?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study ZSM-5 Nano particles with high crystallinity were synthesized using a dry gel conversion technique. An L9 orthogonal array of the Taguchi method was applied to investigate the effect of synthesis parameters, such as crystallization time, gel drying temperature, molar composition of template (TPAOH) and water content in the crystallization stage on crystallinity and particle size of the ZSM-5 catalyst. The products were characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM). Beside short crystallization time, the particle sizes were considerably smaller in comparison with those prepared using the hydrothermal method. The results showed that the particle size and crystallinity increased with increasing water content and crystallization time. The effects of gel drying temperature and molar composition of template were found to be more complex, however. Comparing to hydrothermal method, ZSM-5 samples synthesized with the dry gel conversion exhibited higher selectivity to gasoline than other hydrocarbons.
1. Introduction
Gasoline, an important derivative of oil, is widely used as a transportation fuel in all over the world [Citation1]. High crude oil prices have encouraged worldwide interest in finding and developing alternative ways of fuel production. An alternative commercially proven route is converting syngas to liquid through conversion of methanol to conventional gasoline (MTG). MTG produced gasoline meets the standards and requirements and is completely Sulphur-free gasoline. ZSM-5 zeolite is a promising catalyst in the conversion of fossil and biomass resources to gasoline due to its unique structure and characteristics such as high surface area, high acidity and good thermal stability [Citation2–4]. It is recognized that smaller particles of ZSM-5 zeolite are much more effective than large particles [Citation5]. Higher resistance to coke poisoning, shorter diffusion paths and more active sites are important advantages of ZSM-5 with smaller size in catalytic reaction applications [Citation6,Citation7]. Therefore, it would be interesting to develop a new approach for the synthesis of nano-sized ZSM-5 zeolite.
In general, three approaches have been reported for the synthesis of nano-sized ZSM-5 which are hydrothermal, confined-space and two-step seeded growth synthesis [Citation8–11]. The synthesis of small and uniform ZSM-5 nanoparticles by the hydrothermal method is normally difficult. Recently, the dry gel conversion technique (DGC), as a new synthesis route has been applied to the synthesis of nanosized-ZSM-5 zeolites [Citation12]. In this regard, Tao et al. [Citation13] prepared ZSM-5 zeolites of uniform and small particles with a size of 100–200 nm. The higher nucleation density for ZSM-5 crystals in the dry gel conversion, in comparison with the hydrothermal conditions, leads to the formation of small crystals. The major advantages of the DGC method over conventional hydrothermal routes are as follow [Citation14]:
Higher zeolite yield
Less waste generation and less reactor volume requirement
Rapid crystallization
Reduction of the consumptions of expensive templates
No need for wastewater treatment
Possible continuous production process
Cost effective and eco-friendly route
The elements that are of major concern in the synthesis of nano zeolites are crystal size, synthesis time, and product yield. Several authors have investigated the factors affecting the formation of nano size zeolite crystals, such as the source of alkalinity, water content, temperature, and aging time. It has been revealed that these factors are critical on the size and uniformity of the final product [Citation7,Citation9,Citation15–17].
In this study, ZSM-5 zeolite was synthesized through a new dry gel conversion method as a simple technique, with a detailed parametric study to optimize the experimental procedure. The effects of crystallization time, gel drying temperature, water content added to the crystallization stage and template molar ratio in the composition on crystallinity, shape and particle size were investigated. The catalytic performance of the nominated ZSM-5 sample in the MTG process is also studied to investigate its reactivity.
