1. Introduction
For drug development and approval by regulatory agencies, data from rodent and non-rodent animal models are required; canines and nonhuman primates (NHPs) have been the species of choice as non-rodent large animal models with the NHP generally considered the animal model gold standard by the US Food and Drug Administration (FDA). The pig (swine) model is a large animal model used in a great number of laboratories and pharmaceutical research sites. The pig model has anatomical, physiological, and biochemical similarities to humans, and this model is considered a translational model in biomedical research and development [Citation1]. The pig is specifically appropriate for the investigation of drug disposition since they have a significant number of membrane transport and enzymatic proteins in common with humans.
Pigs can vary greatly in size and characteristics depending on breed. Minipigs were first used for medical research in Europe before being introduced to the United States in the 1980s. Since then, these swine have been used in studies by investigators around the world. Minipigs, which are available from various countries (), were specifically developed for research purposes and as pets since they will typically weigh between 30 and 70 kg at maturity. Conversely, agricultural pigs may grow to weigh more than 320 kg. Minipigs reach sexual maturity at an earlier age than larger domestic pigs. Minipigs are a genetically defined model; the majority of breeds have their entire population history documented, beginning at their early development up to present [Citation2,Citation3]. There are some breed-specific characteristics, such as cytochrome P450 enzyme levels and activities [Citation4].
Table 1. Breeds of minipigs used as animal models for drug evaluation in various countries.
2. Minipig as an animal model for acute radiation syndrome (ARS) and delayed effects of acute radiation exposure (DEARE)
Developing the minipig as an animal model for ARS is critical to expedite radiation countermeasure development and the basic understanding of radiation-induced injury in various organs and tissues. It has been demonstrated that minipigs develop hematopoietic ARS symptoms similar to those observed in humans, NHPs, and canines; the LD50 (lethal dose of radiation for 50% of test animals) of whole body γ-radiation exposure is approximately 1.7–2.0 Gy [Citation5,Citation6]. Euthanized animals display signs of multi-organ dysfunction, including widespread internal hemorrhage. Importantly, the radiomitigative potential of G-CSF and PEGylated G-CSF (polyethylene glycol conjugated G-CSF) has been demonstrated using the minipig model of hematopoietic ARS [Citation7].
Radiation-induced gastrointestinal (GI) damage is characterized by the destruction of crypt cells, a decrease in villus height and number, decrease in mitotic figures, necrosis of epithelial cells and mucosa, diarrhea, hemorrhage, ulceration, and, at later stages, fibrosis and obstruction. Due to limited clinical data for acute radiation damage following high-dose radiation exposure, GI-ARS remains difficult to treat. Hence, identifying the pathological mechanisms of GI injury induced by radiation is crucial [Citation7]. The majority of GI-ARS-focused studies have been performed using rodents and NHPs. However, there are several clinical, physiological, and anatomical differences between humans and rodents [Citation8–Citation10]. Seok et al. (2013) provided highly provocative data that indicates that mouse models poorly mimic the genetic alterations in human inflammatory disease, which is highly relevant to radiation injury [Citation11]. Further, it is difficult to collect sequential pathological data from the same rodent. Finally, Libby (2015) made the bold statement: ‘I challenge trainees and colleagues with the assertion that attempts to model a human disease in mice virtually guarantee failure’ [Citation10]. Comparatively, the minipig model may provide relevant results for radiation-induced GI injury due to similar physiological parameters as humans, including transit time and pH, as well as being less prone to emesis than canines. Using the minipig model, inter-animal variations can be minimized since sequential tissue samples can be taken without euthanizing the animal over the course of the study. Recent reports investigating high radiation dose-induced GI syndrome suggest that plasma citrulline levels in irradiated minipigs may be a biomarker specifically associated with radiation-induced GI damage [Citation12]. In these studies, plasma citrulline levels were positively correlated with microscopic changes and the endoscopic score for mucosal damage. The most apparent changes in the ileum were mucosal atrophy and telangiectasia from day 1 to day 17 after abdominal irradiation. Microscopic changes were identified as architectural disorganization, loss of villi, and inflammation.
