial isolate was estimated following the protocol of Alexander and Zuberer (Citation1991) using a modified CAS assay solution. Hexadecyltrimethylammonium (HDTMA, 21.9 mg) was dissolved in 25 ml of distilled water with constant stirring under low heat. In a 50 ml flask, 1.5 ml of 1 mM FeCl3. 6H20 in 10 mMHCl was added to 7.5 ml of 2 mM CAS. This solution was slowly added to the HDTMA solution and the resultant mixture transferred to a 100 ml flask. A buffer solution was prepared by dissolving 9.76 g of 2-(N-morpholino) ethanesulfonic acid (MES, Merck, SA) in 50 ml distilled water and the pH adjusted to 5.6 with 50% KOH. This buffer solution was then added to the flask containing the dye solution while distilled water was added to get a final volume of 100 ml. A shuttle assay solution was prepared by adding 87.3 mg of 5-sulfosalicyclic acid to the above solution before use. All seven isolates to be tested for siderophore were inoculated in 5 ml sterilized LB medium without added Fe and incubated at 25°C for five (5) days. Bacterial cells were pelleted by centrifugation at 3000 g for 10 min and the supernatant was collected in tubes. The concentration of siderophore in the supernatant was obtained by mixing 100 µl of CAS assay solution with 100 µl of supernatant and allowed to equilibrate for 3–4 h, the absorbance of the 200 µl mixture was measured at 630 nm using a UV spectrophotometer (ThermoSpectronic, Merck chemicals, SA). The percentage siderophore produced was calculated by the equation:
ere Ar = Absorbance of reference at 630 nm (CAS reagent),
As = Absorbance of sample at 630 nm.
ACC deaminase activity
The seven drought tolerant bacterial isolates used in this study were screened for ACC deaminase activity based on their ability to utilize ACC as sole nitrogen source, following the method by Ali et al. (Citation2014). All bacteria were first grown on 5 ml of tryptone-soy broth (TSB, rich medium, Merck, SA) and incubated at room temperature for 48 h. Bacterial cells were harvested by centrifugation at 5000 g for 5 min, washed twice with sterile 0.1 M Tris-HCl (pH 7.5) and resuspended in 1 ml of 0.1 M Tris-HCl (pH 7.5). Washed bacterial cells were spot inoculated on Petri plates containing modified Dworkin and Foster salts minimal medium (Dworkin and Foster Citation1958). Minimal salts medium was composed of 2 g glucose, 2 g gluconic acid, 2 g citric acid, 4 g KH2PO4, 6 g Na2HPO4, 0.2 g MgSO4.7H2O and 10 ml micro nutrient solution (200 mg CaCl2, 200 mg FeSO4.7H2O, 15 mg H3BO3, 20 mg ZnSO4.7H2O, 10 mg Na2MoO4, 10 mg KI, 10 mg NaBr, 10 mg MnCl2, 5 mg COCl2, 5 mg CuCl2, 2 mg AlCl3, 2 mg NiSO4 and 1000 ml distilled H2O) in 990 ml distilled H2O amended with 3 mM ACC as a sole nitrogen source. Negative control for this experiment was Petri plates containing only DF minimal salts medium without ACC while the positive control consisted of plates containing DF minimal salts medium +0.2% (w/v) (NH4)2SO4. Inoculated plates were incubated at 30°C for 7 days. The growth of bacterial isolates on DF minimal plates containing ACC was used to compare those of the positive and negative controls. Petri plates were selected based on bacterial growth by utilizing ACC as sole source of nitrogen. The experiment was performed thrice.
The activity of ACC deaminase was measured by growing all 7 actinomycetes isolates on TSB medium at 30°C for 7 days. The induction of ACC deaminase activity was achieved by collecting bacterial cells by centrifugation at 5000 g for 5 min and washing with 0.1 M Tris-HCl (pH 7.5). Washed cells were resuspended in 2 ml of modified DF minimal medium containing 3 mM concentration of ACC, then incubated under shaking at 30°C for 7 days. ACC deaminase activity was determined by measuring the production of α-ketobutyrate and ammonia generated when ACC cleaved to ACC deaminase (Penrose and Glick Citation2003). Induced bacterial cells were harvested by centrifugation at 5000 g for 10 min, washed twice with 0.1 M Tris-HCl (pH 7.5) solution, then resuspended in 200 µl of 0.1 M Tris-HCl (pH 8.5). Toluene (5% v/v) was added to the cells to labilize and cells were vortexed at highest speed for 30 s. In sterile Eppendorf tubes, fifty (50) µl of labilized cells was collected and 5 µl of 3 mM ACC was added. Tubes were incubated at 30°C for 30 min. For this assay, the negative control consisted of 50 µl of labilized cell suspension without ACC while the blank consisted of 50 µl of 0.1 M Tris-HCl (pH 8.5) with 3 mM ACC. Samples were then thoroughly mixed with 500 µl of 0.56 N HCl by vortexing and cell pellets were removed by centrifugation at 10, 000 g for 10 min. The supernatant (500 µl) was transferred into test tubes and mixed with 400 µl of 0.56 N HCl and 150 µl of 2, 4 DNP solution (0.1 g 2, 4-dinitrophenylhydrazine in 100 ml of 2 N HCl). The mixture was incubated at 30°C for 30 min. One ml of 2 N NaOH was finally added to the samples after which their absorbances were measured at 540 nm using a UV spectrophotometer (ThermoSpectronic, Merck chemicals, SA).
Alpha-ketobutyrate concentration in each sample was determined by comparing with a standard curve generated as follows: alpha-ketobutyrate solutions (500 µl) of 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9 and 1.0 mM were mixed each with 400 µl of 0.56 N HCl and 150 µl of 2,4-DNP solution. One (1) ml of 2 N NaOH was then added and mixed. The absorbance was measured at 540 nm and the values obtained for the absorbance against concentration (mM) were used to generate a standard curve (Renn Citation2013). Note: each standard was replicated 3 times.
Greenhouse experiments
Greenhouse experiments were conducted to: (i) evaluate the effect of bacterial inoculation on the growth of maize plant under three moisture levels [field capacity (FC, 100%), moderately wet (MW, 50%) and completely dry (D, 0%)], and (ii) evaluate the effect of the method of inoculation on drought stress tolerance in maize. Maize as a drought intolerant crop was chosen for this study based on its high nutritional and economic importance in South Africa. Knowledge gained from this study will help to find suitable means of improving maize growth and yield, despite the ravaging drought.
Actinomycetes inoculum preparation
The two actinomycetes isolates A. arilaitensis (MG547869) and S. pseudovenezuelae (MG547870) used in this experiment were based on their outstanding ability to resist drought stress in-vitro as well as their plant growth promoting potentials. The inocula were prepared by growing the bacterial strains in 250 ml conical flasks containing 100 ml of sterilized LB broth. Inoculated flasks were incubated at 30°C under constant shaking (120 rpm) for 7 days. Pellets were collected by centrifugation at 10000 rpm for 20 min and washed twice with sterile distilled water. Pelleted cells were resuspended in 0.01M phosphate buffer at pH 7 and adjusted to an absorbance of 1.2 at 600 nm with a UV spectrophotometer (Thermospectronic, Merck, SA) (Ndeddy Aka and Babalola Citation2016).
Soil collection and preparation for pot experiments
Soil for the trial was collected from the farm behind the Animal Health Center of the North-West University, Mafikeng Campus at 25°S Latitude, 25°E Longitude and elevation of 1278.3 m. The soil was collected at the surface 0–20 cm depth, oven dried at 70°C for 48 h, passed through a 2 mm sieve and autoclaved at 121°C for 15 min. Sterilized soil was allowed to cool for 2 days after which 10 kg of soil was aseptically transferred into plastic pots.