2. Experimental
2.1. ZSM-5 zeolite synthesis
In order to synthesize ZSM-5 zeolite the initial gel composed of tetrapropylammonium hydroxide (TPAOH), aluminum isopropoxide (Al (O-i-Pr)3, Merck), Tetraethylorthosilicate (TEOS, Merck), H2O, and NaOH was prepared at room temperature. Molar composition of the initial gel was as follows:The amounts of x are presented in . The initial solution was stirred to give a homogeneous gel. Despite the hydrothermal method, here, the initial gel was dried at different temperatures. The resultant dried gel was put into an autoclave with a small amount of distilled water. This dry gel synthesis method has minor differences compare to ordinary dry gel conversion method (i.e. vapor phase transport (VPT) and steam-assisted conversion (SAC)). The autoclave was heated at 200°C at different crystallization times, then it was cooled and the solid product was separated and dried at 80°C. Finally, the products were calcined at 550°C for 5 h to remove the template. The obtained sample is called DGC-ZSM-5. For comparison, ZSM-5 zeolite was synthesized hydrothermally (HT-ZSM-5) by the same molar composition as described above, except that the crystallization time was 24 h and there was no added water to the gel.
Table 1. Factors and levels for L9 orthogonal array.
2.2. Characterization
The powder X-ray diffraction patterns (XRD) were recorded by an X-ray diffractometer (Equinox) with a CuKα X-ray source (λ = 1.54) at room temperature. The morphologic analysis of ZSM-5 zeolite was carried out using scanning electron microscopy (SEM, KYKY EM-3200, 30 KV).
2.3. Orthogonal array and experimental parameters
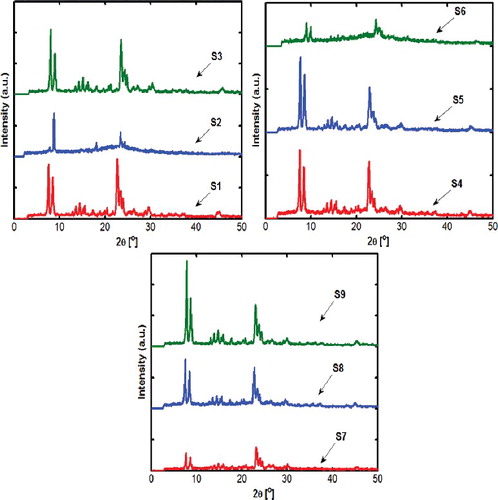
To determine the effects of the parameters on the crystallinity and particle size of nano-sized ZSM-5 and to find the optimum synthesis conditions, Taguchi orthogonal arrays design of experiment with four factors (crystallization time, gel drying temperature, water added at crystallization stage and template molar composition) each at three levels has been performed. In the Taguchi experimental design, the L9 array was considered. The factors and levels for L9 orthogonal array design are listed in . The orthogonal array layout and the measured responses are summarized in . The phase crystallinity and particle size of ZSM-5 were taken as responses. The overall crystallinity was obtained by the XRD results. Based on sample S9 and peak intensity of peak appearing around 2Ѳ = 7.79, 8.69, 23.08, 23.48, 23.78, 24.18 which belongs to ZSM-5, the relative crystallinities of samples were obtained and listed in .
Table 2. L9 orthogonal array layout and the measured response.
2.4. Catalytic performance
The conversion reactions of methanol over the DGC-ZSM-5 catalyst was carried out in a fixed-bed reactor at temperature of 371°C under atmospheric pressure with a mixture containing 83 wt.% methanol in water as the reactor feed (WHSV (MeOH) = 5 h−1). The products were analyzed by a three channel gas chromatograph. A Varian CP 3800 with three detectors and two thermal conductivity detectors (TCD) was used for analyzing H2, CO, CO2, CH4, and other non-condensable gases and a flame ionization detector (FID) was used for analyzing organic liquid products.
