1. Introduction
Sulfur is an ubiquitous heteroatom in medicinal chemistry [Citation1]. Its prevalence is often credited to its ability to exist in many oxidation states, whilst also being capable of bonding to a variety of atoms, including carbon, oxygen, nitrogen, phosphorus and halides. Consequently, a number of familiar sulfur functional groups are routinely encountered in synthetic and medicinal chemistry (), and display a broad spectrum of useful chemical and biological properties.
Figure 1. Prevalent sulfur functional groups in medicinal chemistry and examples of corresponding medicinal agents.
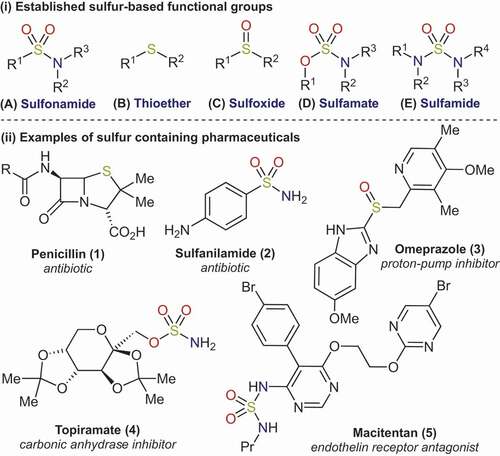
There is a long-standing history of sulfur functionalities in pharmaceutically active molecules. This is exemplified by the revolutionary antibiotic penicillin (1), which has a sulfur atom embedded in the thiazolidine ring. Sulfur atoms are also common in many modern pharmaceuticals; out of the 120 small molecule drugs present in the top 200 drugs by retail sales for 2019 [Citation2], 33% contain a sulfur atom. Amongst these sulfur-containing molecules, certain functional groups are present in higher frequencies, with sulfonamides (A) leading the way, featuring in eight of these drugs. If we rewind to almost a century ago, we again encounter sulfonamides, this time in some of the first antibiotics to be developed, the so-called ‘sulfa drugs’ of the 1930s (2). Today, sulfonamides are found in molecules with a wide range of applications that go far beyond the scope of antibiotics. The longevity of sulfonamides in drug discovery can be attributed to several features: high stability, favorable solubility, and the presence of multiple hydrogen bonding donor and acceptor sites. The latter enables the formation of interactions with metal ions and amino acid residues. Consequently, sulfonamides are highly valued in small molecule drug discovery, and are currently the most acclaimed sulfur pharmacophore.
2. Establishing underexplored sulfur pharmacophores
Given the prevalence of sulfonamides in known medicines, as well as their ease of preparation, it is not surprising that many drug discovery programmes are still drawn to this functional group [Citation3]. Nonetheless, several other sulfur functional groups are also straightforward to prepare, and have been integrated into numerous drug molecules. In particular, the role of thioethers (B) and sulfoxides (C; see Omeprazole 3) as pharmacophores has been examined in detail [Citation4]. In contrast, there are fewer synthetic routes to sulfamates (D) [Citation5], and sulfamides (E) [Citation6]. However, these groups still feature in blockbuster drugs such as topiramate (4) and macitentan (5).
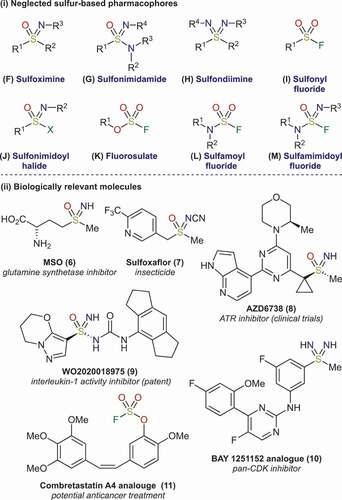
Despite the success of established sulfur pharmacophores, a large proportion of sulfur functional groups remain neglected in drug discovery (). Many of these species offer additional attractive features, including, stereogenic sulfur centers, increased polarity, and additional points of diversification, potentially allowing for the fine-tuning of their physiochemical properties. The limited use of these molecules can also offer attractive intellectual property positions. Recently, increasing attempts have been made to elucidate the biology of these underexplored pharmacophores [Citation7].