Seed viability test in petri plates
Seed germination tests were conducted to evaluate the effect of bacterial inoculation on the germination of the test seeds in the presence of 5% polyethylene glycol (PEG) 8000 (Rincón et al. Citation2008). Prior to the test, drought sensitive maize seeds of the variety S0/8/W/ I137TNW//CML550 obtained from the Agricultural Research Council (ARC), South Africa were first washed with tap water, then sterilized using 70% ethanol for 5 min follwed by 2% sodium hypochlorite (NaClO2) solution for 15 min and severally rinsed with sterile distilled water to remove the remains of the disinfectant (Madhaiyan et al. Citation2007). Thereafter, 4 clean Petri plates (replicated three times) were prepared by placing two filter papers at the bottom of each plate and subsequently 10 ml of each bacterial suspension in 5% PEG 8000 or 10 ml of sterile tap water (in the case of the control) was pipetted in each Petri plate. Sterile seeds were immersed in 10 ml of bacterial suspension containing 5% PEG 8000 for 5 h in a rotary shaker at 150 rpm after which 20 seeds were placed in each petri plate and incubated at 25°C for 10 days. Germinated seeds in each Petri plate were counted and 5 seedlings per plate were randomly selected for growth parameter measurements (shoot length, root length and dry seedling weight). Percentage germination and vigor index were estimated according to the method of Ghorbanpour and Hatami (Citation2014) as follows:
Where n is the number of germinated seeds after 7 days and N is the total number of seeds
Vigor index = % germination × total length of seedling (shoot length + root length)
Preparation of maize seeds for greenhouse study
Drought susceptible maize seeds of the cultivar S0/8/W/I137TNW//CML550 were firstly immersed in 70% ethanol for 15 min and washed three times with sterile distilled water. Thereafter, the seeds were soaked in 2% sodium hypochlorite (NaClO2) solution for 10 min, and then thoroughly rinsed twice with sterile distilled water.
Seed inoculation with bacterial isolates
In this study, two methods of inoculation were employed to enable the bacterial isolates adhere to the surface sterilized maize seeds. This was done to determine the effect of mode of inoculation on drought stress amelioration in maize plants. Firstly, surface sterilized maize seeds were inoculated by direct immersion in bacterial cultures (1.5 OD600/ ml) or 0.01M phosphate buffer (pH 7) for the control treatment for 12 h. Secondly, maize seeds were immersed in bacterial cultures (1.5 OD600/ ml) or 0.01M phosphate buffer (pH 7) in the case of the control for 12 h. Following immersion, seeds were resuspended in 1% carboxylmethyl cellulose (CMC, binder) in a 500 ml conical flask and finely ground and sterilized vermiculite was spread all over the seeds until they were completely coated. Both the directly inoculated and coated seeds were left to dry overnight in a sterile laminar flow chamber prior to being sown in the greenhouse.
Greenhouse evaluation of bacterial induced tolerance to drought stress
In the 2 × 3 × 4 factorial greenhouse experiment, a total of seventy-two (72) pots (23-cm diameter) were used, representing twenty-four (24) treatment combinations based on three (3) experimental factors (seed treatments, bacterial types and soil moisture levels). The three experimental factors used in the present study were:
Two seed treatments (inoculation method): directly inoculated seeds and vermiculite coated seeds as described earlier
Four types of seed inoculation: without bacteria isolate (control), with S. pseudovenezuelae, with A. arilaitensis and combination of both bacterial isolates, and
Three moisture levels: Field capacity (FC, 100%), moderately wet (MW, 50%) and completely dry (DS, 0%).
Data analysis
Data obtained from this study were analyzed by One-way analysis of variance (ANOVA) using the Statistical analysis software (SAS), version 9.4 (SAS Citation2014). For each treatment, generated data were presented as arithmetic means ± S.E. Significantly different means were separated using New Duncan Multiple Range Test (DMRT) at 5% level of significance.
Results
Drought tolerance by actinomycetes isolates
The actinomycetes strains used in this study were those isolated and identified in our previous study due to their high tolerance to higher concentration of PEG 8000. S. pseudovenezuelae (MG547870) and A. arilaitensis (MG547869), chosen for greenhouse studies grew well at the highest PEG 8000 concentration of 20% with a growth of 0.786 ± 0.076 (OD600) for S. pseudovenezuelae MG547870 and 1.379 ± 0.134 for A. arilaitensis MG547869.
Plant growth promoting characteristics of bacterial isolates
All tested isolates produced multiple plant growth promoting characteristics. The results of the qualitative plant growth promoting tests conducted are presented in Results revealed that all seven isolates tested were positive for ammonia, Indole-3-acetic acid, siderophore and ACC deaminase activity, five isolates [S. werraensis (S4), Streptomyces spp. (S12), S. indiaensis (R11), A. arilaitensis (R15) and S. pseudovenezuelae (S20) tested positive for phosphate solubilization by showing clear zones around colonies on petri dishes while only Streptomyces spp. (S12) was positive for hydrogen cyanide activity.
Table 1. Qualitative plant growth promoting abilities of bacterial isolates.
Indole-3-acetic acid production by bacterial isolates
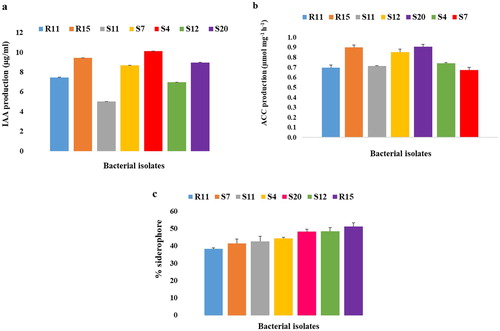
IAA production by the tested bacterial isolates varied ((a)). Streptomyces werraensis showed maximum indole-3-acetic acid production (10.12 ± 0.02 µg/ml) followed by A. arilaitensis (9.44 ± 0.01 µg/ml), S. pseudovenezuelae (8.96 ± 0.03 µg/ml), S. luteogriseus (8.68 ± 0.01 µg/ml), S. indiaensis (7.46 ± 0.02 µg/ml), Streptomyces spp. (6.98 ± 0.02 µg/ml), and M. oxydans (5.03 ± 0.01 µg/ml).
Phosphate solubilization by bacterial isolates
From the results in , five out of the seven tested bacterial isolates solubilized phosphate by showing clear zones around colonies on agar plates. These bacterial isolates include S20, R15, S12, R11 and S4 (S. pseudovenezuelae, A. arilaitensis, Streptomyces spp., S. indiaensis and S. werraensis respectively).
ACC deaminase activity of bacterial isolates
In the present study, the ACC deaminase activity of all tested isolates on agar plates containing ACC as the sole nitrogen source was positive () However, in the quantitative assay, variations were observed in the amount of ACC deaminase activity produced among tested bacterial isolates ((b)). Streptomyces pseudovenezuelae produced the highest amount of ACC deaminase activity (0.903 ± 0.024 µmol αKB mg−1 h−1) followed by A. arilaitensis which produced 0.899 ± 0.023 µmol αKB mg−1 h−1. Streptomyces indiaensis, M. oxydans, Streptomyces spp., S. werraensis and S. luteogriseus respectively produced 0.696 ± 0.028, 0.713 ± 0.003, 0.850 ± 0.032, 0.741 ± 0.004 and 0.671 ± 0.027 µmol αKB mg−1 h−1 of ACC deaminase activity. In our previous work, PCR amplification of ACC gene revealed that the tested isolates amplified the accd gene when run on agarose gel indicating a possible presence of the gene.
Ammonia, siderophore and hydrogen cyanide production by bacterial isolates
In the present study, all tested bacterial isolates produced ammonia. Siderophore production by bacterial isolates on CAS agar plates was positive for all the tested isolates ((c)). In the quantitative siderophore tests, siderophore production was significantly highest (p < 0.05) in A. arilaitensis (51.3 ± 2.11%), followed by Streptomyces spp. (48.6 ± 2.04%), S. pseudovenezuelae (48.3 ± 1.41%), S. werraensis (44.5 ± 0.48%), M. oxydans (42.7 ± 2.97%), S. luteogriseus (41.4 ± 2.57%) while S. indiaensis had the least production (38.3 ± 0.58%).