3. Results and discussions
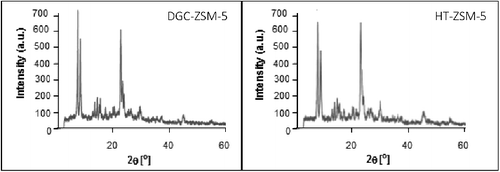
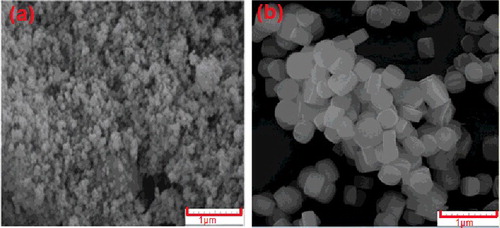
shows the XRD patterns of the samples obtained by the hydrothermal (24 h crystallization) and dry gel conversion (gel drying temperature of 115°C, crystallization time of 8 h and water/dry gel mass ratio of 0.5). Template molar ratios of both samples were 16. Both samples are in good agreement with standard ZSM-5 patterns reported in the literature [Citation18]. According to this figure, DGC-ZSM-5 shows slightly more intense peaks compare to HT-ZSM-5. The crystallinity of samples were also calculated with the aid of PANalytical X'Pert software based on the Rietveld method and it was observed that it DGC-ZSM-5 is more crystalline compare to HT-ZSM-5 (100% compare to 79% relative crystallinity). Furthermore, it is observed from SEM images that the crystal size for the two samples varied considerably (). For the DGC sample, fairly uniform and spherical crystals, with size around of 32–74 nm were formed. For the HT-sample, the crystal size increased to 0.4–1 μm. Appearance of fully crystalline DGC-ZSM-5 with small and uniform spherical particles in lower crystallization time (8 h) in comparison with HT-ZSM-5 shows the advantages of the simple dry gel method to the hydrothermal.
This reason may be attributed to the high nucleation and crystallization rates of zeolite in the dry gel conversion treatment process. Matsukuta et al. [Citation19] attributed faster crystallization in the dry gel conversion method to the enhanced interaction of TPA+ cations with siliceous species and high magnitude of stabilization of TPA+ species. Additionally, compared to the hydrothermal synthesis process, dry gel conversion method used steam to assist heating the precursor and convert the amorphous structure to a crystal structure [Citation12]. Therefore, the DGC sample exhibits higher crystallinity than the HT-sample. The reason for lower particle size is attributed to the high nucleation density in the early stages of synthesis and slow crystal growth after nucleation in the DGC method. These experiments demonstrate that, ZSM-5 zeolites with a high crystallinity and a uniform, small particle size can be synthesized by the simple dry gel conversion method.
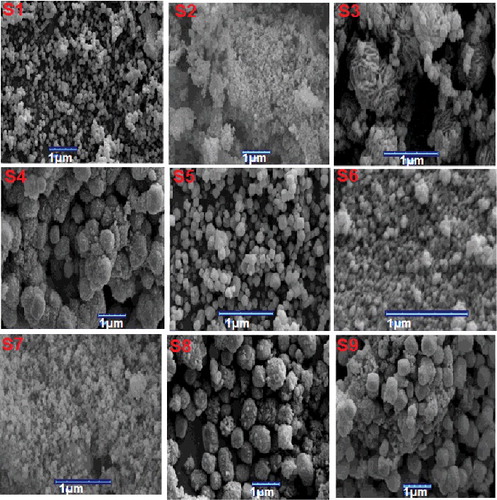
illustrates the XRD patterns of all the synthesized DGC samples according to . In this Figure, XRD patterns match well with the results reported in the literature [Citation19]. Also the SEM images of the synthesized nanoparticles are shown in and according to these images various morphologies and sizes can be observed. Also some of the samples are bimodal, which may be due to agglomeration of smaller particles or simply because of particle growth. These variations in the morphology and size might be associated with the differences in rates of nucleation and crystallization.
