Abstract
Little is known about the reproductive biology of the codfishes (Gadidae). Lacking direct observations, the study of secondary sexual characteristics can provide cues to their reproductive biology and behaviour. We reviewed here published accounts on sexual dimorphisms in 25 gadids in light of their general lifestyle, i.e. pelagic or demersal, and social behaviour. In addition, complementary data on fin lengths and drumming muscle size in haddock (Melanogrammus aeglefinus), saithe (Pollachius virens), blue whiting (Micromesistius poutassou) and cod (Gadus morhua) are presented. Capacity for sound production occurred in almost half of the studied species, but was most prevalent in demersal species, where it is probably used in resource contests and to attract mates. For semi-pelagic gadids, we postulate that sound production may be linked to the formation of male-biased spawning shoals and the attraction of females towards such shoals; we identify candidate species to further test this hypothesis. Although rarely studied, sexual fin dimorphisms occur in several gadids. Cod, saithe and blue whiting males have longer pelvic fins than females, whereas no such dimorphism was observed in haddock. In cod and haddock, males use pelvic fins during courtship of females and agonistic encounters with other males. Pelvic fins probably also have a similar function in other gadids. The hitherto available information on sexually dimorphic traits and/or courtship behaviour in seven gadid species suggests that complex mating systems and non-random mate choice occurs frequently in this important group of exploited fishes.
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
Introduction
Sexual selection, typically through female choice of certain male traits, can lead to the emergence of secondary sexual characteristics or sexual dimorphisms, provided there is a genetic component to the variation in said trait (Ryan Citation1997). Such traits are thought to give females either a direct, e.g. more offspring produced, or indirect, e.g. higher quality offspring, benefit. Sexual selection can result in sexual dimorphism in size, body structure or colour (e.g. Kodric-Brown Citation1990; Hendry & Berg 1999; Gardner Citation2010). Teleost examples abound and include the genus Xiphophorus where males develop ‘swordtails’ or elongated caudal fins (Basolo Citation1990a) and females show preference for males with longer swords (Basolo Citation1990b), whereas dominant black goby Gobius niger Linnaeus, 1758 males have a distinct dark nuptial colouration (Mazzoldi & Razzotto Citation2002). Given that sexual dimorphisms are commonly closely linked to reproductive behaviour, they may therefore provide important insights into the mating systems of species that are poorly understood.
The codfishes (family Gadidae) comprise numerous species of which many are of significant commercial and cultural importance and have been harvested for thousands of years (Cohen et al. Citation1990). Despite this, little is known about their mating behaviour and reproductive systems, because most spawn in the ocean at depths where direct observations of spawning behaviour are difficult. Only Atlantic cod (Gadus morhua Linnaeus, 1758) and haddock (Melanogrammus aeglefinus (Linnaeus, 1758)) have been subject to close scientific scrutiny.
Laboratory studies have demonstrated that during the reproductive period, male cod and haddock court females and there is pronounced aggression between males (Brawn Citation1961a, Citationb; Hutchings et al. Citation1999; Hawkins & Amorim Citation2000). In cod, these behaviours appear to be energetically costly (Skjæraasen & Hutchings Citation2010; Skjæraasen et al. Citation2010a) and linked to individual male reproductive success (Rowe et al. Citation2008), suggesting the presence of female choice (Rowe et al. Citation2008; Skjæraasen et al. Citation2010b). Concurrent with these displays, male cod and haddock also produce sound (Brawn Citation1961a; Hawkins & Amorim Citation2000). In the field, male cod are observed to form dense sex-biased shoals (Morgan & Trippel Citation1996; Nordeide Citation1998), which females appear to visit at the time of mating (Robichaud & Rose Citation2001; Meager et al. Citation2009, Meager et al. Citation2010). The cod mating system has therefore been suggested to resemble a lek (Hutchings et al. Citation1999; Nordeide & Folstad Citation2000; Windle & Rose Citation2007; Meager et al. Citation2010).
Cod and haddock vocalize during reproductive displays with ‘drumming muscles’, large pairs of striated muscles attached to the swim bladder (Brawn Citation1961c; Hawkins & Amorim Citation2000; Nordeide et al. Citation2008). These muscles are larger in cod and haddock males than females during the reproductive period (Hawkins Citation1993; Engen & Folstad Citation1999). Hawkins & Rasmussen (Citation1978) examined sound production and drumming muscles in nine gadid species and found that they were present in all sound producing species, but not in ‘silent’ species. Similarly, the pelvic fins of male cod are also used in both courtship and aggressive behaviours (Brawn Citation1961a, Citationb) and are larger in males than females (Skjæraasen et al. Citation2006). Examination of secondary sexual dimorphisms in combination with insights into shoaling dynamics and lifestyle thus represents a useful tool for making inferences about the reproductive behaviour and thereby sexual selection of species difficult to observe in the field.
