Abstract
Cucurbita pepo L. presents a nonedible biomass potentially valuable for the synthesis of gold nanoparticles (AuNPs). Searching environmental conditions which can affect AuNPs' yield and structure, extracts were obtained from plants treated with excess metal ions in the culture medium. AuNPs one-pot synthesis was conducted at 40°C for 30 min with diluted HAuCl4. Shoot extracts yielded a high number of spherical nanoparticles with lower size than AuNPs obtained from root extracts. Interestingly, root extracts from plants grown in the presence of Cu(II), Ag(I), or Au(III) gave nanoparticles with treatment-dependent shape, whereas using shoot extracts this phenomenon was not observed. This result could be attributed to metal-imposed specific changes in the cell antioxidant pool. Total polyphenol concentration in the extracts was correlated with the differences obtained in nanoparticle production. Atomic clusters from the metal accumulated in the plant, acting as specific nucleation seeds, could contribute to the synthesis of differently shaped nanoparticles.
1. Introduction
Since engineered nanomaterials have moved beyond laboratory settings to the “real world,” more than a thousand of nanotechnology-based products are currently on the market (www.nanotechproject.org, Citation41, Citation42). Along with the interest in their applications, the assessment of potential health and environmental impact of these materials is of equal concern (Citation39).
In the context of nanoparticle synthesis, the so-called Green Chemistry is attracting increasing interest as an emerging technology aimed at reducing or eliminating the use of hazardous substances during large-scale production (Citation11, Citation12), since, if nanotechnology is to realize its full promise and potential, it is essential that these next-generation nanomaterials be produced by a route benign to human health and the environment (Citation4). Green technology is still at an early stage of development, though it is expected to be widely applied and distributed.
A successful method for the generation of metal nanoparticles with different size and shapes is the bottom-up approach (Citation21). If this methodology is carried out by conventional chemistry, it often requires the use of aggressive reducing agents, such as sodium borohydride or hydrazine and may additionally involve a toxic organic solvent, such as chloroform or toluene (Citation41). The stabilizing compounds, named capping agents, are usually less dangerous, but, on the whole, the economic and environmental sustainability of these chemicals is strongly questionable. In this context, Dahl et al. established the three main criteria for a green nanoparticle synthesis: the choice of an environmentally compatible solvent, an eco-friendly reducing agent, and a non-hazardous capping agent (Citation5). Therefore, more cost-effective and environment-friendly alternatives to the conventional existing methods for metal nanoparticle synthesis are needed to be developed following such criteria.
Naturally occurring biodegradable substances can be used as reducing and stabilizing agents to obtain finely dispersed metals or metal oxides. A number of different plant-derived products have already been tested; for example, polyphenol rich plant extracts from tea and coffee were employed in the synthesis of silver and palladium nanoparticles (Citation32), agricultural residual waste (red grape pomace from winery) in the synthesis of various metal nanoparticles, such as Au, Ag, Pd, and Pt (Citation3), and the remnant water collected from soaked Bengal gram in the synthesis of gold nanoparticles (AuNPs; Citation13).
Therefore, plant extracts represent a promising route for the hydrotropic synthesis of nanoparticles, being rich in molecules potentially able to act simultaneously as reducing and capping agents. Among these compounds, a prominent role has been attributed to phytochemicals, like vitamins, small peptides, and polyphenols, due to their well-established antioxidant activity (Citation8, Citation14, Citation34), and to the abundance of chemical groups which can bind the particle surface, thus providing stabilization with reduced toxicity (Citation23, Citation30, Citation38). Extracts also present the advantage of avoiding the preparation and maintenance of cultures (Citation24), which simplifies the synthesis and recovery of nanomaterials.
Gold at the nanoscale has exceptional properties of fundamental interest for research, and it is used in many applications, such as optoelectronic devices, catalysis, biomedicine, including DNA labeling and drug delivery, cell imaging, immunostaining, and biosensors (Citation2). One of the most promising applications of AuNPs is in the environment monitoring and cleanup. In fact, AuNPs can be used in the in situ sensing of pollutants, that is, for example, in the construction of sensors for the detection of heavy metals (Citation26) and in the development of assay for TNT (Citation15). In addition, since AuNPs are able to actively catalyze a wide range of reactions with potential applications in the control of pollution, such as CO oxidation (Citation16), water gas shift reaction (Citation27), and selective oxidation of hydrocarbons (Citation20), they can be used for the removal of contaminants from different environmental matrices (Citation40).
