1. Introduction
Recently, studies have been reported on the successful reproductive cloning of non-human primates (NHP) although the method is still relatively inefficient and expensive. Reproductive cloning of animals has progressively become more commonplace for laboratory and farm animal species [Citation1]. Some of the initial problems seen as inefficiencies of embryonic development during pregnancy, birth defects, and unpredictable postnatal abnormalities and death have largely disappeared with refinements of nuclear transfer methodology [Citation2]. The availability of genetic clones of NHP raises the question as to whether it would improve the chances of finding effective drugs for treating human brain conditions? The answer needs to include consideration of the ethics of making cloned NHP as models for discovery of new and effective drugs, the cost benefit of this approach compared with other approaches and the likely increased probability of discovering effective new drugs by using genetically identical NHP in any experiments proposed.
2. The production of cloned monkeys
It has been difficult to clone NHP [Citation3] but recently Liu et al. [Citation4] reported the birth of two healthy macaque baby clones using fetal fibroblast cells as the nuclear donor in somatic cell nuclear transfer (cloning). However, the overall success rate was <2% of the nuclear transfer attempts. Two other births were also reported from adult ovarian follicle cells (cumulus cells) but neither baby survived long after birth. The success in this study was attributed to improve epigenetic reprogramming of somatic cells using demethylases and a histone deacetylase inhibitor [Citation5]. There is little reason to think there is a really significant difference between fetal and adult cell types with this data but the treatments did enable up regulation of gene expression in epigenetic reprogramming resistant regions of the genome.
3. Cloned animals as models for neurodegenerative disorders
Genetically inbred rodent models and transgenic or gene knock out mice have been the primary source for studies of functional genomics and the genetic basis for neurodegenerative disorders in the human [Citation6,Citation7]. However, there are many complex neurodegenerative diseases where rodent models do not sufficiently well represent the human disease to enable predictable human responses to many drugs or cell therapies. Some large animal models more closely resemble the human condition and allow better analyses of drug response for therapy in neurological disorders because of the genetic, anatomical, physiological and neurological similarities with humans. The closest large animal to the human is the NHP, which is used extensively in Parkinson’s, Alzheimer’s, Huntington’s and motor neuron diseases, multiple sclerosis, spinal cord injury and many other neurological conditions [Citation8,Citation9]. Cloned NHP would have value in reducing the experimental animal numbers needed for data consistency because the animals are genetically identical.
4. Alternative models
Promising drug discovery alternatives for neurological diseases will almost certainly be driven by models evolving around; in silico computer (neuro-computational) modeling of pharmacologic or physiologic process, and a rational extension of controlled in vitro experimentation that simulates the mechanisms of human biology to predict how new therapeutic targets will behave in the human. In vitro testing models can now not only create cellular representations that behave like the original tissue by mimicking the biological and chemical processes but also represent specific human diseases. Induced pluripotent stem cells (iPSCs) derived from patients with neurological conditions [Citation10] and the ease of establishing isogenic iPSCs using accurate gene editing techniques [Citation11], removes background genetic influences and enables precise disease phenotype reproduction. The development of three-dimensional brain organoids from iPSCs potentially further enhances the opportunities for drug discovery because it involves numerous brain cell types in communication and support as happens in the developing brain [Citation12,Citation13]. While not without challenges to produce meaningful patient-specific models in vitro, progress in reproduction of neurological phenotype and drug response in patient derived iPSCs models has been encouraging [Citation14].
5. Ethics and non-human primate cloning
Ethical discussion on cloning has mainly been confined to human reproductive cloning and somatic cell nuclear transfer or therapeutic cloning [Citation15]. There were some concerns about cloning farm animals and their entry into the human food chain but these have disappeared with regulatory approval for their use. Cloning NHP is a technical step closer to the cloning of humans and this might be an ethical concern to some people. Regulatory authorities do not require data from NHP models in preclinical medical research unless the therapeutic risks and efficacy cannot be demonstrated adequately in rodents or other large animals, or in vitro. It is difficult to completely avoid the use of NHP in neurological research because of the need to find models that represent closely human neuronal conditions. NHP research attracts increased opposition from animal activists but the degree of concern varies in different cultures. There is also a general trend by research ethics committees for reducing the number of animals used in experiments in medical research and cloned animals may be considered as supporting this trend because of their genetic homogeneity. However, other practical factors such as cost and availability will also impact on the use of cloned NHP.