Radiation-induced lung injury (DEARE) due to nuclear or radiological exposure remains difficult to treat because of inadequate clinical data [Citation13]. Göttingen minipigs have been used to establish a single-high-radiation-dose-induced lung injury model and to characterize a thoracic computed tomography (CT)-based method to measure the progression of radiation-induced lung damage [Citation14]. Peribronchial opacification, lung volume loss, and interlobular septal thickening are three quantifiable CT parameters for monitoring the advancement of radiation-induced lung injury and appear as valuable tools for preclinical studies. Earlier, investigators have utilized the rodent model for such studies; however, the lung pathology of rodents is quite different than that of the human in respect of lobularity, the thickness of the septa and pleura, and the blood supply to the pleura [Citation13]. Both the NHP and canine have also been utilized to study radiation-induced lung injury; unfortunately, the canine lung differs from that of the human and shares anatomical similarities with rodents. Though there are similarities between the lung of the NHP and the human in respect of physiology, only limited studies have been performed in NHPs. Researchers have identified several volatile organic compounds in the exhaled breath of irradiated minipigs that could potentially serve as candidate biomarkers to assess γ-radiation exposure.
3. Minipig as non-rodent model in toxicology
Over the last three decades, minipigs have transitioned from being an obscure alternative to either the canine or NHP model to fulfill the non-rodent evaluation requirement in nonclinical studies to being an established animal model in regulatory toxicity studies. The pharmaceutical industry highly values the minipig as a non-rodent model for use in toxicity studies due to the similarities to human physiology. Minipigs have been used in nonclinical pharmacology and preclinical studies for single-dose, repeat-dose, teratology, fertility evaluation, and for absorption, distribution, metabolism, and excretion studies. These animals are readily amenable to various methods of drug administration, including inhalation, oral intubation, dietary, dermal, multiple routes of parenteral injections, and continuous intravenous infusion. These characteristics make the minipig similar to both canines and NHPs; however, minipigs are frequently overlooked as an animal model for the pharmacokinetic screenings of drugs [Citation15].
4. Minipig models for other disorders (cardiovascular, gastric, and skin) and pharmacology
Minipigs have been used as animal models for several clinical indications such as cardiovascular, metabolic syndromes, digestive (gastric) and bone disorders, diabetes, heart disease, skin conditions, and acute and chronic intestinal inflammation [Citation9,Citation15]. Cardiovascular disease is widely recognized as a common underlying cause of mortality in developed countries, varying with increased prosperity and longevity. Hypertriglyceridemia, lipemia, and prediabetic status and overt type 2 diabetes are common factors of metabolic syndrome axis that tie metabolic diseases and lipid disorders as contributors to cardiovascular diseases. A number of strains of minipigs have been used in several studies ranging from dyslipidemia, atherogenesis to working of intraarterial stents [Citation16]. A familiar problem is that a large number of studies with minipigs did not use well-defined strains. Minipigs represent a relevant animal model to humans in respect of skin injury and underlying tissue damage following irradiation; mesenchymal stem cells therapy, which could be useful to treat human burns, has been successfully tested in this model. Minipigs are gaining regulatory acceptance as an animal model over canines in pharmacology based on recent studies in the United States, European Union, and Japan.
The anatomic structure of the minipig GI tract, in particular the small intestine and stomach, is analogous to the structures in humans and differs only with respect to the spiral orientation of the minipig colon and the lack of an appendix. Despite such differences, the primary intestinal functions (nutrient and water absorption and microbial fermentation) are comparable to the human GI tract. In addition, several immune cells and processes of the innate and adaptive immune system (mucosal and intraepithelial B and T lymphocytes) and the recognition of activators of innate immunity by macrophages are also comparable to humans, making this animal an excellent model to study intestinal inflammation. The minipig has an abundance of intraepithelial and plasma γδ T-cells in comparison to mice and humans. The higher number of γδ T-cell receptors makes it an effective model to study this T-cell lineage, as the low levels of γδ T-cell receptors in human and mouse models proved to be inadequate to understand how these cells rapidly respond to bacterial antigens in the intestine.