2.1. Sulfoximines
The biological significance of sulfoximines (F) has been apparent since the 1950s, when methionine sulfoximine (MSO, 6) – now a widely studied molecule [Citation8] – was first discovered. Despite this, to date there are no marketed sulfoximine-containing pharmaceuticals. Only a single agrochemical, the insecticide Sulfoxaflor (7), has reached the market, gaining approval in 2013. Several reports have investigated sulfoximines as potential pharmacophores [Citation9]. In particular, the pharmacokinetics of sulfoximine drug analogues have been explored numerous times. A variety of sulfoximine-containing molecules have now entered clinical trials, across a variety of disease areas, for example, the ATR inhibitor 8.
A significant limitation in the development of sulfoximine-containing drugs has been the lack of synthetic routes to access this functional group. Traditional syntheses of these molecules involve the imination of sulfoxides (C). However, this approach is limited by the formation of N-substituted sulfoximines, which require further deprotection and functionalisation. In addition, toxic and explosive reagents are required [Citation9]. In recent years, several breakthroughs have been achieved that allow rapid access to free (N-H) sulfoximines, under milder conditions, including the direct oxidation from thioethers (B) [Citation10,Citation11]. Furthermore, a recent alternative synthesis paired simple organometallic reagents with a high oxidation state sulfur linchpin reagent, ‘BiPhONSO’ [Citation12]. Strategies based on sulfonamidate [Citation13] and sulfinamide [Citation14] intermediates have also enjoyed recent success.
2.2. Sulfonimidamides
Sulfonimidamides (G) are inherently chiral aza-analogues of sulfonamides that can also act as tuneable bioisosteres. Several reports have investigated their qualities as bioisosteres [Citation15], suggesting that they may have advantageous properties such as increased metabolic stability and aqueous solubility. However, this research is still limited, especially with regards to exploring biological activity. Despite there being no general guidelines on best practice for incorporation of sulfonimidamides into drug discovery programmes, it is intriguing to note that their prevalence in the patent literature has rapidly increased in recent years (example 9).
The synthesis of sulfonimidamides has not significantly evolved over the last few decades. Most routes typically employ unstable sulfonimidoyl chloride intermediates (J, X = Cl) in a displacement reaction with amines, although the use of the corresponding sulfonimidoyl fluorides is known [Citation16]. Imination methods have recently been successfully applied to sulfonimidamide syntheses [Citation17,Citation18]. Although sulfonimidamides are inherently chiral molecules, very few methods afford enantioenriched examples [Citation19]. Enantioselectivity is particularly important because stereochemical configuration can have profound effects on drug potency. With more new methodologies being developed [Citation12], the prevalence of sulfonimidamides in medicinal agents is expected to increase.
2.3. Sulfondiimines
Although the synthesis of aza-sulfur compounds is a topical area of research, the sulfondiimine moiety (H) has received little attention in a medicinal chemistry setting. A notable exception to this is sulfondiimine 10, which has been explored as a potential pan-CDK inhibitor [Citation20]. Despite sulfondiimine 10 showing promising results, its synthesis on scale was challenging due to the requirement to use hazardous reagents. Recently, sulfondiimines have been prepared from organometallic reagents [Citation21]. Use of the latter, resulted in a more straightforward preparation of a diverse range of sulfondiimines.
2.4. Functional groups featuring sulfur-fluorine bonds
Chemical probes have become a vital tool for studying biological systems. Amongst probes that feature an electrophilic functional group, sulfonyl fluorides (I) and fluorosulfates (K) have emerged as common electrophilic warheads [Citation22]. The application of these sulfur-fluorine based molecules has provided a wealth of information on the chemoselectivity of their interactions with protein residues. One key feature of these functional groups, and also of the related sulfamoyl fluorides (L), is their relative stability toward hydrolysis [Citation23]. This has led to recent investigations into exploiting these molecules as covalent inhibitors in drug discovery programmes. An additional attractive feature of these groups is that they can be installed into known pharmaceuticals with relative ease. Molecule 11 is a good example of this [Citation24]. In this case, the fluorosulfate displayed increased potency compared to the known phenol derivative, which is itself an anti-cancer drug candidate.