Here we review the literature on the presence of sound production and sexual dimorphisms in gadids (subfamilies Gadinae, Lotinae and Gaidropsarinae, cf. Endo 2002) in the light of their general lifestyle, i.e. pelagic or demersal, and, where such information was available, shoaling behaviour during reproduction (). In addition, we present new data on fin lengths and drumming muscle size in four common North Atlantic gadids: haddock, saithe (Pollachius virens (Linnaeus, 1758)), blue whiting (Micromesistius poutassou (Risso, 1827)) and cod.
Table I. Summary table of information on adult habitat, social behaviour and sexual dimorphism in codfishes (Gadidae); maximum size (total length, cm); reproductive behaviour (RB); presence of, and sexual dimorphism in drumming muscles (D, drumming muscle present, SD, sexually dimorphic drumming muscle), and the presence of sexual pelvic-fin length dimorphism (PFD) sorted according to habitat. Under ‘Habitat’, D denotes demersal, P denotes pelagic, and BeP denotes bentho-pelagic. Under ‘RB’, A denotes the presence of aggressive behaviour and C courtship behaviour during reproduction. Under D/SD the first Y denotes the presence of drumming muscles and * indicates that actual sound production of the species has been recorded. The second Y indicates that the drumming muscle have been shown to be sexually dimorphic. ** Juvenile but not adult saithe possess a drumming muscle. N denotes the absence of a drumming muscle/pelvic-fin dimorphism. ‘–’ Indicates that the species in question has not been examined for this particular trait. Data on habitat and maximum size (cm) were obtained from the FAO species catalogue (Cohen et al. Citation1990).
Material and methods
All fish sampled for the purpose of the present study were sourced from the Institute of Marine Research surveys conducted between February and April 2007. Northeast Arctic (NEA) haddock and saithe (~70°38′N, 20°50′E) were caught in the Barents Sea. Cod were sourced from catches at the main spawning grounds for NEA cod in Lofoten (67°38′N, 1°30′E). Blue whiting were caught in the Faroe–Shetland Channel (~59°48′N, 07°43′W). See for further information on sample sizes. All fish were frozen to –30°C immediately upon capture, and subsequently transported to a freezer room (–30°C) at the University of Bergen until they were measured in June - July 2008.
Table II. Summary of morphological measurements. Mean (M) and coefficient of variation (CV) of total length (TL, cm), total weight (TW, g), drumming-muscle dry weight (DR, g) and lengths (cm) of the first (D1), second (D2), and third dorsal (D3) fin, pectoral (PF), pelvic (PL), pectoral (PF), and first (A1) and second (A2) anal fin (all lengths are given in cm). Numbers in parentheses indicate sample size. Not all fin measurements were conducted on each sample.
Laboratory measurements of fin lengths and drumming muscle size
Fish were first thawed for approximately 16–20 h before total length (± 1 cm) and body weight (± 1 g) was measured. We then measured the length of the longest pelvic and pectoral fin ray from the base of the fin to the tip of the ray with callipers (± 1 mm). For the three dorsal and two anal fins we followed the procedure of Engen & Folstad (Citation1999) and measured the length of the third fin ray along the length of the spine, counting in a head-to-tail direction. This is usually the longest fin ray. The only exception to this procedure was the first dorsal fin of haddock, where we measured the length of the first fin ray, which is the longest fin ray for haddock. Only whole, undamaged fin rays were measured.
Fish were then gutted and sexed based on macroscopic examination of the gonads. All drumming muscles were subsequently removed using forceps. These were placed in numbered aluminium trays and dried at 60°C and weighed daily (± 0.0001 g) until the weight remained stable and no more weight loss occurred to obtain muscle protein weight and exclude water. We took the utmost care to remove and only weigh the drumming muscle itself and not any connective fibres or swimbladder tissue.
Data analyses
In addition to the results of the present study, we examined published research on gadids for records of sexual dimorphisms, drumming muscles, sound production, reproductive behaviour, spawning shoaling dynamics and habitat association.
For the new data, we tested for sexual dimorphisms by comparing pelvic-fin length or drumming-muscle mass between sexes using ANCOVAs. We controlled for the effect of body size by including total length as the covariate for analyses involving fin length as the response variable, and total weight in analyses where drumming muscle mass was the response variable. The initial models also contained an interaction term between the categorical variable sex and slope. If this parameter was not significant, i.e. slopes were homogenous, a standard ANCOVA analysis was applied. All mass and length data, i.e. both the response and covariate variables, were loge-transformed to meet the assumption of normality and to linearize allometric relationships. We also investigated the variability in pelvic-fin length and drumming-muscle mass by comparing the coefficient of variance (CV) for each trait, because theory suggests that sexually selected characters exhibit large individual variation (Andersson Citation1994).