Therefore, the request of a large-scale manufacturing of AuNPs for their diverse applications, joined to the increasing use of gold-based nanotechnologies in environmental remediation, gives raise to the stringent necessity of developing a totally safe route for their production. To date, some information about the processes occurring in AuNP green synthesis is available (Citation31, Citation33, Citation37); however, obtaining nanoparticles with precise control over size distribution, shape selectivity, and stability remains a challenge (Citation4).
This work is aimed at shedding new light on the potential of plant extracts in the green synthesis of AuNPs. The plant chosen for this study was Cucurbita pepo L., a species widely exploited in agricultural practices, whose nonedible biomass still has to find a suitable process of recycling. Abiding by the principles of sustainable chemistry, a completely aqueous route at mild temperature (40°C) was devised for AuNPs preparation.
The distinctive potentialities of extracts from roots and shoots of this species in the production of AuNPs were assessed. To our knowledge, this is the first report in which the addition of different metals in the culture medium has been proposed as a strategy to influence the size, shape, and structural organization of the obtained nanoparticles. Specifically, plant samples were exposed to Au(III), the same metal used for the nanoparticle synthesis, to Ag(I), an element with physicochemical characteristics similar to gold, and to Cu(II), an element with redox properties well-known to induce oxidative stress in plants (Citation28), which is also widely used in phytosanitary practices to prevent fungal infection. Furthermore, Ag(I) and Cu(II) have already been demonstrated to have an effect on AuNPs synthesis in planta (Citation1). The obtained results were promising and opened new routes toward size/shape selectivity in the bio-based synthesis of AuNPs.
2. Materials and methods
2.1. Plant material and experimental conditions
Seeds of Cucurbita pepo L. (var. Faenza) were sown in peat soil, and after two weeks seedlings were transferred to hydroponic culture, in 1-l polyethylene pots (three plants per pot) containing a modified half-strength Hoagland’s solution composed of 3 mM KNO3, 2 mM Ca(NO3)2, 1 mM NH4H2PO4, 0.50 mM MgSO4, 20 µM Fe(Na)-EDTA, 1 µM KCl, 25 µM H3BO3, 2 µM MnSO4, 2 µM ZnSO4, 0.1 µM CuSO4, and 0.1 µM (NH4)6Mo7O24 in milli-Q water buffered with 2 mM 2-morpholinoethanesulfonic acid, pH 5.5, adjusted with KOH (Citation19). Nutrient solutions were renewed weekly and plants were grown in a growth chamber (24/16°C day/night; light intensity 75 µE m–2 s–1, 12 h d–1; relative humidity 60–65%).
Twenty-four hours before harvesting, plants were transferred to hydroponic cultures containing the nutrient background solution described above and a concentration of 100 µM HAuCl4, Ag(NO3)2 and CuSO4.
2.2. Determination of metal concentration
Metal concentrations were determined by digesting oven-dried plant material in a 5–2 (v/v) mixture of HNO3 (Romil, 69%) and HClO4 (Applichem, 70%) in 25 ml beakers at 120–200°C, after which the volume was adjusted to 10 ml with milli-Q water. Metal was determined using inductively coupled plasma optical emission spectroscopy (Optima 2000, PerkinElmer, Waltham, Massachusetts, USA).
2.3. Preparation of plant extracts
Plants were washed three times with distilled water, blotted dry, and separated into roots and shoots. Plant material was ground in 3 ml g–1 fresh weight of distilled water and filtered with Miracloth (Calbiochem).
2.4. Total polyphenols
Total polyphenols were spectrophotometrically determined with the F-C method using chlorogenic acid as reference standard, according to a procedure described elsewhere in detail (Citation9). Briefly, the extract (50 and 150 μL for shoot and root extracts, respectively) was mixed with 200 µL of F-C reagent; after 3 min, 400 µL of a supersaturated sodium carbonate solution was added, and the obtained mixture was made up to 10 mL with milli-Q water. After dark incubation for 1 h, the spectrophotometric determination was carried out at λ = 740 nm.
