1. Introduction
Obesity is a principal driver of chronic diseases and a public health crisis in both developed and developing countries. Obesity has been recognized as a key risk factor that leads to further health concerns and contributes to numerous metabolic diseases, including stroke, diabetes, atherosclerosis, cardiovascular diseases, and some types of cancer [Citation1]. Over the past few decades, various therapeutic options have emerged for preventing and treating overweight and obesity, such as pharmacotherapy, dietary therapy, surgical therapy, and behavioral therapy. Among these therapies, pharmacotherapy is one of the most effective and convenient therapeutic strategies. Over the past few decades, various pharmacotherapies for treating obesity including accelerating energy expenditure (such as blocking adipogenesis and inducing lipolysis) and reduction in energy intake (such as suppressing appetite and delaying or inhibiting lipid absorption) have also been proposed [Citation2]. Notably, blocking the lipid intake has been validated as one of the most feasible strategies to reduce body weight, while pancreatic lipase (PL) inhibitors can block lipid digestion and absorption in the gastrointestinal system, which in turn bring beneficial effects in weight maintenance and obesity treatment. Orlistat, a potent inhibitor of mammalian pancreatic lipase (PL, a key serine hydrolase in the gastrointestinal tract) that is launched on the market for the treatment of overweight and obesity in 1999 [Citation3], has been used clinically as the first-line anti-obesity agent in over 100 countries. As the most commonly used anti-obesity agent, orlistat may lead to some non-negligible side effects (including steatorrhea, fecal spotting, diarrhea, abdominal pain, anal fissures, and acute kidney injury) and increased risk of osteoporosis, which greatly limit its use in special patient groups, such as children, pregnant women, the elderly, and the patients with basic diseases [Citation4]. Thus, discovering and developing the efficacious anti-obesity agents with good safety profiles and desirable pharmacokinetics behaviors is always desirable.
Over the past few decades, increasing evidence has indicated that natural medicines and their constituents display good lipid-lowering and weight-loss effects [Citation5], which have aroused great interest to discover more efficacious anti-obesity agents from natural sources (especially for herbal medicines). Natural products have several inherent advantages for treating obesity-associated metabolic disorders, including good safety profiles, high anti-PL efficacy, as well as high gastrointestinal exposure but extremely low exposure to the circulation system [Citation6]. Considering that most of constituents in natural medicines cannot be well-absorbed into the circulation system and the majority of the absorbed herbal ingredients can be readily metabolized in vivo, the majority of naturally occurring constituents are more likely to exert their lipid-lowering and weight-loss effects via targeting some key therapeutic targets in the gastrointestinal tract (such as PL). In fact, a number of naturally occurring compounds have been found with strong to moderate PL inhibition activity (), parts of them can strongly inhibit multiple mammalian pancreatic lipases, such as porcine pancreatic lipase (pPL) and human pancreatic lipase (hPL). These findings indicate that it is feasible to discover promising lead compounds or drug candidates from natural sources for combating obesity.
2. Strategies for discovering lipase inhibitors from natural sources
Although a variety of herbal or folk medicines have been found with potent anti-PL activity and good weight-loss effects, the key anti-obesity constituents in these medicines and their molecular mechanisms are poorly understood. Over the past few decades, great efforts have been made by the researcher to decipher the key anti-PL constituents in herbal medicines or other natural sources by using the state-of-the-art approaches and interdisciplinary strategies. Particularly, a panel of ligand discovery techniques (such as fluorescence-based biochemical assay, affinity ultrafiltration mass spectrometry, structure-based virtual screening or computer-aided drug discovery, as well as ligand fishing by using enzyme-immobilized magnetic beads or nanoparticles) were developed and used for seeking or screening PL inhibitors, which strongly facilitate the identification of anti-PL constituents from natural sources. Meanwhile, some integrated research strategies were also proposed and used for the highly efficient discovery of PL inhibitors from complex herbal extracts. For example, Chen et al. construct pPL-immobilized nano-scaled metal-organic framework (PPL@MOF) for ligand fishing via a precipitation-cross-linking method, which has been successfully used for the highly efficient discovery of PL inhibitors from Prunella vulgaris L. [Citation7]. More recently, Ma et al. proposed an integrated strategy to identify several potent anti-hPL constituents from the root extract of Rhodiola crenulata, via integrating bioactivity-guided fractionation, mass spectrometry-based chemical profiling, and florescence-based biochemical assay [Citation8].