We then used partial correlation to measure correlation between pelvic-fin length and drumming muscle mass while correcting for total body length. This test determined if there was a trade-off between pelvic-fin length and drumming-muscle size, i.e. do males with large pelvic fins have small drumming muscles after controlling for fish size (e.g. Engen & Folstad Citation1999)? All three variables were loge-transformed to linearize relationships.
Sexual dimorphism in morphological characters
Drumming muscles
Sound production has now been described in more than 800 teleosts worldwide (Kaatz Citation2002). Sound can be produced by various means such as extruding gas through the cloaca (Wilson et al. Citation2004) or rubbing fins together (Fine et al. Citation1996), but the most common mechanism for making sound in teleosts is by contracting muscles attached to the swimbladder wall, i.e. the ‘drumming muscles’ (Ladich & Fine Citation2006).
In accordance with the results of Hawkins & Rasmussen (Citation1978), drumming muscles were present in cod and haddock, but not in saithe and blue whiting (). For haddock, there was a strong, significant difference in drumming muscle size between males and females with males having bigger muscles (F (1,75)=251, P<0.0001, ). Overall, the slopes of the drumming muscle size–body size relationships did not differ between sexes (P>0.05), but this result was strongly influenced by a single point: a male with a very small drumming muscle (A). If this male was excluded from the analysis, the difference in drumming-muscle mass between the sexes increased with size (F (2,73)=251, P < 0.0001, A). For a given body weight, male haddock also had larger drumming muscles than male cod (F (1,95)=80.5, P < 0.0001, AB, ). The partial correlation coefficients did not indicate that males with bigger drumming muscles had shorter pelvic fins for either cod (r = 0.108, P=0.47) or haddock (r = 0.096, P = 0.53).
Figure 1. Drumming muscle mass of male (grey) and female (black) (A) haddock (Melanogrammus aeglefinus) and (B) cod (Gadus morhua). The white point (A) indicates the ‘outlier’ male mentioned in the results. Note the different scales on the y-axes of both graphs.
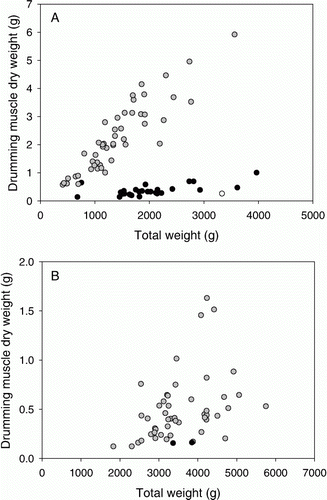
Cod mating sounds consist of calls of short duration, i.e. grunts and hums (Brawn Citation1961c; Finstad & Nordeide Citation2004), whereas haddock can produce long pulses lasting for several seconds with a number of ‘knocks’ (Hawkins & Amorim Citation2000). The larger drumming-muscle size of male haddock compared to similarly sized cod (; Hawkins Citation1993) thus concurs with their larger vocal repertoire. The observed sexual dimorphism in haddock drumming muscle was also noted by Hawkins (Citation1993). Sound production may vary between populations (Mann & Lobel Citation1998; Parmentier et al. Citation2005; Amorim et al. Citation2010) and individuals (Amorim et al. Citation2011); for cod, such differences in the frequency of vocalizations are positively associated with drumming muscle mass (Rowe & Hutchings Citation2006).
The presence of drumming muscles has, to our knowledge, been examined in 25 different gadids (family Gadidae) to date (). Eleven species possess well-developed drumming muscles in the adult stage; eight of these have a predominantly demersal lifestyle. The only clear exceptions were the bentho-pelagic Atlantic cod, walleye pollock (Theragra chalcogramma (Pallas, 1811)) and the pelagic/bentho-pelagic pollack (Pollachius pollachius (Linnaeus, 1758)) (). It seems further likely that sound production during reproduction is not only found in the Gadidae family, but instead could be more widespread in the order Gadiformes. Indeed, drumming muscles have been reported for European hake (Merluccius merluccius (Linnaeus, 1758)) (Groison et al. Citation2011). Many gadiforms are demersal, a lifestyle that appears to favour sound production ().