2.5. Preparation of AuNPs
A total 3 ml of extract was diluted with 3 ml of milli-Q grade water and equilibrated at 40°C before adding 0.5 ml of HAuCl4 stock solution (0.5% w/w in water) dropwise and under stirring. The Au(III) concentration in the reaction vessel was thus 1.1 × 10–3 M. The solution color slowly changed from pale green (shoots) or brownish (roots) to red/purple, indicating that 5–10 min were necessary for the reaction to take place. For the reference samples, 0.15 ml of HCl 0.1 M diluted to 0.5 mL were used instead of HAuCl4.
The heating and stirring were arrested after 30 min, and all samples were stocked in the dark at room temperature.
2.6. UV-vis spectroscopy
UV-vis spectra were recorded with a Perkin Elmer lambda 35 spectrophotometer. Samples were put in quartz cuvettes with a 1 cm path length and diluted with milli-Q water when the optical density was too high (i.e. when the absorbance in the range 500–600 nm was ≥ 3.0). The scan rate was 60 nm/min.
2.7. Electron microscopy
Transmission electron microscopy (TEM) analyses were carried out on a TEM CM 12 PHILIPS, equipped with an OLYMPUS Megaview G2 camera, with an accelerating voltage of 80 keV. Samples for TEM analysis were prepared by suspending in water and deposing a drop of the obtained suspension on a Carbon Cu grid, followed by solvent evaporation. The samples with high amount of organic matrix were performed with a different procedure: the grid was laid down on the surface of a drop of suspension for 1 min, and then, after solvent evaporation, it was introduced into the TEM.
2.8. Dynamic Light Scattering
The mean size of nanoparticles in water was determined by Photon Correlation Spectroscopy, better known as dynamic light scattering (DLS), by using a Zetasizer Nano S (Malvern Instruments Ltd., UK), equipped with a 4 mW He-Ne, laser (632.8 nm), and back scatter detection. The manufacturer’s software was used for data analysis. The particle size distribution and polydispersity index were obtained by the intensity distribution algorithm.
3. Results and discussion
3.1. Metal accumulation
reports Ag, Au, and Cu concentration in the different samples. In untreated plants, Ag and Au concentrations were below the detection limit, in agreement with their absence in the nutrient solution, whereas Cu was represented in physiological amounts (<30 µg g–1, as the limits estimated by 21), as it is a well-known micronutrient for plants (Citation28). As a general finding of all treatments, metal accumulated in root was much higher than that in shoot. However, within the same plant organ, different patterns of metal accumulation were observed, depending on the metal considered. More in detail, in roots, Au and Cu reached remarkable levels of accumulation with respect to Ag. In shoots, Ag was totally excluded, whereas Au and Cu reached levels higher than the untreated plants. These differences could be attributed to a diverse mobility of metals inside the plant, due to element-specific affinity toward the plant homeostatic network for metal uptake and translocation.
Table 1. Metal concentration in metal-treated and untreated Cucurbita pepo plants.
Since treated plants accumulated different amounts of elements according to the considered organ and the metal used, different responses to counteract their toxicity might have been generated. For example, in such condition, plants are known to produce metal chelating compounds for their detoxification and antioxidant compounds to preserve the cell redox status, threatened by the presence of the metals themselves (Citation18, Citation29).
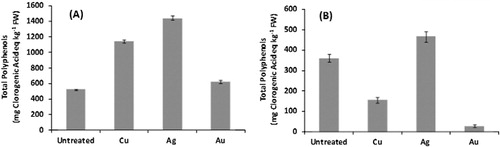
3.2. Polyphenol concentration
In our experimental conditions, total polyphenol concentration varied markedly depending on the organ and the treatment considered as reported in . In shoots, a metal-imposed increase in polyphenols was generally observed, probably as a consequence of a still active plant response to counteract the metal toxic effects. In such organs, the metal concentration was extremely low with respect to roots; thus, shoots could have preserved their ability to synthesize phenols as detoxification strategy toward such a stress. Similar results for a differentially metal-induced phenol production in root and shoot were observed for the response of Nicotiana langsdorffii plants to chromium (Citation7) and of a mangrove species to manganese or lead (Citation43). In fact, phenols are commonly involved in the defense strategy toward different abiotic stresses, including those related to heavy metal exposure (Citation10, Citation25), due to both their redox and metal-chelating properties. Furthermore, phenolics have been recently identified to impart Au(III) tolerance in cowpea through the generation of AuNPs ex planta, i.e. in the culture medium (Citation36).