6. Conclusion
It is unlikely that cloned NHP will be used for human brain drug discovery in the near future, given the low success and high cost of producing cloned animals. In the event that genetic pathways are defined in NHP that consistently reproduce the human brain disease phenotype, the use of cloned NHP may provide an effective model for verifying the human disease response to new drug discoveries using few animals.
7. Expert opinion
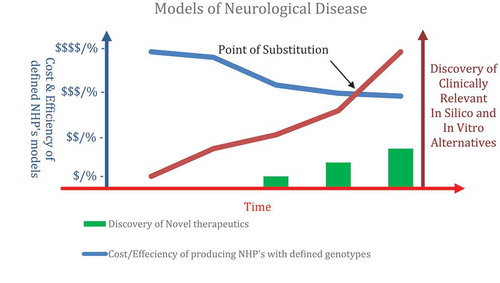
The humane use of animals in scientific research is overseen by the need to: restrict the use of animals; refine experiments to minimize distress; and replace tests with alternative technique (3R’s). These requirements continue to drive a raft of novel experimental techniques that provide alternatives to animal studies. The need for NHP in medical research for neurological conditions rests on their similarity to human physiology and anatomy, and likely meaningful drug and cell therapy response for the human condition. The recent demonstration of cloned NHP would reduce the number of experimental animals needed to show response to therapy but the process is still inefficient and expensive (see ).
Figure 1. Non-human primate (NHP) models with a defined genotype would advance our knowledge of the genetic keystones of disease and aid the development of novel therapies for humans. However, the high cost and low efficiency of the NHP model coupled with the emergence of novel alternative technologies that accurately account for genotype variations will ultimately converge in terms of clinical outcomes. The combination of unique NHP’s as preclinical test models in combination with iPSCs and genomics studies may well be considerably more efficient for the delivery of reliable neurological therapeutics. The societal and research value of NHP studies in terms of neurological discoveries will continue to be judged against the advent of alternative technologies that offer similar outcomes without the use of animal models (Point of Substitution).
Generally, NHP such as the macaque have much larger genetic diversity than the human [see Citation16] and there is also considerable variation in genotype associated with brain disorders [see Citation17], which means that large numbers are required to obtain experimental reproducibility and to reduce variance of response to therapeutic treatments. Using cloned NHP may improve the experimental reproducibility of drug therapies but may not properly represent a heterogeneous human population. New therapies for humans evolving from such experiments may elicit responses that are quite different to that of a line of cloned NHP. Hence the success of any therapeutic medicine in the human will require a thorough understanding of how the genetic variances of NHP primate models contribute to the disease phenotype [Citation18] and confidence that this accurately represents the genetic basis of the human disease condition.
The genetic heterogeneity underlying human neurological disease is also substantial and has further limited the efficiency of drug discovery [Citation19]. When these matters are better understood and a sound basis has been developed for demonstrating specific genetic causes of human brain disease, selection of cloned NHP may provide a real value for mimicking the human response by selecting the monkey genotype that best reflects the human disease phenotype.
NHP are closely related species to the human and that means neurological diseases are likely to be similar and drug or cell therapies medically meaningful. Experimenting on closely related NHP (and their reproductive cloning) is widely considered undesirable in Western culture unless the benefits for human medicine are considerable. Current ethical guidelines, dictate that a substantial benefit must be demonstrated for the use of animals, including NHP (and their cloned counterparts) in preclinical experiments as tools for investigating drugs for neural disorders or other indications in the human diseases. Given the presently relatively low success rates of producing normal cloned NHP infants, genetically identical NHP are unlikely to attract much attention for study of neurological disease or injury. The question still remains that if cloning success rates were dramatically improved and the costs of their production consequently reduced, would there be interest for using genetically identical monkeys in drug discovery and translation in humans? Presently, this is unlikely to be the case for the use of cloned NHP in medical research.