Fin lengths
Sexual fin dimorphisms are found in many teleosts (e.g. Ostrand et al. Citation2001; Park et al. Citation2001) and may take on very elaborate forms (e.g. Kottelat et al. Citation2006; Britz & Conway Citation2009). Notably, we found only the pelvic fin to be sexually dimorphic in the gadids examined. There was no sexual dimorphism in dorsal, anal or pectoral fins for haddock, saithe or blue whiting (P>0.05 for all cases). In contrast, pelvic fins were sexually dimorphic with males having longer fins than females for saithe (F (1,108)=9.09, P<0.01, ) and blue whiting (F (1,29)=17.9, P<0.001, ), but not for haddock (F (1,72)=0.798, P=0.38). The slopes of the fin length–body length relationships did not differ between sexes for either species (P>0.05). Fin lengths were not compared between sexes for Northeast Arctic cod, because only 2 out of the 50 sampled fish were female ), but cod have previously been shown to possess sexually dimorphic pelvic fins (Skjæraasen et al. Citation2006). Fish were generally well above the size at which maturation is expected to occur ().
Figure 2. Pelvic-fin versus total length of male (grey circles) and female (black circles) (A) saithe (Pollachius virens) and (B) blue whiting (Micromesistius poutassou).
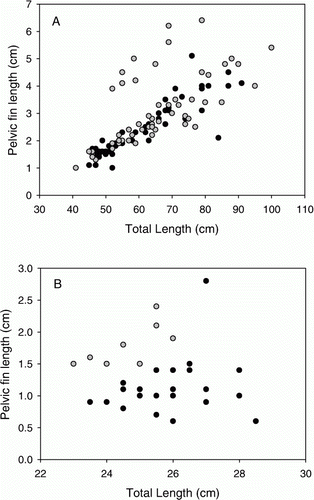
Our results concur with the results of Andersen & Jakupsstova (Citation1978), who detected sexual dimorphism only in the pelvic fins of blue whiting, and Engen & Folstad (Citation1999), who examined the ventral and dorsal fins of Norwegian coastal cod and found them not to be dimorphic. Sexual dimorphism in the pelvic fins is not restricted to gadids, but has also been reported for various other families (e.g. Schenck & Whiteside Citation1977; Barbieri et al. Citation1992; Oliveira & Almada Citation1995; Kottelat et al. Citation2006; Britz & Conway Citation2009; Arbour et al. Citation2010). Yamanoue et al. (Citation2010) proposed that the pelvic fin may be more readily modified by sexual selection than other fins, given their limited use for propulsion during swimming.
In our complementary study the coefficient of variation (CV) was generally lower for fin lengths than for body weight or drumming muscle mass (). We found no indication that males had a larger CV than females in the sexually dimorphic traits (). These findings match the results of Skjæraasen et al. (Citation2006); potential explanations for why this occurs are outlined there and therefore not reiterated here.
Sexual dimorphisms in relation to habitat use, sound production and social behaviour
Sexual dimorphisms can give insights into mating systems. For example, in Paedocypris progenetica (Kottelat et al., 2006) the males possess modified pelvic fins with hypertrophied muscles and a keratinized pad in front of the pelvic girdle (Kottelat et al. Citation2006). This is thought to function as a clasping or holding device used during reproduction to either facilitate internal fertilization, secure the male's position on a spawning site, or give males the possibility to manipulate eggs.
Compiling the limited drumming muscle data and general life-history information of the different gadid species, some patterns emerge. First, drumming muscles, and presumably sound production appear common, but occur predominantly in demersal species (). As in other teleosts, gadids use sound production for social communication (Ladich & Fine Citation2006). The main contexts in which sound production occurs are male mating calls and aggressive vocal displays towards other males during the reproductive period and in food and territorial contests (Hawkins Citation1993). The latter may thus involve both juveniles and adults throughout the year. Arguably, territorial contests are more likely to occur at the seafloor where potential landmarks may make resources defendable (Brawn Citation1961c). Tentatively supporting this, some of the world's most highly vocal fish are both demersal and highly territorial such as Lusitanian toadfish Halobatrachus didactylus (Bloch & Schneider, 1801) and plainfin midshipman Porichthys notatus Girard, 1854 (e.g. Bass et al. Citation2008; Amorim et al. Citation2010). Saithe are interesting as they possess drumming muscles as juveniles when occupying the demersal, benthic habitat, but lack these muscles in the adults that are pelagic (Hawkins & Rasmussen Citation1978). For saithe, the primary function of sound production may thus be to support interference competition for food or shelter or social aggregation formation during the juvenile phase.
Drumming muscles are absent in most pelagic/semi-pelagic gadids examined to date (). The only exceptions were the bentho-pelagic cod and walleye pollock and the pelagic/bentho-pelagic pollack. Interestingly, the pelagic whiting (Merlangius merlangus (Linnaeus, 1758) exhibit similar reproductive behaviour to cod and haddock, but drumming muscles are absent and no sounds are produced during reproduction (Hawkins & Rasmussen Citation1978). Hence, although sound production is associated with courtship and aggression in cod and haddock, it is not an obligatory feature of this particular gadid reproductive behaviour.