3. Expert opinion
From the aforesaid reports it is evident that several sulfur-based functionalities have become privileged pharmacophores. The sulfonamide motif is most notable. However, sulfoximines, sulfonimidamides, sulfondiimines, as well as related sulfur-fluorine compounds, have only recently emerged as useful groups in drug discovery. These under-developed functional groups provide unique opportunities for medicinal chemists to explore new areas of chemical space, which comes with associated patent freedoms. To accelerate the uptake of these less-common functionalities, their pharmacokinetic and pharmacodynamic properties must be elucidated. This is particularly important for groups which have been studied in little detail. For instance, sulfondiimines (H), sulfonimidoyl and sulfamimidoyl fluorides (J, X = F, and M, respectively). The aforementioned properties cannot be ascertained unless there are safe and varied methods to prepare a diverse array of sulfur pharmacophores. It should be noted that several aza-sulfur compounds have only been synthesized a handful of times. It is essential that chemists develop new synthetic strategies to access these species. From a synthetic perspective, new practical methods should avoid cumbersome, stepwise procedures that rely on malodorous thiols. The use of toxic and hazardous reagents should also be avoided. Ideally, these routes should operate under mild conditions, and exploit reagents that allow the divergent synthesis of multiple sulfur functional groups. Attractive routes should also make use of the common building-blocks that are easily accessible to medicinal chemists, for example, aryl halides and amines. The ability to use these components directly in catalytic reactions, to access multiple sulfur pharmacophores, would be a significant breakthrough.
It is also important to develop methodologies that are compatible for late-stage functionalisation of preexisting drug molecules. The introduction of novel sulfur species into known active scaffolds can potentially improve physiochemical properties and potency. In this regard, reactions that focus on interconverting preexisting sulfur functional groups may significantly contribute to the diversification of current drug molecules [Citation25].
Developing methods to prepare neglected sulfur pharmacophores is only one challenge that must be considered. Many of these emerging pharmacophores contain nitrogen atoms and carbon-substituents which can be functionalized. Therefore, in order to gain a fuller picture of the importance and usefulness of these substituents and their general topology, it is vital to develop reactions that can selectively manipulate and modify them. This will allow correlations between a given substituent’s physiochemical and biological effects to be measured.
Overall, sulfur’s role in medicinal chemistry continues to grow. However, many sulfur functionalities remain underused in small molecule drug discovery. This is primarily due to the difficulties associated with their synthesis, and the lack of understanding of their behavior in a biological context. To successfully incorporate new sulfur pharmacophores into drug-discovery programmes, it is crucial that new synthetic methods continue to be developed. The associated biological studies must run alongside these efforts.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Ilardi EA, Vitaku E, Njardarson JT. Data-mining for sulfur and fluorine: an evaluation of pharmaceuticals to reveal opportunities for drug design and discovery. J Med Chem. 2014 April 10;57(7):2832–2842.
- McGrath NA, Brichacek M, Njardarson JT. A graphical journey of innovative organic architectures that have improved our lives. J Chem Educ. 2010 Dec 1;87(12):1348–1349.
- Bingqing FMT, Steven HL, Jiang X. Sulfur containing scaffolds in drugs: synthesis and application in medicinal chemistry. Curr Top Med Chem. 2016;16(17):1200–1216.
- Surur AS, Schulig L, Link A. Interconnection of sulfides and sulfoxides in medicinal chemistry. Arch Pharm. 2019 Jan 1;352(1):1800248.
- Winum J-Y, Scozzafava A, Montero J-L, et al. Sulfamates and their therapeutic potential. Med Res Rev. 2005 Mar 1;25(2):186–228.
- Jun JJ, Xie X-Q. Implementation of diverse synthetic and strategic approaches to biologically active sulfamides. ChemistrySelect. 2021 Jan 20;6(3):430–469.
- Frings M, Bolm C, Blum A, et al. Sulfoximines from a medicinal chemist’s perspective: physicochemical and in vitro parameters relevant for drug discovery. Eur J Med Chem. 2017 Jan;27(126):225–245.
