 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Karstification forms tremendous karst carbon sinks in the Earth. Whether terrestrial higher plants can absorb and utilize bicarbonate, there is a key testimony that karst carbon sinks can be transformed into carbon sequestrations by terrestrial higher plants. The uptake and use of root-derived bicarbonate, photosynthesis, phosphoenolpyruvate carboxylase and ribulose-1,5-bisphosphate carboxylase contents of Broussonetia papyrifera (Bp) and Morus alba L. (Ma) were measured. This study provides the most direct and primary evidence for the transformation using the bidirectional isotope tracer technique. The transformation may result from the synergism in the absorption and utilization of photosynthetic and nonphotosynthetic pathway, and simultaneously strengthen karst carbon sink and carbon sequestrations of plants, while it had no effect on photosynthetic CO2 assimilation in leaves. Differences in the transformation result in the discrepancies of Bp and Ma in the adaptation to karst environments. Karst-adaptable plants can more regulate the entire carbon cycle.
1. Introduction
For a long time, global major carbon pools, carbon sources, carbon sinks and carbon budgets have attracted much attention (Raymond et al. Citation2013; Friedlingstein et al. Citation2020; Luo et al. Citation2020; Ran et al. Citation2021). The overall average time of global carbon turnover is 23 (+7) years (95% confidence interval), and carbon turnover in many semiarid regions is faster (Carvalhais et al. Citation2014). The carbon sinks of exposed areas of carbonate rocks in China account for 177×1011 g CO2/a (Jiang and Yuan Citation1999). With climate warming, high temperatures increase rock weathering, and the CO2 consumption rate of carbonate rock weathering is 6.248×1011 g CO2/a (Liu et al. Citation2021). Plants can effectively promote long-term carbon storage by directly accumulating and fixing organic carbon in their ecosystems. More than one-third of the CO2 in the atmosphere is exchanged annually with the terrestrial biosphere through stomata into leaves and the leaf water solution, and approximately half of this carbon is fixed during photosynthesis (Farquhar et al. Citation1993; Ciais et al. Citation1997). The CO2 exchange of leaves is approximately 15 μmol m−2 s−1 at 25°C, and more than half of the carbon fixed by plants in a year is consumed by plant respiration (Wang et al. Citation2001). The carbon exchange capacity of plants is less affected by environmental changes and temperature at the annual scale, and physiological processes controlling water loss during carbon uptake reach a sustainable balance throughout the year (Law et al. Citation2002), so the carbon exchange of photosynthesis is relatively stable. With the increase of plant coverage, both the net primary productivity (NPP) and net ecosystem productivity (NEP) of land has increased (Cao et al. Citation2003; Zhu et al. Citation2016; Lu et al. Citation2018). In karst regions, above-ground biomass carbon increased by 9% under drought conditions (Tong et al. Citation2018).
The carbon cycle is a key process of terrestrial ecosystem changes. Typically, photosynthesis plays an important role in adjusting to climate change by fixing CO2 in the atmosphere through stomata and converting it into organic matter. However, the rock exposure rate in karst regions is high, and rainwater enters groundwater from rock crevices, resulting in a two-dimensional structure of water and soil separation, which makes water and soil loss occur easily (Wang et al. Citation2004; Van Beynen and Townsend Citation2005; Yan et al. Citation2012). Due to the unique features of karst regions, plants often root in cracks, resulting in a complex plant-soil-rock structure. The plants are thus exposed to for a long time. HCO3- is the main anion present in the soil solution of calcareous soils (Yan et al. Citation2012). The climate in karst regions of Guizhou Province, China, is humid year-round. In humid and anoxic soil environments, the
concentration can reach 5 mM or even 10 mM (Boxma Citation1972; Bloom and Inskeep Citation1986).