It has been suggested that sound production in male haddock may be important in attracting distant females to male-biased spawning aggregations (Hawkins & Amorim Citation2000). This is known as acoustic chorusing, and has been well studied in other taxa such as insects and lekking anurans (e.g. Ryan et al. Citation1981; Castellano et al. Citation2009). Fishing targeted at northeast Atlantic haddock spawning shoals produce catches dominated by males, clearly indicating that haddock do indeed form such sex-biased shoals (Knut Korsbrekke, Institute of Marine Research, Bergen, Norway, pers. comm.).
Male cod also aggregate in reproductive shoals that resemble leks and produce a loud chorus that can be detected several kilometers away (Nordeide & Kjellsby Citation1999; Nordeide & Folstad Citation2000). Formation of similar sex-biased shoals has also been noted for walleye pollock (Baird & Olla Citation1991 and references therein), but has hitherto not been examined in the sound-producing bentho-pelagic/pelagic pollack. Interestingly, the bentho-pelagic gadoid European hake possess drumming muscles (Groison et al. Citation2011), and the closely related Argentinean hake (Merluccius hubbsi Marini, 1933) form sex-biased shoals off the Patagonian coast (Martin Ehrlich, INIDEP, Buenos Aires, Argentina, pers. comm.).
Previously it has been suggested that sound production in gadids may be linked to fish size in relation to predation pressure, i.e. larger gadids are safer from predators and have much lower risk when producing sound (Hawkins & Rasmussen Citation1978), and, second, that it is mostly absent in schooling fish (Hawkins Citation1993). While our comparative analysis does not dismiss such explanations (), we suggest that there is clearly merit in examining whether sound production is also linked to the formation of sex-biased spawning shoals whenever present in semi-pelagic gadids. Obvious candidates for a comparative study are the sound-producing bentho-pelagic/pelagic pollack and the ‘silent’ pelagic saithe ().
The male pelvic fin likely has a special significance during reproduction in gadids. It has been shown to be used prominently in both courtships towards females and during antagonistic interactions between males for cod and haddock (Brawn Citation1961a, Citationb; Hawkins & Amorim Citation2000). Similar reproductive behaviour has also been observed for walleye pollock (Baird & Olla Citation1991; Park et al. Citation1994) and whiting (Hawkins & Rasmussen Citation1978), with the latter also showing the same fin dimorphism ().
Given the observed dimorphism in blue whiting and saithe (), similar courtship and antagonistic displays may be present in these species as well. It is curious that the pelvic fins were not sexually dimorphic in haddock, despite their documented use in haddock reproductive behaviour (Hawkins & Amorim Citation2000) and in contrast to the dimorphisms exhibited by our other study species. We can only hypothesize as to the causes, but it may be that their large investment in drumming muscle size () and the associated, complex (Hawkins & Amorim Citation2000), energetically costly sound production (e.g. Amorim et al. Citation2002) has hindered the development of sexually dimorphic pelvic fins.
Concluding remarks
Our review of previously published accounts indicates that drumming muscles, and as a consequence, sound production, is common in gadids, and seems to be associated primarily with the benthic habitat. Close to the bottom, sound production probably has a function during both contests for food and territories, and for mate attraction and agonistic encounters between males, mainly during the reproductive season. For pelagic/bentho-pelagic gadids, the presence of drumming muscles may be linked to the formation of sex-biased spawning shoals during spawning, but more research is needed to further investigate this assumed function.
The sexually dimorphic pelvic fins are likely to play an important role during reproduction in some North Atlantic gadids, potentially in support of male courtship and aggressive displays. Sexually dimorphic traits and/or courtship behaviour have been studied only in few gadid species so far. Complex mating systems and non-random mate choice may be widespread and hence we encourage morphological studies to shed light into the reproductive biology of these fishes, which includes several heavily harvested species. Such studies should preferably also be designed in a way that makes it possible to further disentangle inter-, intrasexual, and natural selection and their differential influences on dimorphic characters (e.g. Lailvaux & Irschick Citation2006; Bonduriansky Citation2007; Clutton-Brock Citation2009).
Editorial responsibility: Franz Uiblein
Acknowledgements
We thank Marius Moe for his invaluable contribution in the laboratory analyses and the scientists and crew aboard the IMR research vessels for their help in collecting the samples. A special thanks in this regard goes to Asgeir Aglen and Erik Berg at IMR. We also thank J. Nilsson and M. Ehrlich for sharing unpublished results. The study was supported by the Research Council of Norway projects ‘172649’ and ‘190228’ and by the Bergen Research Foundation.