Seed germination tests
The results of the seed germination tests by S. pseudovenezuelae (MG547870) and A. arilaitensis (MG547869) are presented in . Shoot length, root length, germination % and vigor index was significantly highest in treatment M1 (9.27 ± 0.99 cm,10.17 ± 0.84 cm, 94.44 ± 3.21% and 1825.93 ± 133.74) respectively, compared to the other treatments. This was followed closely by treatment S20 with Shoot length, root length, germination % and vigor index of 6.23 ± 0.72 cm, 6.9 ± 0.85 cm, 83.33 ± 3.12% and 1086.3 ± 105.60 respectively. However, treatment C2 which was un-inoculated with PEG 8000 had the least Shoot length, root length, germination % and vigor index (2.5 ± 0.21 cm, 3.17 ± 0.24 cm, 42.59 ± 3.70% and 243.7 ± 31.73) respectively.
Table 2. Seed germination test.
Effect of bacterial inoculation on drought tolerance in maize
The study showed that the plants inoculated with both bacterial isolates (S. pseudovenezuelae and A. arilaitensis) had significantly higher (p < 0.05) chlorophyll content index (CCI) of 10.85 ± 0.87 µ/g compared to the un-inoculated plants (8.17 ± 0.52 µ/g) at field capacity. For the plants that received moderate water, the inoculated plants also performed better than the un-inoculated ones as the highest CCI (8.27 ± 0.35 µ/g) at moderately wet category was observed in the plants co-inoculated with the two bacterial isolates while the lowest (7.37 ± 0.38 µ/g) was seen in the un-inoculated plants (). The results obtained for CCI on the completely drought stressed plants revealed that better CCI values were obtained by the inoculated plants than the un-inoculated plants as the co-inoculated plants produced CCI values of 7.13 ± 0.19 µ/g, the singly inoculated plants (BS and BR) produced CCI values of 6.72 ± 0.19 µ/g and 6.37 ± 0.33 µ/g respectively while the un-inoculated plants produced a CCI of 5.65 ± 0.29 µ/g.
Table 3. Effect of bacterial inoculation on growth parameter measurement of well-watered (FC), moderately watered (MW) and drought stressed (DS) maize plants.
From , the greatest increases in plant heights (relative to the control) were observed in plants that received water at field capacity, followed by the moderately wet plants while the lowest increase was observed in plants that did not receive water at all (completely dry). At field capacity, the highest height of 83.23 ± 2.37 cm was observed in the plants inoculated with the two bacterial strains while the lowest height of 71 ± 2.64 cm was observed in the un-inoculated plants at field capacity. For the moderately wet category, the highest height of 70.87 ± 1.96 cm was observed in the co-inoculated plants followed by the individually inoculated plants whose heights were 69.93 ± 2.0 cm for plants inoculated with S. pseudovenezuelae and 67.65 ± 2.26 cm for plants inoculated with A. arilaitensis while the lowest height of 58.80 ± 2.99 cm was observed in the un-inoculated plants at this water level. Results at the no water level revealed that better plant height of 64.10 ± 2.40 cm was observed in plants whose seeds were co-inoculated with the two bacterial strains followed by plants whose seeds were singly inoculated by either S. pseudovenezuelae (63.07 ± 2.44 cm) or A. arilaitensis (62.80 ± 2.79 cm) while the lowest heights of 46.95 ± 2.52 cm was observed in the plants whose seeds were un-inoculated.
Results on the root lengths followed the same trend, as the longest root was observed in plants whose seeds were co-inoculated with S. pseudovenezuelae and A. arilaitensis with root length of 65.67 ± 11.12 cm at field capacity, 43.13 ± 7.16 cm at moderately wet level and 34.42 ± 4.86 cm at the completely dry level. On the other hand, lower root lengths were observed in the un-inoculated plants (43 ± 7.63 cm) at field capacity, 34.86 ± 5.44 cm at moderately wet and 19.43 ± 1.69 cm at the completely dry level.
The number of leaves on each plant also varied according to treatment as more leaves were observed in the inoculated plants than in the un-inoculated ones. Plants inoculated with the combination of the two bacterial isolates produced approximately 11 ± 0.33 leaves per plant at field capacity, 10 ± 0.33 leaves per plant at moderately wet capacity and 10 ± 0.22 at drought stress capacity. On the other hand, significantly lower leaf number were observed per plant in the un-inoculated plants at moderately wet (10 ± 0.34) and drought stressed (8 ± 0.31) levels. However, there was no significant difference in the number of leaves obtained at field capacity for both inoculated and un-inoculated plants, as approximately 11 leaves per plant were observed at this water level for both treatments. Leaf area of 3991.3 ± 491.67 cm2 was observed in the co-inoculated plants at field capacity, 2801.6 ± 362.7 cm2 at moderately wet capacity and 2074.7 ± 258.17 cm2 was observed in these plants when there was no water application at all. For the un-inoculated plants, total leaf area per plant was 2887.2 ± 422.10 cm2 at field capacity, 2108.3 ± 323 cm2 at moderately wet capacity and 1012 ± 218.23 cm2 at zero water capacity.
Significant differences in dry shoot weights were observed in this study (). Co-inoculated plants produced significantly higher (p < 0.05) shoot weights of 10. 77 ± 0.67 g at field capacity, 6.13 ± 0.41 g at moderate water application and 4.0 ± 0.31 g at zero water application while lower shoot weights were observed in un-inoculated plants as weights of 5.92 ± 0.47 g were obtained at field capacity, 4.23 ± 0.37 g at moderately wet and 1.67 ± 0.16 g at zero water level. Higher dry root weights were also observed in the inoculated plants than the un-inoculated plants as the highest weight of 9.027 ± 1.99 g was observed in the co-inoculated plants at field capacity level while the lowest weights of 4.67 ± 0.54 g were observed in the un-inoculated plants at this capacity. At moderately wet capacity, 4.38 ± 0.60 g was observed in co-inoculated plants while 3.05 ± 0.61 g was observed in un-inoculated plants and at zero water level, the highest dry root weight was observed in co-inoculated plants as 2.75 ± 0.35 g while the lowest was observed in the un-inoculated plants as 1.15 ± 0.19 g.
Effect of inoculation method on growth parameter measurements of maize plants
The mean data on the effect of inoculation method on the growth of maize are presented in . The effect of the two inoculation methods used in the study showed that greater growth parameters were observed in plants whose seeds were bound with carboxylmethyl cellulose and coated with vermiculite than the directly inoculated plants. From the results, treatment S + BR + BS + V produced significantly higher (p < 0.05) CCI value (µ/g) of 8.75 compared to 8.26 µ/g obtained in treatment S + BR + BS. Similarly, treatment S + BS + V gave a CCI of 8.1 µ/g while treatment S + BS gave a CCI value of 7.52 µ/g. Improved CCI was also observed in treatment S + BR + V (7.66 µ/g) compared to treatment S + BR (7.2 µ/g). For the control seeds, results showed that better CCI value was observed in treatment S + V (7.28 µ/g) than in treatment S (6.93 µ/g). The data on plant height, root length, number of leaves per plant, leaf area, dry shoot and root weight also revealed that for all the treatments, plants with seeds immersed in 1% CMC and coated with vermiculite were better in terms of growth and all the parameters measured than the plants with seeds were either directly inoculated with bacteria or distilled water.
Table 4. Effect of inoculation method on growth parameter measurements of maize plants.
Discussion
Plants are continuously exposed to abiotic stresses such as drought, salinity and cold (Bardi and Malusà Citation2012). Drought, being one of the most serious environmental problems affecting the growth and development of plants and subsequently agricultural yields and food supply, has gained research attention over the years. The ravaging effects of drought on plants can be reduced by the action of PGPB with PGP traits. These bacteria are capable of tolerating and surviving under harsh environments through the regulation of phytohormones, production of ACC deaminase activity, accumulation of osmolytes, production of volatile compounds and antioxidant defense (Vurukonda et al. Citation2016).