The interaction between CO2 sequestration of plants and karst carbon sinks has a great impact on the carbon pool of terrestrial ecosystems. In karst regions, maintains a dynamic balance, and it is difficult to determine whether karst carbon sinks are transformed into CO2 sequestrations. Previous work has demonstrated the absorption and utilization of bicarbonate at the leaf level (Wu and Xing Citation2012; Rao and Wu Citation2017), but more direct evidence from roots is lacking. The two Moraceae species studied here, Broussonetia papyrifera (Bp) and Morus alba L. (Ma), have different adaptability to low nutrient and excess
environments, and Bp is a karst-adaptable plant because of its good adaptability to karst soil (Zhao and Wu Citation2017; Yao and Wu Citation2021). Whether plants can absorb and use bicarbonate is one of the key points in proving the transformation of karst carbon sinks into carbon sequestrations of terrestrial higher plants. Moreover, it is of great significance to understand the utilization of inorganic carbon by plants as an additional carbon source for supplementary photosynthesis. This study provides a direct, rapid and nondestructive method for quantifying the bicarbonate absorption capacity of plants and observes the response of plants to photosynthetic CO2 assimilation during bicarbonate absorption under different photoperiods.
2. Materials and methods
2.1. Plant materials
The experiment was conducted in an artificial climate chamber of the Institute of Geochemistry, Chinese Academy of Sciences, Guiyang, China. Seeds of Bp and Ma were germinated in a greenhouse on a mixture of perlite and vermiculite. The germinated seeds of Bp and Ma were subsequently cultivated in 1/2-strength modified Hoagland solution for 10 months and 5 months, respectively, until the plant height was 52–57 cm. Seedlings with a similar size were used for the experiment. All plants for the experiment were grown under controlled environmental conditions in a greenhouse under a 12-h photoperiod (500 ± 50 μmol m−2 s−1, photosynthetically active radiation) and a temperature of 25°C/20°C (light/dark). Relative humidity was maintained at 50%–55%.
2.2. Absorption experiment of bidirectional isotope tracer culture
This experiment was divided into 2 labeling groups, 3 treatments, and 3 replicates for each treatment. The absorption solution was 2 L of the modified Hoagland nutrient solution supplemented with 10 mM NaHCO3 with different δ13C values, 4.00‰ (labeled 1) and −27.07‰ (labeled 2). The pH of the absorption solution was adjusted to 8.30 ± 0.05, which can maintain most in this range (Millero Citation2014). Sixty-five milliliters of absorption solution was used to measure the δ13C of dissolved inorganic carbon (DIC). The 3 treatments were a 12-h photoperiod (control), 24 h of continuous light after 12 h of darkness (24L) and 24 h of continuous dark after 12 h of illumination (24D). The same plants were used for labeled groups 1 and 2. No plant was placed in the bidirectional isotope trace blank culture system (blank). In the control, Bp was selected from the root/shoot ratio (R/S) value of approximately 0.5. In 24L and 24D, Bp was selected from the R/S value of approximately 1.0. The DIC measurement was performed on a gas isotope mass spectrometer (MAT252, Finnigan, Germany). Vacuumed the sealed bottle containing 2–3 ml phosphoric acid and injected absorption solution for full reaction. CO2 produced by the reaction was purified and then measured δ13C values. The volume of solution at the end of the experiment was measured.
2.3. Leaf gas exchange
The CO2 assimilation rate (Ac), stomatal conductance (gs) and transpiration rate (Tr) were measured after treatment (control and 24L) by a portable photosynthesis measurement system (Li-6400; Li-Cor, Lincoln, NE). The PPFD was set at 500 μmol m−2 s−1, and the CO2 concentration was 380 μmol m−2 s−1. The water-use efficiency (WUE) was calculated as follows: WUE = Pn/Tr. The 24D treatment group was exposed to 500 ± 50 μmol m−2 s−1 light for one hour prior to measurement, and the remaining steps were the same.
2.4. Chlorophyll fluorescence
Chlorophyll fluorescence was measured after the determination of leaf gas exchange by a portable photosynthesis system (Li-6400; Li-Cor, Lincoln, NE). First, the minimum initial chlorophyll fluorescence (Fo) and maximum chlorophyll fluorescence (Fm) of the plants were measured after dark adaptation for more than 30 min. Subsequently, the maximum fluorescence () of plants and the steady-state yield of fluorescence (Fs) in the light were measured after the plants were photoactivated for 1 h, at which point the photosynthetic photon flux density (PPFD) was set at 200 μmol m−2 s−1. The photosystem II potential photochemical quantum efficiency (Fv/Fm), the efficiency of photosystem II photochemistry (φPSII) and the electron transfer rate (ETR) can be calculated (Weis and Berry Citation1987; Maxwell and Johnson Citation2000).