3.1. Analysis of variance
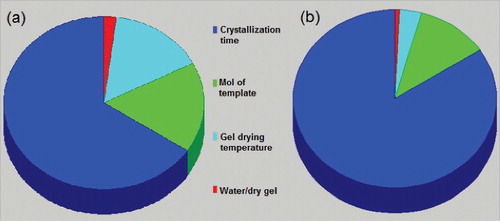
The Taguchi orthogonal array design was used in order to realize those parameters that have the most significant influence on the crystallization procedure of zeolite ZSM-5. and list the signal to noise (S/N) ratio. The relative phase crystallinity and mean particle size (excluding larger particles in bimodal samples) of ZSM-5 are taken as the measured responses. According to the dominating rule [Citation20,Citation21], the parameter with the largest difference indicates the most significant influence on the crystallinity and particle size of ZSM-5. ANOVA software was used to obtain this major parameter. As it was expected, the most important factor that affects the final crystallinity and particle size is crystallization time.
Table 3. The mean response table of S/N ratio for crystallinity.
Table 4. The mean response table of S/N ratio for particle size.
The contributions of each factor on the final ZSM-5 phase crystallinity and particle size are shown in (a,b). These results imply that the kinetics of the crystal growth is time dependent, and the time is the most effective parameter on the crystal size compared to the others.
3.2. Effect of the main parameters
3.2.1. Effect of water/dry gel mass ratio
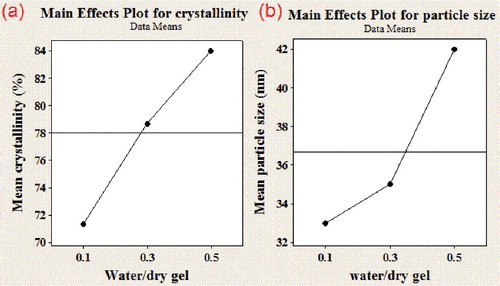
In dry gel conversion, the amount of water added to gel is a critical factor for the crystallization process [Citation22]. In order to determine the effect of the water amount, the ZSM-5 zeolites were obtained over a large range of water content according to .
shows the main effects of water content on crystallinity and crystal size. In this investigation, minimum crystallinity of 78% and maximum crystal size between 36–38 nm were chosen as critical values and are shown in all figure by line. According to (a), there is an enhancement in the crystallinity when water content in the autoclave is increased. The amount of additional water is essential to produce a sufficient amount of steam for successful crystallization of dry gel. According to literature [Citation14,Citation23], the appropriate water content may enhance the crystallization via improving the free diffusion of substrates, controlling the distance between the nutrients for effective interactions, stabilizing TPA+ ion, helping in migration and participation of silicate intermediate species over the dry gel surface for progressive crystal growth. (b) also shows increase in the particle size by increasing water content, because mass transport and crystal growth rates improves.
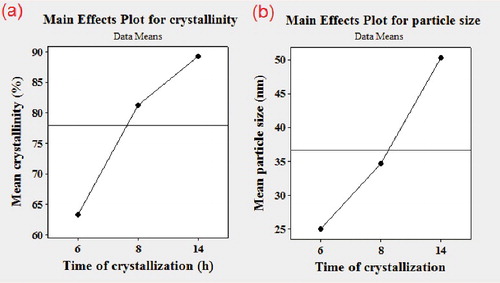
3.2.2. Effect of crystallization time
Crystallization was carried out at different times and the results are illustrated in . Increasing the crystallization time from 6 h to 14 h improved ZSM-5 crystallinity and also promotes zeolite growth. In hydrothermal synthesis, the minimum crystallization time is at least 14 h, however, in this study a pure and full crystalline sample was synthesized for 8 h (sample S9) applying dry gel method. Regarding the crystal size, with the increasing time from 6 h to 14 h, the average crystal size increased from 25 nm to 50 nm ((b)). Prolonging the time of crystallization allows the crystals to get larger. However, at longer crystallization time, the speed of crystal growth is decreased which can be attributed to presence of two different steps. In first 8 h, the nucleation was taking place and crystals were forming and growing rapidly because there were sufficient nutrients in the system for crystals. However, after 8 h, nucleation was already stopped and further crystal growth slowed down since nutrients in the systems were not as much as the beginning of the process. In this step, usually agglomeration of particles takes place which leads to larger size without increase in crystallinity.