3.3. Nanoparticle production and characterization
The systems and procedures studied in this work enabled to obtain a variety of AuNPs, which differed in number, size, and shape, depending on the starting material. The synthesis yield of nanoparticles strongly depended on the plant organ and the metal to which the plants were exposed during growth, before being collected for extraction.
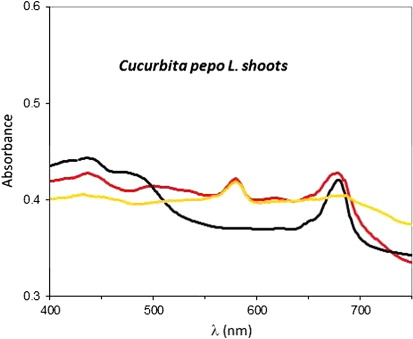
The presence of nanoparticles was assessed in the extracts from both untreated and metal-treated plants to which HAuCl4 was added at 40°C for 30 min, owing to the presence of a characteristic surface plasmon resonance peak of AuNPs in the range 500–600 nm. This range is particularly suited to study the absorption spectra of plant extracts, since it is the only region of the visible spectrum free from chlorophyll signals. shows the absorption spectrum of AuNPs obtained from the shoot extract of untreated plants in comparison with the spectrum of its reference sample. This latter sample was obtained by reaction of the same shoot extract with a diluted HCl solution under the same experimental conditions of temperature and reaction time, to mimic the reaction environment and avoid possible effects of pH variations. In both spectra, the peak at about 680 nm was attributed to the contribution of chlorophyll, and therefore it was not analyzed further. In both spectra, the peaks in the ranges 400–530 nm and 600–750 nm were due to plant pigments (e.g. chlorophylls), which are subject to time-dependent degradation, whereas the plasmon peak at ~570 nm was stable over days, as shown by the spectra recorded at different time interval after the synthesis ().
shows the time evolution of the AuNP plasmon resonance peak for untreated and gold-treated Cucurbita pepo L. roots. The wavelength value of the maximum is known to depend on size and shape of AuNPs, while peak intensity and line width are primarily related to the concentration and polydispersity of nanoparticles (Citation6). Extracts from control roots produced more nanoparticles and in less time than metal-treated plants. This result was inferred from the comparison of the plasmon peak intensity (see as an example) of many replicates and TEM images.
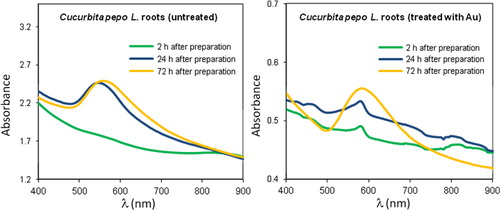
As shown by the comparison of and in samples obtained from Cucurbita pepo roots, the absorption band was broader (from about 500 to 700 nm) than for shoot extracts, indicating in the former case a wider distribution in the size and shape of AuNPs. The temporal evolution of the plasmon peak consisted of an intensity increase and a small shift to higher wavelengths from the freshly synthesized samples throughout the following 2–3 days. After this time interval, no further appreciable aging effect was observed ().
Similar aging behavior on the plasmon resonance peak was obtained from shoot extracts, even though in this case the interpretation of visible spectra was less straightforward, due to the crowding by the peaks of colored pigments in this region. It is also to be noted that metal clusters different from the ones formed by gold atoms were not detected in samples obtained from plants exposed to heavy metals, indicating that they were absent, or small, or diluted enough to escape detection in our experimental conditions.
The size and morphology of nanoparticles were investigated by TEM.