Concomitantly development of alternative drug discovery platforms may change this. Given the extent of pleiotropy in the human genome, careful evaluation of causal relationships between genes and disease requires careful analyses in large-scale genomics data bases [Citation20]. Combining iPSCs with genomics tools can address these problems and makes an extremely powerful platform for drug discovery [Citation21]. While many of these alternatives still need refining, they continue to help narrow down potential therapeutic candidates or confirm results already obtained from animals. The precision and accuracy of predictions based on these technologies may well be most effectively verified in the NHP using cloned animals with carefully selected genotype. Would this be a more efficient process than the use of outbred animals and heterogeneous human patients? Given the very high failure rate of the present drug discovery process, the use of cloned NHP as preclinical test models in combination with predictions based on iPSCs and genomics studies may well be considerably more efficient for the delivery of reliable neurological therapeutics than the present approach of using rodent models and in vitro assays for assessing clinical effectiveness.
Declaration of interest
A Trounson is supported by an Australian Federal Government Grant for Immunotherapy. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Wilmut I, Bai Y, Taylor J. Somatic cell nuclear transfer: origins, the present position and future opportunities. Phil Trans R Soc B. 2015;370:20140366.
- Loi P, Iuso D, Czernik M, et al. A new dynamic era for somatic cell nuclear transfer? Trends Biotechnol. 2016;34:791–797.
- Mitalipov SM, Zhou Q, Byrne JA, et al. Reprogramming following somatic cell nuclear transfer in primates is dependent upon nuclear remodeling. Hum Reprod. 2007;22:2232–2242.
- Liu Z, Cai Y, Wang Y, et al. Cloning of macaque monkeys by somatic cell nuclear transfer. Cell. 2018;172:881–887.
- Cibelli JB, Gurdon JB. Custom-made oocytes to clone non-human primates. Cell. 2018;172:647–649.
- Puzzo D, Gulisano W, Palmeri A, et al. Rodent models for Alzheimer’s disease drug discovery. Expert Opin Drug Discov. 2015;10:703–711.
- Li W, Sayana P, Jankovic J. Animal models of Parkinson’s disease: a gateway to therapeutics. Neuro Therapeut. 2014;11:92–110.
- Yang S-H, Cheng P-H, Banta H, et al. Towards a transgenic model of Huntington’s disease in a non-human primate. Nature. 2008;453:921–924.
- Jennings CG, Landman R, Zhou Y, et al. Opportunities and challenges in modeling human brain disorders in transgenic primates. Nat Neurosci. 2016;19:1123–1130.
- Grobarczyk B 1, Franco B, Hanon K, et al. Generation of isogenic human iPS cell line precisely corrected by genome editing using the CRISPR/Cas9 system. Stem Cell Rev. 2015;11:774–787.
- Brennand K, Marchetto MC, Benvenisty N, et al. Creating patient-specific, neural cells for the in vitro study if brain disorders. Stem Cell Rep. 2015;5:933–945.
- Tomaskovic-Crook E, Crook JM. Clinically amendable, defined, and rapid induction of brain organoids from induced pluripotent stem cells. Methods Mol Biol. 2017. DOI:10.07/7651_2017_95
- Wang Z, Wang SN, Xu TY, et al. Organoid technology for brain and therapeutic research. CNS Neurosci Ther. 2017;23:771–778.
- Trounson A, Shepherd KA, DeWitt ND. Human disease modeling with induced pluripotent stem cells. Curr Opin Genes Dev. 2012;22:509–516.
- Trounson AO. Future and application of cloning. Methods Mol Biol. 2006;348:319–332.
- Yuan Q, Zhou Z, Lindell SG, et al. The rhesus macaque is three times as diverse but more closely equivalent in damaging coding variation as compared to the human. BMC Genet. 2012;13:52.
- Rogers J, Raveendran M, Fawcett GL, et al. CRHR1 genotypes, neural circuits and the diathesis for anxiety and depression. Mol Psychiatry. 2013;18:700–707.
- Haus T, Ferguson B, Rogers J, et al. Genome typing of nonhuman primate models: implications for biomedical research. Trends Genet. 2014;30:482–487.
- Dugger SA, Platt A, Goldstein DB. Drug discovery in the era of precision medicine. Nat Rev Drug Discov. 2018;17:183–196.
- Verbanck M, Chen CY, Neale B, et al. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nature Genet. 2018. DOI:10.1038/s41588-018-0099-7.
- Zhang J, Li H, Trounson A, et al. Combining hiPSCs and human genetics: major applications in drug development. Cell Stem Cell. 2017;21(2):161–165.