2.5. PEPC and Rubisco
The phosphoenolpyruvate carboxylase (PEPC) and bibulose-1,5-bisphosphate carboxylase (Rubisco) contents under the 3 treatments (control, 24L and 24D) were measured by a double antibody one-step cascade enzyme-linked immunosorbent assay (ELISA). Samples, standards and horseradish peroxidase (HRP)-labeled detection antibodies were successively added to micropores precoated with PEPC and Rubisco antibodies, which were incubated and thoroughly washed. Tetramethylbenzidine (TMB) is a chromogenic substrate and changes color from blue to yellow. The intensity of color was positively correlated with the concentrations of PEPC and Rubisco in the sample. The optical density (OD) was measured at 450 nm using a microplate spectrophotometer. The concentrations of PEPC and Rubisco in the sample were then determined by comparing the OD of the samples to the standard curve. The measured samples were fresh roots, stems and leaves of plants stored in a refrigerator at −80°C until analysis.
2.6. Calculation of bicarbonate absorption
The strong fractionation characteristics of stable carbon isotopes facilitate the identification of different inorganic carbon sources. The bivariate isotope-mixture model can be expressed as
(1)
(1) where δi is the δ13C value of inorganic carbon in the culture medium at a certain period of time in cultured plants, δCa is the δ13C value of inorganic carbon dissolved in the culture solution from CO2 in the air, δCi is the δ13C value of bicarbonate in the initial culture solution, and fBi is the proportion of added exogenous bicarbonate accounting for the total inorganic carbon sources in the culture solution at a certain period of time.
For labeled 1, Eq. 1 can be rewritten as follows:
(2)
(2) For labeled 2, Eq. 1 can be rewritten as follows:
(3)
(3)
Comparing Eqs. 2 and 3, fB = fB1 = fB2; thus, Eqs. 2 and 3 can be used for the following calculations:
(4)
(4) where fB is the proportion of added exogenous bicarbonate accounting for the total inorganic carbon sources in the plant culture system at a certain period of time, δ1 is the δ13C value of inorganic carbon in the culture medium at a certain period of time in labeled 1, δ2 is the δ13C value of inorganic carbon in the culture medium at a certain period of time in labeled 2, δC1 is the δ13C value of bicarbonate in the initial culture solution in labeled 1, and δC2 is the δ13C value of bicarbonate in the initial culture solution in labeled 2.
In the blank culture system, the added exogenous bicarbonate ions can be exchanged with CO2 in the air. After a period of consumption, the proportion of added exogenous bicarbonates accounting for the total inorganic carbon source in the blank culture system can be represented as follows:
(5)
(5) In the bidirectional isotope trace plant and blank culture system under the different time periods Eqs. 4 and 5 can be rewritten as
(6)
(6)
(7)
(7) where i is the sampling time.
In the bidirectional isotope trace blank culture system under the different time periods, the cumulative bicarbonate consumption amount (mi) can be calculated as follows:
(8)
(8) where c is the initial concentration of bicarbonate set at 10 mM, v0i is the volume of the culture solution at different times, f00 is the proportion of the labeled bicarbonate in the initial culture solution, and
is cumulative sampling consumption.
In the bidirectional isotope trace plant culture system under the different time periods, the cumulative bicarbonate consumption amount (pi) can be rewritten as
(9)
(9) where c is the initial concentration of bicarbonate set at 10 mM, v1i is the volume of the culture solution at different times, fB0 is the proportion of the labeled bicarbonate in the initial culture solution, and d1i is cumulative sampling consumption.
In the bidirectional isotope trace blank culture system under the different time periods, can be calculated as follows:
(10)
(10) where vd is the sampling volume,
is 0, and
is the last cumulative sampling consumption.
In the bidirectional isotope trace plant culture system under the different time periods, can be written as
(11)
(11) where vd is the sampling volume,
is 0, and
is the last cumulative sampling consumption.