Plants’ developmental processes are being regulated by the production of phytohormones in their various parts. The phytohormone, indole-3-acetic acid, plays a major role in plant development and its supply is capable of supporting its host during stress conditions like drought and pathogenic attacks (Bardi and Malusà Citation2012; Sathya et al. Citation2017). It also improves seedling growth, and cell differentiation, as well as enhancing both elongation and development of lateral roots in plants (Vurukonda et al. Citation2016; Sathya et al. Citation2017).
Several rhizospheric bacteria have been documented for their ability to produce IAA as well as their different biosynthetic pathways of IAA production (Cassán et al. Citation2014; Duca et al. Citation2014; Vijayabharathi et al. Citation2016). Results from the present study revealed that all tested bacterial isolates produced indole-3-acetic acid. However, the amount of this acid produced varied in bacterial isolates tested. The highest IAA production was obtained in S. werraensis (10.12 ± 0.02 µg/ml) while the least (5.03 ± 0.01 µg/ml of IAA) was produced by M. oxydans. The increased amount of IAA produced by these bacteria in the medium used was due to the presence of L-tryptophan, as corroborated by Idris et al. (Citation2007) who revealed that the secretion of IAA can be increased by the addition of tryptophan in medium. In this regard, inoculating maize plants with IAA producing bacteria can improve the growth and development of the plant under drought stress. The IAA result of this study is in agreement with previous reports of IAA production by bacteria. Studies have shown that the endophytic IAA producing Streptomyces species (astrovirens, olivaceoviridis, rimosus, rochei and viridis) improved seed germination, growth and root elongation in plants (El-Tarabily Citation2008; Khamna et al. Citation2010). Matsukawa et al. (Citation2007) also reported that IAA triggered cell differentiation, sporulation and hyphal elongation in Streptomyces atroolivaceus.
Solubilization of phosphate is an important mechanism of plant growth promotion (Richardson Citation2001). Bacteria are capable of increasing the availability of phosphorus (P) to plants through mechanisms such as the secretion of phosphatase to free P bound in organic matter and the production of organic acids / chelating substances that helps to decrease rhizosphere pH (Rashid et al. Citation2004; Chen et al. Citation2006). Several bacterial species including Pseudomonas and Bacillus have also been reported to solubilize inorganic phosphates. In a study by Chabot et al. (Citation1993), growth of lettuce and maize were enhanced by certain microorganisms capable of solubilizing mineral phosphate. Rodríguez and Fraga (Citation1999) also reported phosphate solubilization in Pseudomonas striata and Bacillus polymyxa. In this regard, the result from the present study conforms to other previous studies on phosphate solubilization.
The introduction of drought tolerant ACC deaminase producing bacteria to drought stressed soils helps to improve stress tolerance in plants by lowering the production of stress induced ethylene. Several studies have reported the production of ACC deaminase activity bacteria (Glick et al. Citation2007; Rashid et al. Citation2012; Glick Citation2014). Drought tolerant bacteria are capable of surviving in dry environments by adhering to the roots of developing seedlings or on seed coats of plants causing the deamination of ACC (the immediate precursor of ethylene in plants) by ACC deaminase which decreases the level of plant ethylene and consequently enhances plant growth and development (Glick Citation2005; Ali et al. Citation2014). The mechanism of action of ACC deaminase producing bacteria in the improvement of both biotic and abiotic stresses is by the reduction of stress ethylene level through the activity of the enzyme ACC deaminase which breaks down ACC into α-ketobutyrate and ammonia instead of ethylene (Arshad et al. Citation2008). Bacteria producing ACC deaminase activity are known to improve the growth of a wide range of plants under stressful conditions like drought, salinity, heavy metals and flooding (Belimov et al. Citation2009; Shakir et al. Citation2012; Ali et al. Citation2014; Vurukonda et al. Citation2016). They also play major roles in plant nodulation processes in different leguminous plant species (Belimov et al. Citation2009).
The methods used by bacteria to inhibit pathogenic growth may include the secretion of volatile compounds like ammonia and other antifungal enzymes, the production of HCN, competitive secretion and the production of siderophores (Brimecombe et al. Citation2000). The production of low molecular weight metal chelators (siderophore) by the tested bacterial isolates offers them a competitive advantage to be used as biocontrol agents and to contribute to disease suppression in plants due to insufficient supply of essential trace minerals in natural environments (Laslo et al. Citation2012). A stimulated biosynthesis may cause these tested bacterial isolates to directly secrete antimicrobial compounds. In antagonism effect development, siderophore production and antifungal effect play major roles, although antifungal effects encompass other features (Selvakumar et al. Citation2008). Results obtained from the present study concur with the work of Laslo et al. (Citation2012) who reported that 36.2% of tested isolates produced siderophore. Quan et al. (Citation2010) detected different types of siderophores in Pseudomonas species. Furthermore, Plant growth promoting bacteria are able to produce ammonia as secondary metabolite, also playing a major role in antagonistic effects (Compant et al. Citation2005). Among all tested bacterial isolates for HCN activity, only Streptomyces spp. produced HCN, indicating its potential for use as a biocontrol agent.
Plant growth promoting rhizobacteria capable of colonizing both the surface and inner parts of plant roots play essential roles that directly or indirectly influence plant growth and development (Gerhardt et al. Citation2009). In this study, maize seed treatment with the two selected bacterial strains S. pseudovenezuelae and A. arilaitensis significantly improved the emergence and growth of the seedlings. Different mechanisms have been proposed for the promotion of plant growth by PGPB, which include the indirect enhancement of seed germination and vigor index by reduction in the incidence of seed mycoflora that can negatively affect plant growth (Begum et al. Citation2012). In a study by Duarah et al. (Citation2011), amylase activity was increased during rice and legume germination after treatment with PGPB. Starch is hydrolyzed by amylase to metabolizable sugars to provide the roots and shoots of germinating seeds with the energy to grow. The production of phytohormones such as IAA is another commonly reported mechanism of plant growth promotion (Patten and Glick Citation2002). In this study, all tested isolates produced IAA. Studies have shown that several PGPB produce IAA (Ng et al. Citation2012; Zahid Citation2015). IAA promotes root development and nutrient uptake making it a very important mechanism of plant growth promotion.
Drought stress is a serious environmental problem in agriculture as it causes severe loss in plant yield, depending on its severity (Farooq et al. Citation2009). In this study, maize survival and growth was affected when drought stress was introduced. However, under drought stress conditions, better growth was observed in bacterial inoculated maize plants than the un-inoculated plants as better survival, dry root and shoot weight, root and shoot length and chlorophyll content were observed. Over the years, PGPB have been used mostly to promote plant growth because of their ability to stimulate plant growth through certain mechanisms such as the production of plant growth regulators and fixation of nitrogen (Lucy et al. Citation2004). Studies have demonstrated other beneficial effects of PGPB on plants including their ability to enhance tolerance toward several abiotic stresses such as drought (Yang et al. Citation2009; Wang et al. Citation2012; Yandigeri et al. Citation2012; Vurukonda et al. Citation2016). A study by Creus et al. (Citation2004) demonstrated that there was significant increase in growth, water content, water potential, relative water content and apoplastic water function in roots and shoots of wheat plants primed with Azospirillum brasilense Sp245 compared to un-primed plants. In addition, Pereyra et al. (Citation2012) reported that inoculation of Azospirillum on maize seedlings under osmotic stress enhanced better water status of the seedlings indicated by the morphological modifications of the coleoptile xylem architecture. Their results were also attributed to Azospirillum ability to produce IAA and improved bacterial IAA synthesis.