These results indicate that the size and crystallinity of the particles strongly depend on crystallization time; therefore, its adjustment becomes essential for controlling the size and crystallinity of nano zeolite.
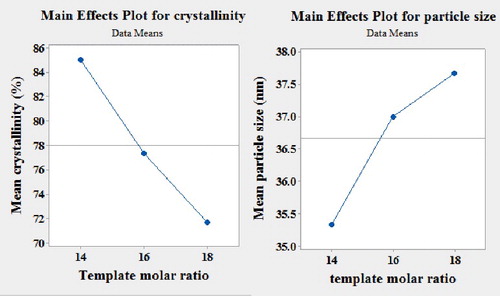
3.2.3. Effect of template molar ratio in precursor gel
The template concentration has a strong effect on the final product crystallinity and morphology. There is a minimum amount of template content that is necessary for making highly crystalline ZSM-5 zeolite. The results for different template concentrations are presented in . Increasing the template content raises the alkalinity of the mixture, which leads to a decrease in the crystallization rate and crystallinity [Citation24]. Moreover, due to the decrease in the incorporation of Al at high pH and considering the role of Al species in the nucleation process, a higher pH induces a significant increase in the crystallite size [Citation11]. As shown in (a), by increasing template molar ratio, the relative crystallinity of ZSM-5 decreased, while the average particle size increased ((b)). Therefore, the dry gel method requires a lower amount of template in comparison with the hydrothermal method, which is economically preferable.
3.2.4. Effect of gel drying temperature
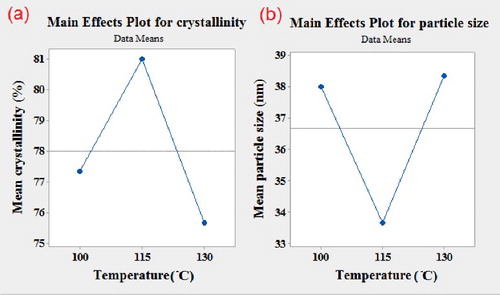
Another important parameter in the synthesis of DGC-ZSM-5 zeolites is gel drying temperature. This process has been carried out before the crystallization stage to produce a clear supersaturated solution. indicates the effect of gel drying temperature on particle size and crystallinity. The results reveal that, at temperatures of 100°C and 130°C, low crystallinity ZSM-5 zeolite with large particles were obtained. However, at 115°C highly crystalline ZSM-5 zeolite is synthesized. Therefore, the 115 °C can be considered as an optimum in the synthesis of ZSM-5 zeolite using the dry gel conversion method. Increasing gel drying temperature from 100 to 115 °C increases the dissolution of nutrients and thus the interaction between species is enhanced. However, further increasing of temperature over 115 °C indicated to have negative effect on the crystallinity with increase in crystal size, which can be due to unfavorable reactions between species or change in ingredients properties.