In the bidirectional isotope trace blank culture system under the different time periods, the cumulative consumption amount of dissolved bicarbonate from the air (ni) can be calculated as follows:
(12)
(12) In the bidirectional isotope trace blank culture system under the different time periods, the cumulative consumption amount of total bicarbonate (a0) can be calculated as follows:
(13)
(13)
In the bidirectional isotope trace plant culture system under the different time periods, the cumulative consumption amount of dissolved bicarbonate from the air (qi) can be written as
(14)
(14) In the bidirectional isotope trace plant culture system under the different time periods, the cumulative consumption amount of total bicarbonate (b0) can be written as
(15)
(15)
A linear relationship model between a0, b0 and absorption time was constructed, and the slope of the model was the consumption rate of the total bicarbonate in the bidirectional isotope trace culture systems. The bicarbonate absorption rate (Vb) of the plant was calculated as
(16)
(16) where V1 is the consumption rate of the total bicarbonate in the bidirectional isotope trace plant culture systems, and V0 is the consumption rate of the total bicarbonate in the bidirectional isotope trace blank culture systems.
After the treatment, the shoot fresh weight (Sfw) was measured. The bicarbonate absorption rate of shoots (VSFW) was calculated as follows:
(17)
(17)
2.7. Statistical analysis
All measurements were subjected to analysis of variance (ANOVA). The means of the different groups were compared via Duncan’s multiple range tests. Tests were considered significant at the p < 0.05 level. Data are shown as the mean ± the standard error (SE). Statistical analyses were carried out with SPSS software (version 25, IBM.). Linear fitting analyses and graphing were carried out with Origin software (version 9.4, OriginLab.).
3. Results
3.1. Absorption of bicarbonate by plants
The linear fitting results of the changes in a0 and b0 showed discrepancies in the different treatments of the two plant species (). In the control, the Vb of Bp is higher than that of Ma. The Vb of Bp was higher under the control and 24L than 24D, and there was no significant difference between the control and 24L. The Vb of Bp under the control and 24L were 2.5 times that under 24D. The Vb of Ma was higher under the control and 24L than 24D, there is no significant difference between the control and 24L, and the Vb of Ma under the control and 24L were 5 and 7 times that under 24D, respectively. Through the results of the linear fitting analysis (), there is an excellent linear proportional relationship between VSFW and absorbed time, and the square values of the correlation coefficient (R2) exceed 0.94. In the control, there is no significant difference between the VSFW of Bp and Ma. The VSFW of Bp was highest under the control and was 1.5 and 3.8 times that of 24L and 24D, respectively. The VSFW of Bp under 24L was 2.5 times that of 24D. The VSFW of Ma was highest in 24L and was 1.4 and 5.9 times that of the control and 24D, respectively. The VSFW of Ma under the control was 4.3 times that of 24D.
Figure 1. a0 and b0 of Bp and Ma in the fitting linear relationship between the cumulative amount of total bicarbonate consumed and absorbed time under the control (A and B), 24L (C and D) and 24D (E and F), respectively.
Note: The shadow part represents the plant absorption rate of bicarbonate (Vb). a0, the cumulative consumption amount of total bicarbonate in the bidirectional isotope trace blank culture system; b0, the cumulative consumption amount of total bicarbonate in the bidirectional isotope trace palnt culture system.
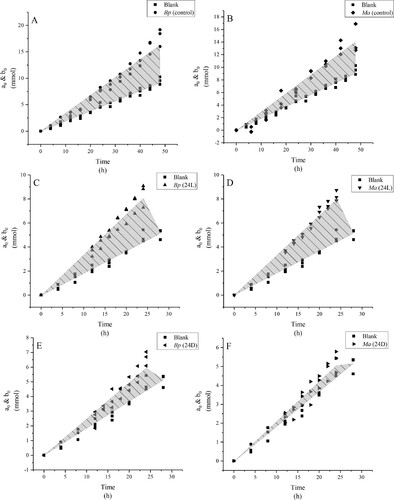
Table 1. The consumption rate of the total bicarbonate in blank and plant culture system under the control, 24L and 24D.
3.2. Photosynthetic CO2 assimilation
Leaf gas exchange and chlorophyll fluorescence represent photosynthetic CO2 assimilation. As shown in , the Ac, gs, and Tr of Bp were highest, while the WUE was lowest under the control. The Ac of Bp under 24L was lower than that under 24D. gs, Tr and WUE of Bp were not significantly different between 24L and 24D. The Ac of Ma was highest under 24D and had no significant differences between the control and 24L. gs, Tr and WUE of Ma had no significant differences among the 3 treatments. φPSII and ETR of the two plant species had no significant differences among the 3 treatments. The Fv/Fm of Bp under the control and 24D treatments was significantly higher than that under 24L. The Fv/Fm of Ma was highest under the control.