Notes
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
References
- Almada , VC , Amorim , MCP , Pereira , E , Almada , F , Matos , R and Godinho , R. 1996 . Agonistic behaviour and sound production in Gaidropsarus mediterraneus (Gadidae) . Journal of Fish Biology , 49 : 363 – 66 .
- Amorim , MCP , McCracken , ML and Fine , ML. 2002 . Metabolic costs of sound production in the oyster toadfish, Opsanus tau . Canadian Journal of Zoology , 80 : 830 – 38 .
- Amorim , MCP , Simoes , JM , Mendonca , N , Bandarra , NM , Almada , VC and Fonseca , PJ. 2010 . Lusitanian toadfish song reflects male quality . Journal of Experimental Biology , 213 : 2997 – 3004 .
- Amorim , MCP , Simões , J , Almada , V and Fonseca , PJ. 2011 . Stereotypy and variation of the mating call in the Lusitanian toadfish, Halobatrachus didactylus . Behavioral Ecology and Sociobiology , 65 : 707 – 16 .
- Andersen KP , Jákupsstova SH. ICES 1978 . Sexual dimorphism and morphological differences in blue whiting (Micromesistius poutassou) . ICES Document CM 1978/H:46. Copenhagen . 22 pages
- Andersson M. 1994 . Sexual Selection . Princeton , NJ : Princeton University Press . 599 pages .
- Arbour , JH , Avendaño , P and Hutchings , JA. 2010 . Aspects of the ecology and life history of Alligatorfish Aspidophoroides monopterygius . Environmental Biology of Fishes , 87 : 353 – 62 .
- Baird , TA and Olla , BL. 1991 . Social and reproductive behaviour of a captive group of walleye pollock, Theragra chalcogramma . Environmental Biology of Fishes , 30 : 295 – 301 .
- Barbieri , LR , Dossantos , RP and Andreata , JV. 1992 . Reproductive biology of the marine catfish, Genidens genidens (Siluriformes, Ariidae), in the Jacarepagu'a Lagoon system, Rio De Janeiro, Brazil . Environmental Biology of Fishes , 35 : 23 – 35 .
- Basolo , AL. 1990a . Female preference predates the evolution of the sword in swordtail fish . Science , 250 : 808 – 10 .
- Basolo , AL. 1990b . Female preference for male sword length in the green swordtail, Xiphophorus helleri (Pisces, Poeciliidae) . Animal Behaviour , 40 : 339 – 49 .
- Bass , AH , Gilland , EH and Baker , R. 2008 . Evolutionary origins for social vocalization in a vertebrate hindbrain–spinal compartment . Science , 321 : 417 – 21 .
- Bonduriansky , R. 2007 . Sexual selection and allometry: A critical reappraisal of the evidence and ideas . Evolution , 81 : 838 – 49 .
- Brawn , VM . 1961a . Aggressive behaviour in the cod (Gadus callarias L.) . Behaviour , 18 : 107 – 47 .
- Brawn , VM . 1961b . Reproductive behaviour of the cod (Gadus callarias L.) . Behaviour , 18 : 177 – 98 .
- Brawn , VM. 1961c . Sound production by the cod (Gadus callarias L.) . Behaviour , 18 : 239 – 55 .
- Britz , R and Conway , KW. 2009 . Osteology of Paedocypris, a miniature and highly developmentally truncated fish (Teleostei: Ostariophysi: Cyprinidae) . Journal of Morphology , 270 : 389 – 412 .
- Castellano , S , Zanollo , V , Marconi , V and Berto , G. 2009 . The mechanisms of sexual selection in a lek-breeding anuran, Hyla intermedia . Animal Behaviour , 77 : 213 – 24 .
- Clutton-Brock , T. 2009 . Sexual selection in females . Animal Behaviour , 77 : 3 – 11 .
- Cohen DM , Inada T , Iwamoto T , Scialabba N. 1990 . FAO species catalogue . Gadiform fishes of the world (Order Gadiformes) . An annotated and illustrated catalogue of cods, hakes, grenadiers and other gadiform fishes known to date FAO Fisheries Synopsis 125 10 Rome . 442 FAO
- Endo , H. 2002 . Phylogeny of the Order Gadiformes (Teleostei, Paracanthopterygii) . Memoirs of the Graduate School of Fisheries Sciences, Hokkaido University , 49 : 75 – 149 .
- Engen , F and Folstad , I. 1999 . Cod courtship song: A song at the expense of dance? . Canadian Journal of Zoology , 77 : 542 – 50 .
- Fine , ML , McElroy , D , Rafi , J , King , CB , Loesser , KE and Newton , S. 1996 . Lateralization of pectoral stridulation sound production in the channel catfish . Physiology and Behavior , 60 : 753 – 57 .