A number of abiotic stresses are associated with ROS species accumulation in plant cells. Reactive oxygen species react with DNA, membrane lipids and proteins and are capable of causing severe oxidative damage to plant tissues (Reddy et al. Citation2004). For plants to be able to survive under drought stress, they need to avoid oxidative damage. These species can be removed by certain enzymes: catalase and peroxidases such as glutathione peroxidase (GPX) and ascorbate peroxidase (APX) (Gong et al. Citation2005). In our previous study, S. pseudovenezuelae and A. arilaitensis showed the presence of glutathione peroxidase, indicating their capability of withstanding drought stress by the avoidance of oxidative damage. This could also be the reason behind the greener leaves of the inoculated plants over the un-inoculated ones. We also observed that the severity of drought stress showed more on the un-inoculated plants than the inoculated plants as the un-inoculated plants showed more signs of wilting than the inoculated plants. The increase in the severity of drought stress causes increase in enzyme (GPX and APX) activity. Koussevitzky et al. (Citation2008) demonstrated that APX activity and APXI transcript levels were increased in Arabidopsis thaliana plants after their exposure to drought stress. They concluded that APX is necessary to protect plant chloroplast from increased levels of ROS during drought as APX helps in scavenging ROS. Omar et al. (Citation2009) also recorded lower peroxidase and catalase activities in the leaves of barley plants primed with Azospirillum brasilense under salinity stress.
Damage to plant proteins often results from stress exposure, therefore it is necessary to maintain proteins in their functional forms to enable plants to survive under stress conditions (Wang and Huang Citation2004). Plant proteins like the Heat-shock proteins (HSP), Malic-enzyme proteins (ME), glycine-rich RNA binding proteins (GRP) and desiccation protectant proteins are often synthesized during stress conditions and are recognized as mechanisms of stress tolerance in plants (Wahid et al. Citation2007). They play major roles in translocation, protein folding, degradation and assembly in several cellular processes. They can also assist in stabilizing and refolding of proteins under different conditions of stress (Wang and Huang Citation2004). From our previous study on drought tolerant genes, S. pseudovenezuelae and A. arilaitensis were observed to possess the proteins glycine-rich RNA binding protein, and Malic-enzyme and this could have also contributed to their better survival under drought stress.
The growth of plants depends highly on differentiation, enlargement and cell division. Also, drought stress affects the physiological, morphological, ecological and genetic processes of plant growth (Taiz and Zeiger Citation2002; Farooq et al. Citation2009; Vurukonda et al. Citation2016). According to Farooq et al. (Citation2009), severe water limitation causes an inhibition in cell elongation as a result of water flow interruption from the xylem to surrounding elongation cells leading to cell mitosis and cell expansion; and finally resulting in reduced plant growth. The growth and formation of lateral roots may be stimulated by PGPB, thereby increasing the capacity of water uptake of inoculated plants. Studies have described the roles of PGPB in modifying plant metabolism under normal and abiotic stress conditions by mechanisms including indole-3-acetic acid production, ACC deaminase activity, nitrogen fixation and antioxidant production (Dimkpa et al. Citation2009; Bashan and De-Bashan Citation2010). Plant growth promoting bacteria are also capable of producing compatible solutes (glycine-betaine and proline) that assist in the processes of osmoregulation (Dimkpa et al. Citation2009). In the present study, better tolerance to drought stress conditions was observed in bacterial inoculated plants than un-inoculated plants. This could be as a result of production of IAA, ACC deaminase and glycine-rich protein by these bacterial isolates.
Besides the inoculation of plants with single strains of PGPB, co-inoculation or combination of two or more strains also induces drought stress tolerance in plants to an even greater extent (Wang et al. Citation2012). From the results obtained in the present study as shown in , better tolerance was observed in the co-inoculation of S. pseudovenezuelae (MG547870) and A. arilaitensis (MG547869) in maize plants as better shoot and root lengths, dry shoot and root weights, chlorophyll content and numbers of leaves were observed in the plants. Moreover, wilting of leaves was observed to be lower in the co-inoculated plants than those inoculated with either S. pseudovenezuelae or A. arilaitensis, which were much better than the control. The results from this study are in agreement with the study of Wang et al. (Citation2012), who observed enhanced drought tolerance in cucumber plants when the seeds were inoculated with a Microbial consortia consisting of the PGPB Bacillus subtilis SM21, Bacillus cereus AR156 and Serratia sp. XY21 (BBS). According to Wang et al. (Citation2012), darker green leaves, lighter wilting symptoms, relative electrical conductance, increased leaf proline and chlorophyll content and intension of root recovery were observed in BBS treated plants after water was withheld for 13 days. In a similar study, exopolysaccharide producing bacterial strains Proteus penneri (Pp1), Pseudomonas aeruginosa (Pa2) and Alcaligenes faecalis (AF3) exhibited better tolerance to drought stress in maize compared to individual PGPB strains (Naseem and Bano Citation2014). Results obtained from the study showed that maize physiological parameters were enhanced by the inoculation with both bacterial isolates, as well as with individual isolates, as better growths were observed in inoculated maize plants than the un-inoculated ones. This confirms the effectiveness of bacteria inoculation on growth and drought tolerance in plants.
The effect of inoculation method on the growth and drought tolerance potential of S. pseudovenezuelae (MG547870) and A. arilaitensis (MG547869) in maize seeds revealed that plants whose seeds were co-inoculated with S. pseudovenezuelae and A. arilaitensis and coated with vermiculite showed better increase in growth parameter measurements compared to the plants whose seeds were directly co-inoculated with the bacterial isolates. Similarly, better growth was observed when seeds were un-inoculated but coated with vermiculite compared to the plants with seeds merely immersed in distilled water. In all, seed inoculation with the combination of the two bacterial strains was more effective than inoculation with individual bacterial strains. The better results obtained from the growth parameter measurements of the vermiculite coated seeds when compared to the un-coated seeds of both inoculated and un-inoculated seeds on the effect of inoculation method used in this study, could have been due to the presence of 1% carboxylmethyl cellulose (CMC), as this adhesive may have facilitated the better binding of the bacterial isolates to the seeds. Carboxymethyl cellulose is an adhesive that has also been used in drug and food industries (Williams and Phillips Citation2004; Delcour and Poutanen Citation2013; Ibarra et al. Citation2016). It plays major roles in binding inoculants to seeds and also protects the seeds from desiccation. It may also provide nourishment for the inoculated plants (Elegba and Rennie Citation1984). On the other hand, vermiculite has a neutral pH with a good buffering capacity. It does not produce organic by-products and does not undergo any change in structure when sterilized. At extreme temperature, it exfoliates and kills contaminants as its mineral nature does not support the growth of microbes. During fermentation, vermiculite provides space for microbial growth, good aeration and quick temperature equilibration. It has good sticking properties which makes it widely used as seed coats. Therefore, the coating of the seeds with vermiculite in this study may have helped to protect the seeds from possible insect and pathogen attacks. From the results obtained in the present study, it is encouraged that for efficient tolerance to drought stress, inoculated seeds should be bound as well as coated with good binding and coating agents as this will reduce pest attacks, preserve seeds for longer periods and enhance easy delivery.
Conclusion
Changes in climatic conditions can bring about undesirable environmental conditions, including drought which is responsible for several physiological and morphological changes in plants leading to mass decrease in agricultural productivity. Soil harbors numerous bacteria that can be beneficial in agriculture to facilitate growth and abiotic stress tolerances in plants. Plant growth promoting bacteria facilitate plants’ growth and help them to resist and adapt to harsh and dry environmental conditions (drought), and could also play a major role in solving the problem of global food insecurity. Tolerance to drought stress by PGPB can be enhanced through a variety of mechanisms ranging from phytohormonal modifications, ACC deaminase activity, to alteration in root morphology and molecular techniques. Drought stress tolerance by bacteria is an emerging technology that is cost effective and efficient to help solve the problem of low crop productivity and yield. This study has shown that the inoculation of drought tolerant S. pseudovenezuelae (MG547870) and A. arilaitensis (MG547869) strains increases plant growth as well as reduces the undesirable effect of drought stress when used as bioinoculants on maize plant. Streptomyces pseudovenezuelae and Arthrobacter arilaitensis with high IAA and ACC production will be beneficial isolates for biofertilization of crops especially under drought stress conditions, as they mitigated the impact of drought on the maize plants and increased plant biomass and physiological parameters.