Table 2. Leaf gas exchange and chlorophyll fluorescence under the control, 24L and 24D.
3.3. PEPC and Rubisco
PEPC and Rubisco were found in roots, stems and leaves under the 3 treatments, but there was a discrepancy between the two plant species under different treatments. As shown in , the PEPC of the stems and leaves of the two plant species were not significantly different among the 3 treatments. The PEPC of Bp roots was not significantly different among the 3 treatments. The PEPC of Ma roots under 24L was 78% that under 24D. There was more PEPC in the shoots of plants than in the roots. Rubisco in the roots had no significant difference between Bp and Ma among the 3 treatments. Rubisco of Bp stems was lower under 24D than 24L. The Rubisco of Ma stems was higher under the 24L than control. The Rubisco of Bp leaves under 24L was 79% that under 24D. The Rubisco of Ma leaves had no significant difference among the 3 treatments. There was more Rubisco in the shoots of plants than in the roots.
Table 3. The content of PEPC and Rubisco in roots (R), stems (S) and leaves (L) under the control, 24Land 24D.
4. Discussion
This study demonstrates that plants absorb and utilize considerable amounts of bicarbonate under both light and dark conditions, indicating that karst carbon sinks can be converted into carbon sequestrations by plants during all times of day. The absorption of bicarbonate with first-order reaction kinetics reveals that plants can absorb and utilize root-derived in addition to use CO2 from the air (). Meanwhile, bicarbonate can be absorbed and utilized by the nonphotosynthetic pathway in the dark in addition to via photosynthesis. Inorganic carbon sequestration via these two pathways is a key mechanism for plant adaptation to karst environments. However, the bicarbonate uptake of Bp and Ma was different (), which may cause discrepancies in the adaptation to the karst environment in the two plant species.
This study also showed that inorganic carbon sequestration from karst carbon sinks was independent of CO2 assimilation. Bp had greater photosynthetic CO2 assimilation than Ma under the control, but there was no significant difference between Ma and Bp in the absorption rate of bicarbonate (, ). The changes in VSFW and photosynthetic CO2 assimilation capabilities of the two plant species under 24L and 24D were inconsistent. Non parallel in bicarbonate uptake and photosynthetic CO2 assimilation of plants indicated that the conversion of karst carbon sinks into photosynthetic carbon sequestrations did not affect photosynthetic CO2 assimilation per unit leaf area.
The absorption and utilization of bicarbonate by plants throughout the day may result from the PEPC and Rubisco contents (). It has been reported that DIC carbon fixation in roots is related to PEPC (Vuorinen et al. Citation1989; Vuorinen et al. Citation1992). The role of PEPC in root bicarbonate absorption and utilization is related to the conversion of malic acid. Malic acid is formed from PEPC and/or phosphoenolpyruvate carboxykinase (PEPCK) in the roots (Popp et al. Citation1982). Bicarbonate is absorbed and utilized by plants via the function of PEPC, which is considered a nonphotosynthetic pathway.
Figure 2. Hypothetical schematic model of PEPC and Rubisco response to bicarbonate in plants.
Note: Bicarbonate uptake by plants via photosynthetic and nonphotosynthetic pathways under light, and only nonphotosynthetic pathway under dark. IC, inorganic carbon; RUBP, ribulose-1,5-bisphosphate; 3-PGA, 3-phosphoglycerate; G3P, glyceraldehyde 3-phosphate; OAA, oxaloacetic acid; TCA cycle, tricarboxylic acid cycle.