In the area of medical devices, the minipig is viewed as an appropriate animal model based on the hematological and cardiovascular similarities to humans [Citation8]. The minipig is also suitable for assessing local effects after implantation.
5. Expert opinion
NHPs are the species most likely to be closest to humans in terms of genetic homology. Canines are easy to handle and have comprehensive background data for toxicology and safety evaluation available. However, canines are susceptible to emesis making them less appropriate for oral drug evaluation. Even though minipigs have been used considerably less frequently than either the canine or NHP model in pharmaceutical research, the Göttingen minipig has gained popularity among researchers within the last decades, due to its small size, convenience in handling, well-characterized genotype, and the gene sequence homology between swine and humans [Citation17].
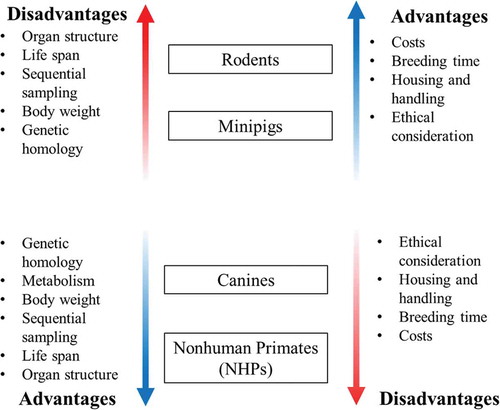
It has been repeatedly demonstrated that no single animal model can appropriately portray human conditions for all parameters. Therefore, it is imperative to be familiar with the various animal models available in order to select the most appropriate model for the specific study need depending on anatomy, physiology, metabolic reactions, genetics, and immune response (). The traditional use of the canines and NHPs may be the preferred choice of models; however, there are some circumstances when the minipig may be the ideal choice. For example, in studies involving a cutaneous component, the minipig may mimic the human response more closely than any other animal model. If a candidate drug is metabolized by aldehyde oxidase, N-acetyltransferase, or certain cytochrome P450 enzymes which are not expressed in canines, the minipig may also be the model of choice [Citation18]. Canines lack the organic anion transporter 3 (OAT3), therefore, requiring higher exposures of some drugs; on the other hand, minipigs may not be the optimal choice if sulfation is an important pathway in the human metabolism of the candidate drug.
Figure 1. Advantages and disadvantages of various animal models in biomedical research. Metabolism has not been mentioned in the upper panel since swine metabolism is very similar to humans and it is a distinct advantage over rodents.
To expand the minipig model for translational research, the different breeds of minipigs need to be evaluated as various disease models. Though recent attempts to develop the minipig as an animal model for ARS have progressed satisfactorily, the relative radiosensitivity of the Göttingen minipig raises concerns. A recent study suggests that radiation-induced disseminated intravascular coagulation may be a contributing factor to the radiosensitivity of minipigs [Citation19]. Though the referred study has been conducted using X-rays, it is believed that the findings are comparable for X-rays, γ-rays, and solar particle event radiation. These recent studies have used a 30-day observation period after irradiation; it will be prudent to use a longer observation period and to use supportive care for such studies. The radiosensitivity of other minipig breeds needs to be evaluated, in addition to use of younger animals to replicate pediatric conditions.
Another concern is the lack of experience the regulatory agencies have with the minipig model. This is particularly important in the context of drug development for chemical, biological, radiological, and nuclear threats following the FDA Animal Efficacy Rule [Citation20].
The nonpharmaceutical sectors are reluctant to accept this model and the model is rarely recommended in the guidelines. In order to help regulatory agencies to adopt this model for drug development, additional characterization is crucial as major gaps still exist, such as development and qualification of additional biomarkers, and the development of kits and reagents for analysis of various parameters. Once these gaps are filled, it is likely that minipigs will become an increasingly important animal model for research and pharmaceutical development.
Declaration of interest
KD Thrall is an employee of SNBL US. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interests or conflicts with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Helke KL, Swindle MM. Animal models of toxicology testing: the role of pigs. Expert Opin Drug Metab Toxicol. 2013;9:127–139. doi:10.1517/17425255.2013.739607.