However, for efficient results, it is highly encouraged that inoculated seeds are bound as well as coated as this will ensure that bacteria are well adhered to the seeds and also protected from insect attacks. Further studies are required to understand the exact molecular mechanisms of plant-bacterial interactions in the rhizosphere for plant growth and drought tolerance, as understanding of these mechanisms is necessary for efficient elicitation of drought stress in plants by soil bacteria.
Author contribution statement
All authors contributed in data collection, wet laboratory, analyses, and drafting of the manuscript for publication.
Disclosure statement
No potential conflict of interest was reported by the author(s).
ORCID
Chinenyenwa Fortune Chukwuneme http://orcid.org/0000-0002-3995-208X
Olubukola Oluranti Babalola http://orcid.org/0000-0003-4344-1909
Funso Raphael Kutu http://orcid.org/0000-0002-8162-0329
Omena Bernard Ojuederie http://orcid.org/0000-0003-0474-6697
Additional information
Notes on contributors
Chinenyenwa Fortune Chukwuneme
Chinenyenwa Fortune Chukwuneme is a doctoral candidate in the Department of Microbiology, North-West University, South Africa. Her research focused on Actinomycetes impacts on drought stress in maize. Her research interests are on microbial biotechnology, metagenomics, molecular plant-microbe interactions, rhizosphere biology, rhizosphere microbiome, bioinformatics, and biofertilizer production. Her present research focuses on the analyses of bacterial communities and functional genes in maize rhizosphere using a metagenomic approach to understand the composition, structure, biogeographic distributions, and the functions of various bacteria associated with maize rhizosphere around the North West Province of South Africa.
Olubukola Oluranti Babalola
Prof Olubukola Oluranti Babalola (Pr. Sci. Nat, MASSAF) hold BSC, MSc, PhD (Microbiology) and a MBA in Sci. Leadership. She is a product of the Int. Institute of Tropical Agric. (IITA), the Org for Women in Science for the Developing World (OWSD), the Weizmann Institute of Sci., Israel, and the Univ. of the Western Cape (UWC), South Africa. She is the Research Director of Food Security and Safety Niche Area at North-West Univ. (NWU), the Vice President of OWSD and without reservation leading, as the Principal Investigator, a Microbial Biotech lab. Her lab is a mini united nation, with students from within and outside Africa. She has graduated 14 PhDs, 16 masters and 48 Honors students. Babalola is a prolific author with ~200 publications. She has over 40 professional certificates from the University of California, Berkeley, USA; University of Mauritius, Reduit; NWU, South Africa and Bradford University, UK to mention a few. Her wealth of int. experience spans through Americas, Asia, Europe, and Oceania.
Funso Raphael Kutu
Dr. Funso Raphael Kutu (Pr. Sci. Nat) is a professor and Head of the School of Agricultural Sciences, at the University of Mpumalanga, South Africa. He is a seasoned agronomist with over 25 years of research experience. His research focuses on improved agronomic practices, cropping systems, soil-crop interactions, compost science and utilization, nutrient recycling in agro-ecosystem, and soil health improvement.
Omena Bernard Ojuederie
Dr. Omena Bernard Ojuederie (Pr. Sci. Nat) is a seasoned researcher and academic with expertise in plant genetics and biotechnology. His research focuses on biodiversity assessment and phylogenetic studies of underutilised crops, plant tissue culture, germplasm conservation, molecular biology, molecular plant-microbe interactions, and improving food security in Africa. He is currently a postdoctoral research fellow at the Food Security and Safety Niche Area of North-West University, South Africa researching on the exploitation of rhizobacteria for the mitigation of drought stresses in maize. He is a member of the Editorial Board of the Journal of Underutilized Legumes and a grantee of the International Foundation for Science (IFS) Sweden.
References
- Adegboye MF, Babalola OO. 2013. Actinomycetes: a yet inexhaustive source of bioactive secondary metabolites. In: Mendez-Vilas A, editor. Microbial pathogens and strategies for combating them: science, technology and education. Microbiology Book Series - 4, Vol. 2. December 2013 ed. Spain: Formatex Research Center. p. 786-795. ISBN-13. 978-84-942134-0-3.
- Adegboye MF, Babalola OO. 2015. Evaluation of antibiotic biosynthetic potential of actinomycete isolates to produce antimicrobial agents. British Microbiol Res J. 7(5):243–254. doi:10.9734/BMRJ/2015/14627.
- Alexander D, Zuberer D. 1991. Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertil Soils. 12:39–45. doi: 10.1007/BF00369386
- Ali SZ, Sandhya V, Venkateswar Rao L. 2014. Isolation and characterization of drought-tolerant ACC deaminase and exopolysaccharide-producing fluorescent Pseudomonas spp. Ann Microbiol. 64:493–502. doi: 10.1007/s13213-013-0680-3
- Arshad M, Shaharoona B, Mahmood T. 2008. Inoculation with Pseudomonas spp. containing ACC-deaminase partially eliminates the effects of drought stress on growth, yield, and ripening of pea (Pisum sativum L.). Pedosphere. 18:611–620. doi: 10.1016/S1002-0160(08)60055-7
- Ashraf M. 2010. Inducing drought tolerance in plants: recent advances. Biotechnol Adv. 28:169–183. doi: 10.1016/j.biotechadv.2009.11.005
- Babalola OO. 2010. Beneficial bacteria of agricultural importance. Biotechnol Lett. 32(11):1559–1570. ISSN: 0141-5492 E-ISSN: 1573-6776. doi:10.1007/s10529-010-0347-0.
- Babalola OO, Glick BR. 2012. The use of microbial inoculants in African agriculture: current practice and future prospects. J Food Agric Environ (Finland). 10(3 & 4):540–549. (ISSN: 1459-0263).
- Bakker AW, Schippers B. 1987. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp-mediated plant growth-stimulation. Soil Biol Biochem. 19:451–457. doi: 10.1016/0038-0717(87)90037-X
- Bardi L, Malusà E. 2012. Drought and nutritional stresses in plant: alleviating role of rhizospheric microorganisms. In: Haryana Nikhil, Punj Shreya, editors. Abiotic stress: new research. Hauppauge, NY, USA: Nova Science; p. 1–57.
- Bashan Y, De-Bashan LE. 2010. How the plant growth-promoting bacterium Azospirillum promotes plant growth-a critical assessment. In: Sparks Donald L., editor. Advances in Agronomy. Vol. 108. Academic Press; p. 77–136.
- Begum M, Rai VR, Lokesh S. 2012. Effect of plant growth promoting rhizobacteria on seed borne fungal pathogens in okra. Indian Phytopathol. 56:156–158.
- Belimov AA, Dodd IC, Hontzeas N, Theobald JC, Safronova VI, Davies WJ. 2009. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytol. 181:413–423. doi: 10.1111/j.1469-8137.2008.02657.x
- Brimecombe MJ, De Leij FA, Lynch JM, Pinton R, Varanini Z, Nannipieri P. 2000. The effect of root exudates on rhizosphere microbial populations. Biochemistry and organic substances at the soil-plant interface. In: Pinton R, Varaninin Z, Nannipieri P, editors. The Rhizosphere, biochemistry and organic substances at the soil-plant interface. New York: Marcel Dekker. p. 95–140.