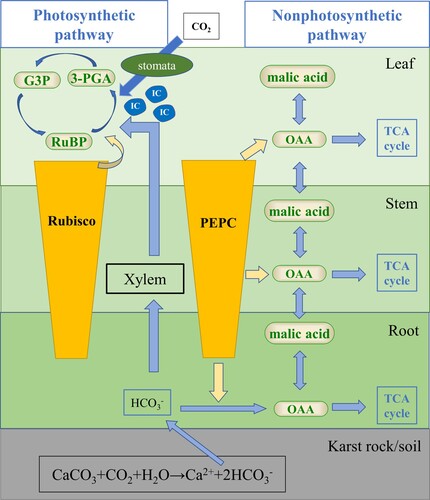
A previous study proved that Rubisco in leaves can combine CO2 from the conversion of root-derived bicarbonate to form carbohydrates during photosynthesis under light (Wu and Xing Citation2012; Rao and Wu Citation2017). In this study, we found that there were considerable amounts of Rubisco in the leaves under 24D, even more than under 24L (), which seemed to question the role of Rubisco in bicarbonate absorption and utilization. However, it has been reported that the expression of the Rubisco chaperone gene in cyanobacterial blooms reached its highest expression during darkness and was downregulated during the daytime (Sandrini et al. Citation2016). Therefore, Rubisco reacts and is consumed only under light and does not function as carboxylation in the dark. Bicarbonate is absorbed and utlized by plants via the function of Rubisco, which is considered a photosynthetic pathway.
The synergism in the absorption and utilization of photosynthetic pathway and nonphotosynthetic pathway dominated the transformation of the karst carbon sinks of plants. Ma can absorb more bicarbonate than Bp (). However, bicarbonate is greatly alkaline, and too much absorption and too little utilization by plants will cause damage to plants. In addition to less absorption of bicarbonate than Ma, the absorbed bicarbonate of Bp can be more readily used as a carbon source for photosynthetic organs to further reduce the damage of bicarbonate to plants because the capability of Bp to convert bicarbonate into CO2 and water by carbonic anhydrase (CA) is stronger than that of Ma. A previous study showed that approximately 30% of the photosynthetic products of Bp come from CO2 transformed from bicarbonate by CA, while only 0%–15% of those of Ma arise this way (Wu and Xing Citation2012). The involvement of the photosynthetic pathway vs the nonphotosynthetic pathway in bicarbonate uptake was greater in Bp than in Ma, which prevented the accumulation of organic acids from diminishing the adverse effect of growth in Bp. The moderate absorption and massive utilization of bicarbonate by Bp greatly reduced the damage. Ma is difficult to grow in karst regions, which is related to its high nonphotosynthetic absorption and low photosynthetic utilization of bicarbonate. This result is consistent with previous findings that Bp is a karst-adaptable plant (Zhao and Wu Citation2018).
The two plant species absorbed and utilized more bicarbonate under 24L than 24D (), indicating that light can promote the absorption and utilization of bicarbonate by plants, which proves that uptake of bicarbonate occurs through the xylem with transpiration flow to the shoots. It has been previously reported that bicarbonate may be incorporated into xylem proteins and transported to the shoots (Mengel et al. Citation1994; Wegner and Zimmermann Citation2004), and in xylem sap may be the carbon source of Rubisco in leaves (Popp et al. Citation1982; Stringer and Kimmerer Citation1993).
The greater bicarbonate uptake in Bp with a smaller R/S suggested that the absorption and utilization of bicarbonate is related to transpiration in leaves. Bp with a smaller R/S absorbed more bicarbonate, which will cause more free and increase the toxic effect in the plants (). Therefore, Bp with a larger R/S has better karst adaptation than that with a smaller R/S.
One of 2 moles of bicarbonate taken up by plants originated from CO2 in the air during karst corrosion (). This part of the carbon flux should be included in the global carbon budget. This pathway may lead to a larger carbon flow during all times of day and play an important role in the global carbon cycle, a role which has previously been underestimated. On the one hand, plants use bicarbonate to drive the corrosion of the carbonate and accelerate the formation of a carbon sink of corrosion, which makes a huge contribution to carbon neutrality. On the other hand, this is conducive to plant growth, increases the carbon sequestration capacity of plants, and provides organic matter and energy for the ecosystem. Karst-adaptable plants are especially able to regulate the entire carbon cycle. Therefore, quantifying the transformation of karst carbon sinks into carbon sequestrations by plants can not only provide new knowledge for the carbon metabolism of plants but also provide basic data for screening karst-adaptable plants and may ultimately provide a new solution for carbon peaks and carbon neutrality. The coupled relationship between the plant inorganic and organic carbon cycles is the key to modifying the carbon circulation model, especially in karst regions.