- Alloosh M, Sturek M, Wenzel J, et al. Ossabaw Island miniature swine: cardiometabolic syndrome assessment. In: Swindle MM, Smith AC, editors. Ossabaw Island miniature swine: metabolic syndrome and cardiovascular assessment. Boca Raton (FL): CRC Press; 2007. p. 397–402.
- McAnulty PA, Dayan AD, Ganderup N, et al. The minipig in biomedical research. Boca Raton (FL): CRC Press; 2012.
- Skaanild MT, Friis C. Cytochrome P450 sex differences in minipigs and conventional pigs. Pharmacol Toxicol. 1999;85:174–180.
- Moroni M, Lombardini E, Salber R, et al. Hematological changes as prognostic indicators of survival: similarities between Gottingen minipigs, humans, and other large animal models. PLoS One. 2011;6:e25210. doi:10.1371/journal.pone.0025210.
- Moroni M, Coolbaugh TV, Lombardini E, et al. Hematopoietic radiation syndrome in the Gottingen minipig. Radiat Res. 2011;176:89–101.
- Singh VK, Newman VL, Berg AN, et al. Animal models for acute radiation syndrome drug discovery. Expert Opin Drug Discov. 2015;10:497–517. doi:10.1517/17460441.2015.1023290.
- Schwartz Longacre L, Kloner RA, Arai AE, et al. New horizons in cardioprotection: recommendations from the 2010 National Heart, Lung, and Blood Institute Workshop. Circulation. 2011;124:1172–1179. doi:10.1161/CIRCULATIONAHA.111.032698.
- Sturek M, Tune JD, Alloosh M. Ossabaw Island miniature swine: metabolic syndrome and cardiovascular assessment. In: Swindle MM, Smith AC, editors. Swine in the laboratory: surgery, anesthesia, imaging, and experimental techniques. Boca Raton (FL): CRC Press; 2015. p. 451–465.
- Libby P. Murine “model” monotheism: an iconoclast at the altar of mouse. Circ Res. 2015;117:921–925. doi:10.1161/CIRCRESAHA.115.307523.
- Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110:3507–3512. doi:10.1073/pnas.1222878110.
- Shim S, Jang W-S, Lee S-J, et al. Development of a new minipig model to study radiation-induced gastrointestinal syndrome and its application in clinical research. Radiat Res. 2014;181:387–395. doi:10.1667/RR13207.1.
- Williams JP, Brown SL, Georges GE, et al. Animal models for medical countermeasures to radiation exposure. Radiat Res. 2010;173:557–578. doi:10.1667/RR1880.1.
- Lee J-G, Park S, Bae C-H, et al. Development of a minipig model for lung injury induced by a single high-dose radiation exposure and evaluation with thoracic computed tomography. J Radiat Res. 2016;57:201–209. doi:10.1093/jrr/rrv088.
- Stricker-Krongrad A, Shoemake CR, Pereira ME, et al. Miniature swine breeds in toxicology and drug safety assessments: what to expect during clinical and pathology evaluations. Toxicol Pathol. 2016;44:421–427. doi:10.1177/0192623315613337.
- Russell JC, Proctor SD. Small animal models of cardiovascular disease: tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis. Cardiovasc Pathol. 2006;15:318–330. doi:10.1016/j.carpath.2006.09.001.
- Forster R, Ancian P, Fredholm M, et al. The minipig as a platform for new technologies in toxicology. J Pharmacol Toxicol Methods. 2010;62:227–235. doi:10.1016/j.vascn.2010.05.007.
- Dalgaard L. Comparison of minipig, dog, monkey and human drug metabolism and disposition. J Pharmacol Toxicol Methods. 2015;74:80–92. doi:10.1016/j.vascn.2014.12.005.
- Krigsfeld GS, Shah JB, Sanzari JK, et al. Evidence of disseminated intravascular coagulation in a porcine model following radiation exposure. Life Sci Space Res (Amst). 2014;3:1–9. doi:10.1016/j.lssr.2014.07.001.
- Nightingale SL, Prasher JM, Simonson S. Emergency use authorization (EUA) to enable use of needed products in civilian and military emergencies, United States. Emerging Infect Dis. 2007;13:1046–1055. doi:10.3201/eid1307.061188.