3. The efficacious hPL inhibitors with high safety profiles are highly desirable
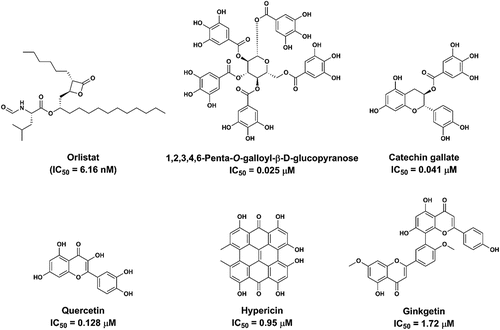
Over the past few decades, with the help of modern analytical methods and efficient drug screening assays, a wide range of natural compounds with diverse structures have been reported with strong to moderate anti-PL effects, such as anthrones extracted from Hypericum perforatum, bioflavones isolated from Ginkgo biloba, flavonoids isolated from Cortex Mori, and alkaloids extracted from Piper retrofractum Vahl [Citation9,Citation10]. Notably, most of the reported PL inhibitors were tested by using low-cost pPL to replace human pancreatic lipase (hPL) as the enzyme source. Although pPL and hPL have high amino acid sequence identity (86%) and a conserved catalytic triad (Asp-His-Ser), the species differences in inhibitor response toward these two lipases have been reported [Citation11,Citation12]. Thus, it is unreliable to use pPL as the enzyme source to explore the anti-PL constituents in herbal medicines and apply them to the human body. As shown in , some reported pPL inhibitors cannot inhibit hPL with similar activity. For example, licochalcone A is a strong pPL inhibitor (IC50 = 1.75 µM) but this agent acts as a moderate inhibitor against hPL, with the IC50 value of 18.03 µM in hPL. By contrast, epicatechin gallate and catechin gallate, two bioactive compounds isolated from the various tea extracts and other edible herbs, show excellent hPL inhibition activity (IC50 = 0.79 µM for epicatechin gallate and IC50 = 0.041 µM for catechin gallate) but these two agents hardly inhibit pPL (IC50 > 100 µM). It is evident from these data that some reported pPL inhibitors cannot inhibit hPL with similar activity and further speculate that some potent hPL inhibitors may be missed when the researchers use pPL to replace hPL as the enzyme source. In other hand, some identified potent pPL inhibitors may display moderate or weak hPL inhibition activity, which may mislead the medicinal chemists to choose these agents as the lead compounds for subsequent structural modification and druglikeness optimization. Thus, the inhibitory effects of the reported pPL inhibitors against hPL should be re-investigated to confirm their anti-hPL effects. Notably, hPL is now commercially available and can be easily expressed and purified in laboratory; the medicinal chemists can use hPL as the enzyme source for screening and characterizing anti-hPL compounds. It should also be noted that most of reported hPL inhibitors (especially the naturally occurring hPL inhibitors) displayed moderate anti-PL effects (IC50 values at the micromole level); more strategies should be used to find more efficacious anti-hPL agents with high safety profiles in future.
Table 1. The inhibitory effects of orlistat and eight natural compounds against human pancreatic lipase (hPL) and porcine pancreatic lipase (pPL).
4. Expert opinion
PL is an ideal target for designing and developing orally administrated anti-obesity agents, owing to its unique tissue distribution (in the gastrointestinal tract) and its crucial role in the hydrolysis of dietary lipids [Citation13]. An ideal orally administrated hPL inhibitor should meet the following requirements, good safety profile, excellent potency, high local exposure to gastrointestinal tract, and very low exposure to the circulatory system, which may influence the endogenous metabolism in the liver or other deep organs [Citation6]. Interestingly, several newly identified naturally occurring hPL inhibitors (such as catechin gallate and 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose) isolated from herbs meet almost all requirements of an ideal orally administrated hPL inhibitor. It is well-known that most polyphenols have good safety profiles and very poor oral bioavailability, which means these agents are highly exposed in the gastrointestinal system but difficult to be absorbed into the blood. Thus, it is a feasible way to find ideal hPL inhibitors from naturally occurring polyphenols or their derivatives.