5. Conclusion
The bidirectional isotope tracer technique can quantify the absorption and utilization of root-derived bicarbonate. The tremendous karst carbon sinks can be transformed into carbon sequestrations by plants via photosynthetic and nonphotosynthetic pathway. Karst-adaptable plants can increase carbon sequestrations through the transformation to strengthen both karst and vegetation carbon sinks in the karst ecosystem.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Lei Fang
Lei Fang is a PhD student studying in the Institute of Geochemistry, Chinese Academy of Sciences.
Yanyou Wu
Yanyou Wu is a Professor working in the Institute of Geochemistry, Chinese Academy of Sciences.
References
- Bloom P, Inskeep W. 1986. Factors affecting bicarbonate chemistry and iron chlorosis in soils. J Plant Nutr. 9(3):215–228.
- Boxma R. 1972. Bicarbonate as the most important soil factor in lime-induced chlorosis in Netherlands. Plant Soil. 37(2):233–243.
- Cao M, Prince SD, Li K, Tao B, Small J, Shao X. 2003. Response of terrestrial carbon uptake to climate interannual variability in China. Glob Change Biol. 9(4):536–546.
- Carvalhais N, Forkel M, Khomik M, Bellarby J, Jung M, Migliavacca M, Mu M, Saatchi S, Santoro M, Thurner M, et al. 2014. Global covariation of carbon turnover times with climate in terrestrial ecosystems. Nature. 514(7521):213–217.
- Ciais P, Denning AS, Tans PP, Berry JA, Randall DA, Collatz GJ, Sellers PJ, White JWC, Trolier M, Meijer HAJ, et al. 1997. A three-dimensional synthesis study of δ18O in atmospheric CO2: 1. Surface fluxes. J Geophys Res Atmos. 102(D5):5857–5872.
- Farquhar GD, Lloyd J, Taylor JA, Flanagan LB, Syvertsen JP, Hubick KT, Wong SC, Ehleringer JR. 1993. Vegetation effects on the isotope composition of oxygen in atmospheric CO2. Nature. 363(6428):439–443.
- Friedlingstein P, O'Sullivan M, Jones MW, Andrew RM, Hauck J, Olsen A, Peters GP, Peters W, Pongratz J, Sitch S, et al. 2020. Global Carbon Budget 2020. Earth Syst Sci Data. 12(4):3269–3340.
- Jiang ZC, Yuan DX. 1999. CO2 source-sink in karst processes in karst areas of China. Episodes. 22(1):33–35.
- Law BE, Falge E, Gu L, Baldocchi DD, Bakwin P, Berbigier P, Davis K, Dolman AJ, Falk M, Fuentes JD, et al. 2002. Environmental controls over carbon dioxide and water vapor exchange of terrestrial vegetation. Agric For Meteorol. 113(1-4):97–120.
- Liu J, Zhong J, Chen S, Xu S, Li SL. 2021. Hydrological and biogeochemical controls on temporal variations of dissolved carbon and solutes in a Karst River, South China. Environ Sci Europe. 33(1).
- Lu F, Hu HF, Sun WJ, Zhu JJ, Liu GB, Zhou WM, Zhang QF, Shi PL, Liu XP, Wu X, et al. 2018. Effects of national ecological restoration projects on carbon sequestration in China from 2001 to 2010. Proc Natil Acad Sci U S A. 115(16):4039–4044.
- Luo ZK, Luo YQ, Wang GC, Xia JY, Peng CH. 2020. Warming-induced global soil carbon loss attenuated by downward carbon movement. Glob Chang Biol. 26(12):7242–7254.
- Maxwell K, Johnson GN. 2000. Chlorophyll fluorescence—a practical guide. J Exp Botany. 51(345):659–668.
- Mengel K, Planker R, Hoffmann B. 1994. Relationship between leaf apoplast pH and iron chlorosis of sunflower (Helianthus annuus L.). J Plant Nutr. 17(6):1053–1065.
- Millero FJ. 2014. Physico-chemical controls on seawater. In: Heinrich DH, Karl KT, editors. Treatise on geochemistry. 2nd ed. Miami, FL, USA: Elsevier; p. 1–18.
- Philip VB, Townsend K. 2005. A disturbance index for karst environments. Environ Manage. 36(1):101–116.