- Brusilow WSA, Peters TJ. Therapeutic effects of methionine sulfoximine in multiple diseases include and extend beyond inhibition of glutamine synthetase. Expert Opin Ther Targets. 2017 May 4;21(5):461–469.
- Mäder P, Kattner L. Sulfoximines as rising stars in modern drug discovery? Current status and perspective on an emerging functional group in medicinal chemistry. J Med Chem. 2020 Dec 10;63(23):14243–14275.
- Tota A, Zenzola M, Chawner SJ, et al. Synthesis of NH-sulfoximines from sulfides by chemoselective one-pot N- and O-transfers. Chem Commun. 2017;53(2):348–351.
- Yu H, Li Z, Bolm C. Iron(II)-catalyzed direct synthesis of NH sulfoximines from sulfoxides. Angew Chem Int Ed. 2018 Jan 2;57(1):324–327.
- Davies TQ, Tilby MJ, Ren J, et al. Harnessing sulfinyl nitrenes: a unified one-pot synthesis of sulfoximines and sulfonimidamides. J Am Chem Soc. 2020 Sept 9;142(36):15445–15453.
- Matos PM, Lewis W, Moore JC, et al. Sulfonimidates: useful synthetic intermediates for sulfoximine synthesis via C-S bond formation. Org Lett. 2018 Jun 15;20(12):3674–3677.
- Aota Y, Kano T, Maruoka K. Asymmetric synthesis of chiral sulfoximines via the S-arylation of sulfinamides. J Am Chem Soc. 2019 Dec 11;141(49):19263–19268.
- Chinthakindi PK, Naicker T, Thota N, et al. Sulfonimidamides in medicinal and agricultural chemistry. Angew Chem Int Ed. 2017 Apr 3;56(15):4100–4109.
- Gao B, Li S, Wu P, et al. SuFEx chemistry of thionyl tetrafluoride (SOF4) with organolithium nucleophiles: synthesis of sulfonimidoyl fluorides, sulfoximines, sulfonimidamides, and sulfonimidates. Angew Chem Int Ed. 2018 Feb 12;57(7):1939–1943.
- Izzo F, Schafer M, Stockman R, et al. A new, practical one-pot synthesis of unprotected sulfonimidamides by transfer of electrophilic NH to sulfinamides. Chem Eur J. 2017 Oct 26;23(60):15189–15193.
- Briggs EL, Tota A, Colella M, et al. Synthesis of sulfonimidamides from sulfenamides via an alkoxy-amino-lambda(6) -sulfanenitrile intermediate. Angew Chem Int Ed. 2019 Oct 1;58(40):14303–14310.
- Greed S, Briggs EL, Idiris FIM, et al. Synthesis of highly enantioenriched sulfonimidoyl fluorides and sulfonimidamides by stereospecific sulfur-fluorine exchange (SuFEx) reaction. Chem Eur J. 2020 Oct 1;26(55):12533–12538.
- Lücking U. Neglected sulfur(vi) pharmacophores in drug discovery: exploration of novel chemical space by the interplay of drug design and method development. Org Chem Front. 2019;6(8):1319–1324.
- Zhang Z-X, Davies TQ, Willis MC. Modular sulfondiimine synthesis using a stable sulfinylamine reagent. J Am Chem Soc. 2019 Aug 21;141(33):13022–13027.
- Barrow AS, Smedley CJ, Zheng Q, et al. The growing applications of SuFEx click chemistry. Chem Soc Rev. 2019;48(17):4731–4758.
- Dong J, Krasnova L, Finn MG, et al. Sulfur(VI) fluoride exchange (SuFEx): another good reaction for click chemistry. Angew Chem Int Ed. 2014 Sep 1;53(36):9430–9448.
- Liu Z, Li J, Li S, et al. SuFEx click chemistry enabled late-stage drug functionalization. J Am Chem Soc. 2018 Feb 28;140(8):2919–2925.
- Fier PS, Maloney KM. NHC-catalyzed deamination of primary sulfonamides: a platform for late-stage functionalization. J Am Chem Soc. 2019 Jan 30;141(4):1441–1445.