Although a wide range of natural compounds have been identified as PL inhibitors, the inhibitory mechanisms and the binding modes on target enzyme are poorly investigated. In future, the inhibitory mechanisms, the binding modes, and ligand-binding sites of the newly identified hPL inhibitors should be carefully investigated by performing a set of assays, such as inhibition kinetics, docking simulations, and mass spectrometry-based serine modification assays. To the best of our knowledge, most of naturally occurring PL inhibitors (such as bioflavones, flavonoids, and polyphenols) are reversible PL inhibitors (acts as competitive or noncompetitive inhibitors), which is quite different from the action mechanism of orlistat (acts by covalent binding on the serine residue of PL). In view of the fact that the agents with different mechanisms of action may bring synergistic inhibitory effects, the drug combinations of orlistat (acts as covalent hPL inhibitor) and naturally occurring polyphenols (acts as reversible inhibitor) or the combinations of two hPL inhibitors with distinct ligand-binding sites should be designed and developed for combating obesity [Citation14,Citation15]. In future, more in-depth investigations are needed to efficiently discover more efficacious hPL inhibitors from natural sources and to exactly identify the binding modes or inhibitory mechanisms of these inhibitors interacting with hPL. All these investigations are very helpful for better understanding the action mechanisms of hPL inhibitors, which will strongly facilitate the medicinal chemists to design and develop more efficacious natural-derived hPL inhibitors or drug combinations as novel anti-obesity agents.
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Lin X, Li H. Obesity: epidemiology, pathophysiology, and therapeutics. Front Endocrinol (Lausanne). 2021;12:706978.
- Stuby J, Gravestock I, Wolfram E, et al. Appetite-suppressing and satiety-increasing bioactive phytochemicals: a systematic review. Nutrients. 2019;11(9):2238.
- Weibel EK, Hadvary P, Hochuli E, et al. Lipstatin, an inhibitor of pancreatic lipase, produced by Streptomyces toxytricini. I. Producing organism, fermentation, isolation and biological activity. J Antibiot (Tokyo). 1987;40(8):1081–1085.
- Shamarao N, Chethankumar M. Antiobesity drug-likeness properties and pancreatic lipase inhibition of a novel low molecular weight lutein oxidized product, LOP6. Food Funct. 2022;13(11):6036–6055.
- Liu H, Liu M, Jin Z, et al. Ginsenoside Rg2 inhibits adipogenesis in 3T3-L1 preadipocytes and suppresses obesity in high-fat-diet-induced obese mice through the AMPK pathway. Food Funct. 2019;10(6):3603–3614.
- Olennikov DN, Chirikova NK, Vasilieva AG, et al. LC-MS profile, gastrointestinal and gut microbiota stability and antioxidant activity of rhodiola rosea herb metabolites: a comparative study with subterranean organs. Antioxidants (Basel). 2020;9(6):526.
- Chen X, Xue S, Lin Y, et al. Immobilization of porcine pancreatic lipase onto a metal-organic framework, PPL@MOF: a new platform for efficient ligand discovery from natural herbs. Anal Chim Acta. 2020;1099:94–102.
- L-J M, Hou X-D, Qin X-Y, et al. Discovery of human pancreatic lipase inhibitors from root of Rhodiola Crenulata via integrating bioactivity-guided fractionation, chemical profiling and biochemical assay. J Pharm Anal. 2022;12(4):683–691.
- Rajan L, Palaniswamy D, Mohankumar SK. Targeting obesity with plant-derived pancreatic lipase inhibitors: a comprehensive review. Pharmacol Res. 2020;155:104681.
- Hou XD, Guan XQ, Cao YF, et al. Inhibition of pancreatic lipase by the constituents in St. John’s Wort: in vitro and in silico investigations. Int J Biol Macromol. 2020;145:620–633.
- Point V, Pavan Kumar KV, Marc S, et al. Analysis of the discriminative inhibition of mammalian digestive lipases by 3-phenyl substituted 1,3,4-oxadiazol-2(3H)-ones. Eur J Med Chem. 2012;58:452–463.
- Abousalham A, Verger R. Egg yolk lipoproteins as substrates for lipases. Biochim Biophys Acta. 2000;1485(1):56–62.
- Birari RB, Bhutani KK. Pancreatic lipase inhibitors from natural sources: unexplored potential. Drug Discov Today. 2007;12(19–20):879–889.
- George G, S˙n˙c˙ S, Paul AT. Investigation of synergistic potential of green tea polyphenols and orlistat combinations using pancreatic lipase assay-based synergy directed fractionation strategy. S Afr J Bot. 2020;135:50–57.
- Li S, Pan J, Hu X, et al. Kaempferol inhibits the activity of pancreatic lipase and its synergistic effect with orlistat. J Funct Foods. 2020;72:104041.