- Popp M, Osmond CB, Summons RE. 1982. Pathway of malic acid synthesis in response to ion uptake in wheat and lupin roots evidence from fixation of 13C and 14C. Plant Physiol. 69:1289–1292.
- Ran L, Butman DE, Battin TJ, Yang X, Tian M, Duvert C, Hartmann J, Geeraert N, Liu S. 2021. Substantial decrease in CO2 emissions from Chinese inland waters due to global change. Nat Commun. 12(1):1730.
- Rao S, Wu YY. 2017. Root-derived bicarbonate assimilation in response to variable water deficit in Camptotheca acuminate seedlings. Photosynth Res. 134(1):59–70.
- Raymond PA, Hartmann J, Lauerwald R, Sobek S, McDonald C, Hoover M, Butman D, Striegl R, Mayorga E, Humborg C, et al. 2013. Global carbon dioxide emissions from inland waters. Nature. 503(7476):355–359.
- Sandrini G, Tann RP, Schuurmans JM, van Beusekom SA, Matthijs HC, Huisman J. 2016. Diel variation in gene expression of the CO2-concentrating mechanism during a harmful cyanobacterial bloom. Front Microbiol. 7:551.
- Stringer JW, Kimmerer TW. 1993. Refixation of xylem sap CO2 in Populus deltoide. Physiol Plant. 89(2):243–251.
- Tong X, Brandt M, Yue Y, Horion S, Wang K, Keersmaecker WD, Tian F, Schurgers G, Xiao X, Luo Y, et al. 2018. Increased vegetation growth and carbon stock in China karst via ecological engineering. Nat Sustainability. 1(1):44–50.
- Vuorinen AH, Vapaavuori EM, Lapinjoki SP. 1989. Time-course of uptake of dissolved inorganic carbon through willow roots in light and in darkness. Physiol Plant. 77:33–38.
- Vuorinen AH, Vapaavuori EM, Raatikainen O, Lapinjoki SP. 1992. Metabolism of inorganic carbon taken up by roots in Salix plants. J Exp Botany. 43(251):789–795.
- Wang S, Grant RF, Verseghy DL, Black TA. 2001. Modelling plant carbon and nitrogen dynamics of a boreal aspen forest in CLASS — the Canadian land surface scheme. Ecol Modell. 142(1-2):135–154.
- Wang SJ, Liu QM, Zhang DF. 2004. Karst rocky desertification in southwestern China: geomorphology, landuse, impact and rehabilitation. Land Degrad Dev. 15(2):115–121.
- Wegner LH, Zimmermann U. 2004. Bicarbonate-induced alkalinization of the xylem sap in intact maize seedlings as measured in situ with a novel xylem pH probe. Plant Physiol. 136(3):3469–3477.
- Weis E, Berry JA. 1987. Quantum efficiency of photosystem II in relation to ‘energy’-dependent quenching of chlorophyll fluorescence. Biochim Biophys Acta. 894(2):198–208.
- Wu YY, Xing DK. 2012. Effect of bicarbonate treatment on photosynthetic assimilation of inorganic carbon in two plant species of Moraceae. Photosynthetica. 50(4):587–594.
- Yan JH, Li JM, Ye Q, Li K. 2012. Concentrations and exports of solutes from surface runoff in Houzhai Karst Basin, southwest China. Chem Geol. 304-305:1–9.
- Yao K, Wu YY. 2021. Rhizospheric bicarbonate improves glucose metabolism and stress tolerance of Broussonetia papyrifera L. seedlings under simulated drought stress. Russian J Plant Physiol. 68(1):1021–4437.
- Zhao K, Wu YY. 2017. Effects of Zn deficiency and bicarbonate on the growth and photosynthetic characteristics of four plant species. Plos One. 12(1). e0169812.
- Zhao K, Wu YY. 2018. Effect of Zn deficiency and excessive bicarbonate on the allocation and exudation of organic acids in two Moraceae plants. Acta Geochimica. 37(1):125–133.
- Zhu Z, Piao S, Myneni RB, Huang M, Zeng Z, Canadell JG, Ciais P, Sitch S, Friedlingstein P, Arneth A, et al. 2016. Greening of the Earth and its drivers. Nat Climate Change. 6(8):791–795.