- Cassán F, Vanderleyden J, Spaepen S. 2014. Physiological and agronomical aspects of phytohormone production by model plant-growth-promoting rhizobacteria (PGPR) belonging to the genus Azospirillum. J Plant Growth Regul. 33:440–459. doi: 10.1007/s00344-013-9362-4
- Chabot R, Antoun H, Cescas MP. 1993. Stimulation de la croissance du maïs et de la laitue romaine par des microorganismes dissolvant le phosphore inorganique. Can J Microbiol. 39:941–947. doi: 10.1139/m93-142
- Chen Y, Rekha P, Arun A, Shen F, Lai W-A, Young C. 2006. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl Soil Ecol. 34:33–41. doi: 10.1016/j.apsoil.2005.12.002
- Chukwuneme CF. 2018. Actinomycetes impacts on drought stress in maize [Dissertation - Masters]. South Africa: North-West University..
- Coleman-Derr D, Tringe SG. 2014. Building the crops of tomorrow: advantages of symbiont-based approaches to improving abiotic stress tolerance. Front Microbiol. 5:283–296. doi:10.3389/fmicb.2014.00283.
- Compant S, Duffy B, Nowak J, Clément C, Barka EA. 2005. Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol. 71:4951–4959. doi: 10.1128/AEM.71.9.4951-4959.2005
- Creus CM, Sueldo RJ, Barassi CA. 2004. Water relations and yield in Azospirillum-inoculated wheat exposed to drought in the field. Can J Botany. 82:273–281. doi: 10.1139/b03-119
- Delcour JA, Poutanen K, editors. 2013. Fibre-rich and wholegrain foods: improving quality. Woodhead, Cambridge. p. 1–459.
- Dimkpa C, Weinand T, Asch F. 2009. Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 32:1682–1694. doi: 10.1111/j.1365-3040.2009.02028.x
- Duarah I, Deka M, Saikia N, Deka Boruah HP. 2011. Phosphate solubilizers enhance NPK fertilizer use efficiency in rice and legume cultivation. Biotechnol. 1:227–238.
- Duca D, Lorv J, Patten CL, Rose D, Glick BR. 2014. Indole-3-acetic acid in plant–microbe interactions. Antonie Van Leeuwenhoek. 106:85–125. doi:10.1007/s10482-013-0095-y.
- Dworkin M, Foster J. 1958. Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol. 75:592–603. doi: 10.1128/JB.75.5.592-603.1958
- Eisenstein M. 2013. Discovery in a dry spell. Nature. 501:S7–S9. doi:10.1038/501S7a.
- El-Tarabily KA. 2008. Promotion of tomato (Lycopersicon esculentum Mill.) plant growth by rhizosphere competent 1-aminocyclopropane-1-carboxylic acid deaminase-producing streptomycete actinomycetes. Plant Soil. 308:161–174. doi: 10.1007/s11104-008-9616-2
- Elegba M, Rennie R. 1984. Effect of different inoculant adhesive agents on rhizobial survival, nodulation, and nitrogenase (acetylene-reducing) activity of soybeans (Glycine max (L.) Merrill). Can J Soil Sci. 64:631–636. doi: 10.4141/cjss84-063
- Farooq M, Wahid A, Kobayashi N, Fujita D, Basra S. 2009. Plant drought stress: effects, mechanisms and management. Agron Sustainable Dev. 29:185–212. doi: 10.1051/agro:2008021
- Figueiredo MV, Burity HA, Martínez CR, Chanway CP. 2008. Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl Soil Ecol. 40:182–188. doi: 10.1016/j.apsoil.2008.04.005
- Gerhardt KE, Huang X-D, Glick BR, Greenberg BM. 2009. Phytoremediation and rhizoremediation of organic soil contaminants: potential and challenges. Plant Sci. 176:20–30. doi: 10.1016/j.plantsci.2008.09.014
- Ghorbanpour M, Hatami M. 2014. Biopriming of salvia officinalis seed with growth promoting rhizobacteria affects invigoration and germination indices. J. Biol Environ Sci. 8:29–36.
- Glick BR. 2005. Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol Lett. 251:1–7. doi: 10.1016/j.femsle.2005.07.030
- Glick BR. 2014. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res. 169:30–39. doi: 10.1016/j.micres.2013.09.009
- Glick BR, Todorovic B, Czarny J, Cheng Z, Duan J, McConkey B. 2007. Promotion of plant growth by bacterial ACC deaminase. Crit Rev Plant Sci. 26:227–242. doi: 10.1080/07352680701572966
- Gong H, Zhu X, Chen K, Wang S, Zhang C. 2005. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 169:313–321. doi: 10.1016/j.plantsci.2005.02.023
- Gusain YS, Singh U, Sharma A. 2015. Bacterial mediated amelioration of drought stress in drought tolerant and susceptible cultivars of rice (Oryza sativa L.). Afr J Biotechnol. 14:764–773. doi: 10.5897/AJB2015.14405
- Ibarra VG, Sendón R, de Quirós AR-B. 2016. Antimicrobial food packaging based on biodegradable materials. In: Barros-Velazques Jorge, editor. Antimicrobial food packaging. San Diego, USA: Academic Press; p. 363–384.
- Idris EE, Iglesias DJ, Talon M, Borriss R. 2007. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol Plant-Microbe Interact. 20:619–626. doi: 10.1094/MPMI-20-6-0619
- Islam MR, Madhaiyan M, Deka Boruah HP, Yim W, Lee G, Saravanan VF, Fu Q, Hu H, Sa T. 2009. Characterization of plant growth-promoting traits of free-living diazotrophic bacteria and their inoculation effects on growth and nitrogen uptake of crop plants. J Microbiol Biotechnol. 19(10):1213–1222. doi:10.4014/jmb.0903.3028.
- Jongdee B, Pantuwan G, Fukai S, Fischer K. 2006. Improving drought tolerance in rainfed lowland rice: an example from Thailand. Agric Water Manag. 80:225–240. doi: 10.1016/j.agwat.2005.07.015
- Khamna S, Yokota A, Peberdy JF, Lumyong S. 2010. Indole-3-acetic acid production by Streptomyces sp. isolated from some Thai medicinal plant rhizosphere soils. EurAsian J. BioSci. 4:23–32. doi: 10.5053/ejobios.2010.4.0.4
- Khantsi M, Adegboye MF, Babalola OO. 2013. 1-Aminocyclopropane-1-carboxylate deaminase activity as a marker for identifying plant-growth promoting rhizobacteria in cultivated soil. Asian Life Sci. 9:199–211. ISSN 0117-3375.
- Koussevitzky S, Suzuki N, Huntington S, Armijo L, Sha W, Cortes D, Shulaev V, Mittler R. 2008. Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J Biol Chem. 283:34197–34203. doi: 10.1074/jbc.M806337200
- Langridge P, Reynolds MP. 2015. Genomic tools to assist breeding for drought tolerance. Curr Opin Biotechnol. 32:130–135. doi: 10.1016/j.copbio.2014.11.027
- Laslo É, György É, Mara G, Tamás É, Ábrahám B, Lányi S. 2012. Screening of plant growth promoting rhizobacteria as potential microbial inoculants. Crop Prot. 40:43–48. doi: 10.1016/j.cropro.2012.05.002
- Lucy M, Reed E, Glick BR. 2004. Applications of free living plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek. 86:1–25. doi: 10.1023/B:ANTO.0000024903.10757.6e
- Maazou A-RS, Tu J, Qiu J, Liu Z. 2016. Breeding for drought tolerance in maize (Zea mays L.). Am J Plant Sci. 7:1858–1870. doi: 10.4236/ajps.2016.714172
- Madhaiyan M, Poonguzhali S, Sa T. 2007. Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere. 69:220–228. doi: 10.1016/j.chemosphere.2007.04.017
- Matsukawa E, Nakagawa Y, Iimura Y, Hayakawa M. 2007. Stimulatory effect of indole-3-acetic acid on aerial mycelium formation and antibiotic production in Streptomyces spp. Actinomycetologica. 21:32–39. doi: 10.3209/saj.SAJ210105
- Naseem H, Bano A. 2014. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J Plant Interact. 9:689–701. doi: 10.1080/17429145.2014.902125
- Ndeddy Aka RJ, Babalola OO. 2016. Effect of bacterial inoculation of strains of Pseudomonas aeruginosa, Alcaligenes feacalis and Bacillus subtilis on germination, growth and heavy metal (Cd, Cr, and Ni) uptake of Brassica juncea. Int J Phytorem. 18:200–209. doi: 10.1080/15226514.2015.1073671
- Ndeddy Aka RJ, Babalola OO. 2017. Identification and characterization of Cr-, Cd- and Ni- tolerant bacteria isolated from mine tailings. Biorem J. 21:1–19. doi:10.1080/10889868.2017.1282933.