3.3. Catalytic activity test
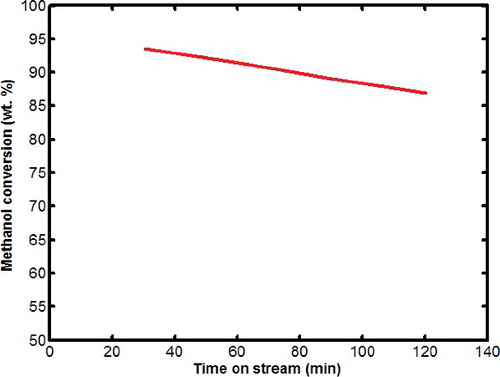
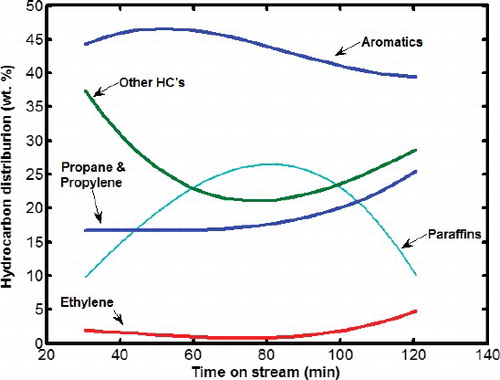
The catalytic performance of sample S9 that revealed the highest crystallinity in MTG reaction is shown in . Also the product distributions of methanol conversion reaction in different time on stream are shown in . It can be seen from that, there was minor changes in methanol conversion from 94% percent to 88% during the period of reaction. In other words, over 120 min the catalyst conversion was almost stable. The uniform and small crystals of ZSM-5 reduce the diffusion path and increase the total numbers of acid sites which lead to near 90% conversion. Although sample S9 has high selectivity for gasoline range hydrocarbons such as aromatics and paraffins (52%–64%), however, selectivity for light olefins such as ethylene is too low (15%--17%). Comparison between DGC-ZSM-5 catalyst with conventional ZSM-5 which is studied in other researches [Citation25–28], shows the superiority of DGC-ZSM-5 in high conversion of methanol and high selectivity to gasoline range hydrocarbons due to its higher surface and active area.
4. Conclusion
In this study, nanosized-ZSM-5 catalysts were successfully synthesized by dry gel conversion technique using an L9 orthogonal array of the Taguchi method. The effects of the main parameters such as crystallization time, gel drying temperature, molar composition of template (TPAOH) and water content added in the crystallization stage on crystallinity and particle size of ZSM-5 catalysts were studied. Unlike the conventional hydrothermal method, which needs high crystallization time (24 h) and yields particle with large crystal size (about several micrometers), the dry gel conversion method requires lower crystallization time (8 h) to produce well crystalline ZSM-5 with a small average particle size of 38 nm (S9). Analysis of the experiment data indicated that an increase in the crystallization time and amount of water added at the crystallization stage resulted in increasing crystallinity and particle size. The optimum crystallization temperature were found to be 115°C. By increasing the molar composition of the template, the crystallinity decreased and the particle size increased. Therefore, the dry gel method requires a lower amount of template in comparison with the hydrothermal method which is economically beneficial. According to ANOVA results, the most important variable to adjust the crystallinity and particle size is time of crystallization. DGC-ZSM-5 sample also showed high methanol conversion and gasoline range of hydrocarbons selectivity.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Gujar AC, Guda VK, Nolan M, et al. Reactions of methanol and higher alcohols over H-ZSM-5. Appl Catal A Gen. 2009;363:115–121.
- Yilmaz B, Müller U. Catalytic applications of zeolites in chemical industry. Top Catal. 2009;52:888–895.
- Davis ME. Ordered porous materials for emerging applications. Nature. 2002;417:813–821.
- Cundy CS, Cox PA. The hydrothermal synthesis of zeolites: history and development from the earliest days to the present time. Chem Rev. 2003;103:663–702.
- Mohammadparast F, Halladj R, Askari S. The crystal size effect of nano-sized ZSM-5 in the catalytic performance of petrochemical processes: a review. Chem Eng Commun. 2014;202:542–556.
- Miar Alipour S. Recent advances in naphtha catalytic cracking by nano ZSM-5: a review. Cuihua Xuebao/Chinese J Catal. 2016;37:671–680.
- Miar Alipour S, Halladj R, Askari S, Effects of the different synthetic parameters on the crystallinity and crystal size of nanosized ZSM-5 zeolite. Rev Chem Eng. 2014;30:289–322.
- Kim SD, Noh SH, Park JW, et al. Organic-free synthesis of ZSM-5 with narrow crystal size distribution using two-step temperature process. Microporous Mesoporous Mater. 2006;92:181–188.
- Aguado J, Serrano DP, Escola JM, et al. Low temperature synthesis and properties of ZSM-5 aggregates formed by ultra-small nanocrystals. Microporous Mesoporous Mater. 2004;75:41–49.