- Finstad , JL and Nordeide , JT. 2004 . Acoustic repertoire of spawning cod, Gadus morhua . Environmental Biology of Fishes , 70 : 427 – 33 .
- Gardner , H. 2010 . Mate choice in fish: A review . Plymouth Student Scientist , 3 : 281 – 88 .
- Groison , AL , Kjesbu , OS and Suquet , M. 2011 . Sexual dimorphism of drumming muscles in European hake (Merluccius merluccius) Environmental Biology of Fishes , 91 : 7 – 13 .
- Hawkins AD. 1993 . Underwater sound and fish behaviour . Chapter 5 in : Pither TJ Behaviour of Teleost Fishes . London : Chapman and Hall , p 129 – 71 .
- Hawkins , AD and Amorim , MCP. 2000 . Spawning sounds of the male haddock, Melanogrammus aeglefinus . Environmental Biology of Fishes , 59 : 29 – 41 .
- Hawkins , AD and Rasmussen , KJ. 1978 . The calls of gadoid fish . Journal of the Marine Biological Association UK , 58 : 891 – 911 .
- Hendry , AP and Berg , OK. 1990 . Secondary sexual characters, energy use, senescence, and the cost of reproduction in sockeye salmon . Canadian Journal of Zoology , 77 : 1663 – 75 .
- Hutchings , JA , Bishop , TD and McGregor-Shaw , CR. 1999 . Spawning behaviour of Atlantic cod, Gadus morhua: Evidence of mate competition and mate choice in a broadcast spawner . Canadian Journal of Fisheries and Aquatic Sciences , 56 : 97 – 104 .
- Kaatz , IM. 2002 . Multiple sound producing mechanisms in teleost fishes and hypotheses regarding their behavioural significance . Bioacoustics , 12 : 230 – 33 .
- Kodric-Brown , A. 1990 . Mechanisms of sexual selection – insights from fishes . Annales Zoologici Fennici , 27 : 87 – 100 .
- Kottelat , M , Britz , R , Hui , TH and Witte , KE. 2006 . Paedocypris, a new genus of Southeast Asian cyprinid fish with a remarkable sexual dimorphism, comprises the world's smallest vertebrate . Proceedings of the Royal Society Series B – Biological Sciences , 273 : 895 – 99 .
- Ladich , F and Fine , M. 2006 . “ Sound-generating mechanisms in fishes: A unique diversity in vertebrates. Chapter 1 ” . In Communication in Fishes , Edited by: Ladich , F , Collin , SP , Moller , P and Kapoor , BG . 1 – 43 . Enfield , NH : Science Publishers, USA .
- Lailvaux , SP and Irschick , DJ. 2006 . A functional perspective on sexual selection: Insights and future prospects . Animal Behaviour , 72 : 263 – 73 .
- Mann , DA and Lobel , PS. 1998 . Acoustic behavior of the damselfish Dascyllus albisella: Behavioral and geographic variation . Environmental Biology of Fishes , 51 : 421 – 28 .
- Mazzoldi , C and Rasotto , MB. 2002 . Alternative male mating tactics in Gobius niger . Journal of Fish Biology , 61 : 157 – 72 .
- Meager , JJ , Skjæraasen , JE , Fernö , A , Karlsen , Ø , Løkkeborg , S Michalsen , K . 2009 . Vertical dynamics and reproductive behaviour of farmed and wild Atlantic cod Gadus morhua . Marine Ecology Progress Series , 389 : 233 – 43 .
- Meager , JJ , Skjæraasen , JE , Fernö , A and Løkkeborg , S. 2010 . Reproductive interactions between fugitive farmed and wild Atlantic cod (Gadus morhua) in the field . Canadian Journal of Fisheries and Aquatic Sciences , 67 : 1221 – 31 .
- Milic , D. and Kraljevic , M. 2011 . Biometry analysis of the whiting, Merlangius merlangus (Linneaus, 1758) from the northern Adriatic Sea . Acta Adriatica , 52 : 125 – 136 .
- Morgan , MJ and Trippel , EA. 1996 . Skewed sex ratios in spawning shoals of Atlantic cod (Gadus morhua) . ICES Journal of Marine Science , 53 : 820 – 26 .
- Nordeide , JT. 1998 . Coastal cod and north-east Arctic cod – Do they mingle at the spawning grounds in Lofoten? . Sarsia , 83 : 373 – 79 .
- Nordeide , JT and Folstad , I. 2000 . Is cod lekking or a promiscuous group spawner? . Fish and Fisheries , 1 : 90 – 93 .
- Nordeide , JT and Kjellsby , E. 1999 . Sound from spawning cod at their spawning grounds . ICES Journal of Marine Science , 56 : 326 – 32 .