- Ng L, Sariah M, Sariam O, Radziah O, Zainal Abidin M. 2012. Rice seed bacterization for promoting germination and seedling growth under aerobic cultivation system. Austr J Crop Sci. 6:170–175.
- Omar M, Osman M, Kasim W, El-Daim IA. 2009. Improvement of salt tolerance mechanisms of barley cultivated under salt stress using Azospirillum brasilense. In: Ashraf M, Ozturk M, Habib-ur-Rehman A, editors. Salinity and water stress improving crop efficiency. Netherlands: Springer. p. 133–147.
- Passari AK, Mishra VK, Saikia R, Gupta VK, Singh BP. 2015. Isolation, abundance and phylogenetic affiliation of endophytic actinomycetes associated with medicinal plants and screening for their in vitro antimicrobial biosynthetic potential. Front Microbiol. 6:273. doi:10.3389/fmicb.2015.00273.
- Patten CL, Glick BR. 2002. Regulation of indoleacetic acid production in Pseudomonas putida GR12-2 by tryptophan and the stationary-phase sigma factor RpoS. Can J Microbiol. 48:635–642. doi: 10.1139/w02-053
- Penrose DM, Glick BR. 2003. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant. 118:10–15. doi: 10.1034/j.1399-3054.2003.00086.x
- Pereyra M, Garcia P, Colabelli M, Barassi C, Creus C. 2012. A better water status in wheat seedlings induced by Azospirillum under osmotic stress is related to morphological changes in xylem vessels of the coleoptile. Appl Soil Ecol. 53:94–97. doi: 10.1016/j.apsoil.2011.11.007
- Philippot L, Raaijmakers JM, Lemanceau P, Van Der Putten WH. 2013. Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol. 11:789–799. doi: 10.1038/nrmicro3109
- Quan C, Wang X, Fan S. 2010. Antifungal compounds of plant growth promoting rhizobacteria and its action mode. In: Maheshwari DK, editor. Plant growth and health promoting bacteria. Berlin-Heidelberg: Springer, Verlag. p. 117–156.
- Rashid S, Charles TC, Glick BR. 2012. Isolation and characterization of new plant growth-promoting bacterial endophytes. Appl Soil Ecol. 61:217–224. doi: 10.1016/j.apsoil.2011.09.011
- Rashid M, Khalil S, Ayub N, Alam S, Latif F. 2004. Organic acids production and phosphate solubilization by phosphate solubilizing microorganisms (PSM) under in vitro conditions. Pak J Biol Sci. 7:187–196. doi: 10.3923/pjbs.2004.187.196
- Reddy AR, Chaitanya KV, Vivekanandan M. 2004. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol. 161:1189–1202. doi: 10.1016/j.jplph.2004.01.013
- Renn, AL. 2013. Bioinformatic and functional analysis of 1-aminocyclopropane-1-carboxylate (ACC) deaminase homologues in strains of sinorhizobium. Minnesota: Hamline University. Departmental Honors Projects. 4. https://digitalcommons.hamline.edu/dhp/4
- Richardson AE. 2001. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Funct Plant Biol. 28:897–906. doi: 10.1071/PP01093
- Rincón A, Valladares F, Gimeno TE, Pueyo JJ. 2008. Water stress responses of two Mediterranean tree species influenced by native soil microorganisms and inoculation with a plant growth promoting rhizobacterium. Tree Physiol. 28:1693–1701. doi: 10.1093/treephys/28.11.1693
- Rodríguez H, Fraga R. 1999. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv. 17:319–339. doi: 10.1016/S0734-9750(99)00014-2
- SAS. 2014. SAS 9.4 output delivery system: user guide. Cary (NC): SAS institute.
- Sathya A, Vijayabharathi R, Gopalakrishnan S. 2017. Plant growth-promoting actinobacteria: a new strategy for enhancing sustainable production and protection of grain legumes. 3 Biotechnol. 7:102–112. Springer-Verlag Berlin, Heidelberg.
- Schwyn B, Neilands J. 1987. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 160:47–56. doi: 10.1016/0003-2697(87)90612-9
- Selvakumar G, Mohan M, Kundu S, Gupta A, Joshi P, Nazim S, Gupta H. 2008. Cold tolerance and plant growth promotion potential of Serratia marcescens strain SRM (MTCC 8708) isolated from flowers of summer squash (Cucurbita pepo). Lett Appl Microbiol. 46:171–175. doi: 10.1111/j.1472-765X.2007.02282.x
- Shakir MA, Asghari B, Muhammad A. 2012. Rhizosphere bacteria containing ACC-deaminase conferred drought tolerance in wheat grown under semi-arid climate. Soil Environ. 31:108–112.
- Sreevidya M, Gopalakrishnan S, Kudapa H, Varshney R. 2016. Exploring plant growth-promotion actinomycetes from vermicompost and rhizosphere soil for yield enhancement in chickpea. Braz J Microbiol. 47:85–95. doi: 10.1016/j.bjm.2015.11.030
- Taiz L, Zeiger E. 2002. Plant physiology. 5th ed. Massachusetts: Sinauer Associates, Inc.
- Vijayabharathi R, Sathya A, Gopalakrishnan S. 2016. A Renaissance in plant growth-promoting and biocontrol agents by endophytes. In: Singh Dhananjaya P., Singh Harikesh P., Prabha Ratna, editors. Microbial inoculants in sustainable agricultural productivity. New Delhi: Springer; p. 37–60. doi:10.1007/978-81-322-2647-5.
- Vurukonda SSKP, Vardharajula S, Shrivastava M, SkZ A. 2016. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol Res. 184:13–24. doi: 10.1016/j.micres.2015.12.003
- Wahid A, Gelani S, Ashraf M, Foolad MR. 2007. Heat tolerance in plants: an overview. Environ Exp Bot. 61:199–223. doi: 10.1016/j.envexpbot.2007.05.011
- Wang Z, Huang B. 2004. Physiological recovery of Kentucky bluegrass from simultaneous drought and heat stress. Crop Sci. 44:1729–1736. doi: 10.2135/cropsci2004.1729
- Wang C-J, Yang W, Wang C, Gu C, Niu D-D, Liu H-X, Wang Y-P, Guo J-H. 2012. Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS One. 7:e52565–e52577. doi: 10.1371/journal.pone.0052565
- Williams P, Phillips G. 2004. Effect of hydrocolloids on emulsion stability, In Gums and Stabilizer’s for the food industry. J Agric Food Chem. 53:3594–4040.
- Yandigeri MS, Meena KK, Singh D, Malviya N, Singh DP, Solanki MK, Yadav AK, Arora DK. 2012. Drought-tolerant endophytic actinobacteria promote growth of wheat (Triticum aestivum) under water stress conditions. Plant Growth Regul. 68:411–420. doi: 10.1007/s10725-012-9730-2
- Yang J, Kloepper JW, Ryu C-M. 2009. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 14:1–4. doi: 10.1016/j.tplants.2008.10.004
- Zahid M. 2015. Isolation and identification of indigenous plant growth promoting rhizobacteria from Himalayan region of Kashmir and their effect on improving growth and nutrient contents of maize (Zea mays L.). Front Microbiol. 6:207. doi: 10.3389/fmicb.2015.00207
- Zhang H, Kim M-S, Sun Y, Dowd SE, Shi H, Paré PW. 2008. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol Plant- Microbe Interact. 21:737–744. doi: 10.1094/MPMI-21-6-0737