- Song W, Justice RE, Jones Ca, et al. Synthesis, characterization, and adsorption properties of nanocrystalline ZSM-5. Langmuir. 2004;20:8301–8306.
- Van Grieken R, Sotelo JL, Menéndez JM, et al. Anomalous crystallization mechanism in the synthesis of nanocrystalline ZSM-5. Microporous Mesoporous Mater. 2000;39:135–147.
- Yue MB, Yang N, Jiao WQ, et al. Dry-gel synthesis of shaped binderless zeolites composed of nanosized ZSM-5. Solid State Sci. 2013;20:1–7.
- Tao H, Yang H, Liu X, et al. Highly stable hierarchical ZSM-5 zeolite with intra- and inter-crystalline porous structures. Chem Eng J. 2013;225:686–694.
- Niphadkar PS, Tangale NP, Joshi PN, et al. Crystallization kinetics of Sn-MFI molecular sieve formation by dry gel conversion method. Microporous Mesoporous Mater. 2013;182:73–80.
- Sang S, Chang F, Liu Z, et al. Difference of ZSM-5 zeolites synthesized with various templates. Catal Today. 2004;93–95:729–734.
- Kumar N, Nieminen V, Demirkan K, et al. Effect of synthesis time and mode of stirring on physico-chemical and catalytic properties of ZSM-5 zeolite catalysts. Appl Catal A Gen. 2002;235:113.
- Mohamed RM, Fouad OA, Ismail AA, et al. Influence of crystallization times on the synthesis of nanosized ZSM-5.Materials Letters. 2005;59:3441–3444.
- Argauer ANJRJ, Kensington Md, Landolt GR, US Pat. 1. 1972.
- Matsukata M, Osaki T, Ogura M, et al. Crystallization behavior of zeolite beta during steam-assisted crystallization of dry gel. Microporous Mesoporous Mater. 2002;56:1–10.
- Dargahi M, Kazemian H, Soltanieh M, et al. High temperature synthesis of SAPO-34: applying an L9 Taguchi orthogonal design to investigate the effects of experimental parameters. Powder Technol. 2012;217:223–230.
- Liu G, Tian P, Zhang Y, et al. Synthesis of SAPO-34 templated by diethylamine: crystallization process and Si distribution in the crystals. Microporous Mesoporous Mater. 2008;114:416–423.
- Niphadkar PS, Kotwal MS, Deshpande SS, et al. Tin-silicalite-1: Synthesis by dry gel conversion, characterization and catalytic performance in phenol hydroxylation reaction. Mater Chem Phys. 2009;114:344–349.
- Zhdanov SP. Some Problems of Zeolite Crystallization. Advances in Chemistry. 1974; 101: 20–43.
- Fouad OA, Mohamed RM, Hassan MS, et al. Effect of template type and template/silica mole ratio on the crystallinity of synthesized nanosized ZSM-5. Catal Today. 2006;116:82–87.
- Rownaghi AA, Rezaei F, Hedlund J. Uniform mesoporous ZSM-5 single crystals catalyst with high resistance to coke formation for methanol deoxygenation. Microporous Mesoporous Mater. 2012;151:26–33.
- Rownaghi AA, Rezaei F, Stante M, et al. Selective dehydration of methanol to dimethyl ether on ZSM-5 nanocrystals. Appl Catal B Environ. 2012;119-120:56–61.
- Rownaghi AA, Hedlund J. Methanol to gasoline-range hydrocarbons: influence of nanocrystal size and mesoporosity on catalytic performance and product distribution of ZSM-5. Ind Eng Chem Res. 2011;50:11872–11878.
- Rownaghi AA, Rezaei F, Hedlund J. Yield of gasoline-range hydrocarbons as a function of uniform ZSM-5 crystal size. Catalysis communication. 2011;14:37–41.