- Nordeide , JT , Solberg , C , Willumsen , L and Amble , S. 2008 . Seasonal variation and condition-dependence of the drumming muscle of cod, Gadus morhua L. An experimental approach . Journal of Experimental Marine Biology and Ecology , 363 : 66 – 74 .
- Oliveira , RF and Almada , VC. 1995 . Sexual dimorphism and allometry of external morphology in Oreochromis mossambicus . Journal of Fish Biology , 46 : 1055 – 64 .
- Onuki , A and Somiya , H. 2006 . Spinal nerve innervation to the sonic muscle in Walleye Pollack, Theragra chalcogramma (Gadidae: Gadiformes) . Copeia , 2006 : 116 – 19 .
- Ostrand , KG , Wilde , GR , Strauss , RE and Young , RR. 2001 . Sexual dimorphism in plains minnow, Hybognathus placitus . Copeia , 2001 : 563 – 65 .
- Park , YS , Sakurai , Y , Mukai , T and Sano , N. 1994 . Sound production related to the reproductive behavior of captive walleye pollock Theragra chalcogramma (Pallas) . Nippon Suisan Gakkaishi , 60 : 467 – 72 .
- Park , IS , Zhang , CI and Lee , YD. 2001 . Sexual dimorphism in morphometric characteristics of cocktail wrasse . Journal of Fish Biology , 58 : 1746 – 49 .
- Parmentier , E , Lagardere , JP , Vandewalle , P and Fine , ML. 2005 . Geographical variation in sound production in the anemonefish Amphiprion akallopisos . Proceedings of the Royal Society Series B – Biological Sciences , 272 : 1697 – 703 .
- Robichaud , D and Rose , GA. 2001 . Multiyear homing of Atlantic cod to a spawning ground . Canadian Journal of Fisheries and Aquatic Sciences , 58 : 2325 – 29 .
- Rowe , S and Hutchings , JA. 2006 . Sound production by Atlantic cod during spawning . Transactions of the American Fisheries Society , 135 : 529 – 38 .
- Rowe , S , Hutchings , JA , Skjæraasen , JE and Bezanson , L. 2008 . Morphological and behavioural correlates of reproductive success in Atlantic cod Gadus morhua . Marine Ecology Progress Series , 354 : 257 – 65 .
- Ryan , MJ , Tuttle , MD and Taft , LK. 1981 . The costs and benefits of frog chorusing behaviour Behavioral Ecology and Sociobiology , 8 : 273 – 78 .
- Ryan , MJ. 1997 . “ Sexual selection and mate choice ” . In Behavioural Ecology: An Evolutionary Approach , 4th edition , Edited by: Krebs , JR and Davies , NB . 179 – 202 . Oxford : Blackwell .
- Sakurai , Y and Hattori , T. 1996 . Reproductive behavior of Pacific cod in captivity . Fisheries Science , 62 : 222 – 28 .
- Schenck , JR and Whiteside , BG. 1977 . Reproduction, fecundity, sexual dimorphism and sex-ratio of Etheostoma fonticola (Osteichthyes: Percidae) . American Midland Naturalist , 98 : 365 – 75 .
- Skjæraasen , JE and Hutchings , JA. 2010 . Shifting reproductive success in a shoal of Atlantic Cod, Gadus morhua L . Environmental Biology of Fishes , 88 : 311 – 18 .
- Skjæraasen , JE , Rowe , S and Hutchings , JA. 2006 . Sexual dimorphism in pelvic fin length of Atlantic cod . Canadian Journal of Zoology , 84 : 865 – 70 .
- Skjæraasen , JE , Meager , JJ and Hutchings , JA. 2010a . A cost of reproduction in male Atlantic cod (Gadus morhua) . Canadian Journal of Zoology , 88 : 595 – 600 .
- Skjæraasen , JE , Meager , JJ , Karlsen , Ø , Mayer , I , Dahle , G Rudolfsen , G . 2010b . Mating competition between farmed and wild cod Gadus morhua . Marine Ecology Progress Series , 412 : 247 – 58 .
- Wilson , B , Batty , RS and Dill , LM. 2004 . Pacific and Atlantic herring produce burst pulse sounds . Proceedings of the Royal Society of London Series B – Biological Sciences , 271 : S95 – 97 .
- Windle , MJS and Rose , GA. 2007 . Do cod form spawning leks? Evidence from a Newfoundland spawning ground . Marine Biology , 150 : 671 – 80 .
- Yamanoue , Y , Setiamarga , DHE and Matsuura , K. 2010 . Pelvic fins in teleosts: Structure, function and evolution . Journal of Fish Biology , 77 : 1173 – 208 .