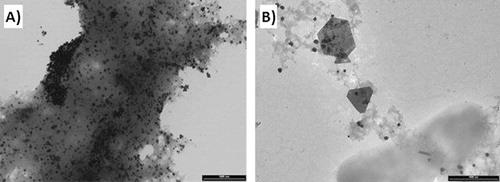
shows the TEM images taken from Cucurbita pepo L. root extracts of untreated (A), Ag-treated (B), Au-treated (C), and Cu-treated (D) samples. The most abundant and smaller AuNPs were obtained by reducing HAuCl4 with the extracts from untreated and Ag-treated samples. Root extracts from untreated plants produced almost exclusively globular AuNPs (), while Ag-treated specimens gave also some triangular AuNPs (). Extracts from plants treated with Cu(II) and Au(III) showed a marked tendency to produce larger and fewer AuNPs. In particular, sharp-edged particles (polygons) were found to be a characteristic of nanoparticles obtained from Au(III)-treated plants (), while in the case of Cu, the ion pretreatment also allowed forming fractal like structures, known as “raspberry” nanoparticles (). These latter structures were not detected in other samples. It has been shown that in the case of bottom-up preparation, anisotropic nanoparticles or particles with nonglobular structure (e.g. plates) can result from seed-mediated growth (Citation35). Other possible sources of anisotropy are capping agents which selectively bind to specific crystal faces (Citation17). The experiments carried out in this work were more in line with the former hypothesis, since the presence of metal ions in the culture medium strongly favored the formation of anisotropic shapes. Although grains formed by reduced metal ions could not be directly evidenced by TEM or UV-vis spectroscopy, it is possible that in planta produced metal seeds were too small to be detected by these techniques.
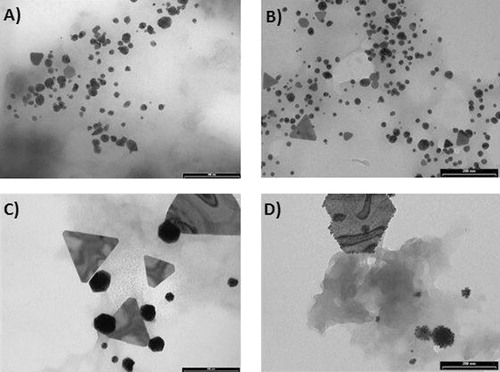
DLS indicated that particle distributions with different mean size existed in solution. As an example, the intensity-averaged particle size distribution in the sample obtained from Cu(II)-treated roots (the TEM image of which is shown in ) is reported in . No particles with diameter as large as 600–800 nm were observed in TEM micrographs; therefore, the larger scattering objects detected in solution were due to clustering of smaller particles mediated by the capping agents in the organic matrix.
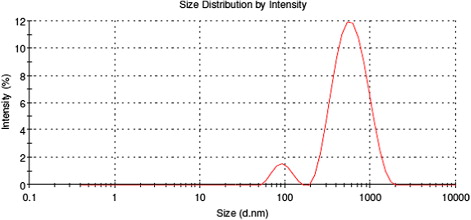
In general, DLS data were not easily obtainable, due to the high quantity of organic material covering and stabilizing the AuNPs, which rendered the solution optically turbid.
Samples from shoot extracts presented a high amount of organic matrix, which formed a sort of thick gel entrapping the nanoparticles, as exemplified by the TEM image reported in , which shows nanoparticles synthesized by Au-treated shoot extracts. However, AuNPs were unambiguously detected in all the samples, owing to the high contrast provided in TEM by gold with respect to the embedding organic matrix. A general finding was that a greater number of AuNPs was produced from shoots with respect to the corresponding root samples and that less shape differentiation occurred. This is illustrated, for instance, by the comparison between and which were taken from different organs of the same plant. Indeed, as reported above, the roots of Au- and Cu-treated plants exhibited lower polyphenol concentrations than untreated and Ag-treated samples, probably due to their very high metal accumulation that could have determined the oxidations of these compounds. Therefore, the higher yield of small-size AuNPs obtained with untreated and Ag-treated root extracts seems to be related with the total polyphenol concentration. Shoot extracts showed higher polyphenol concentration than root extracts, thus concurring to explain the differences in the Au reductive process that determined the above-mentioned diversity in the formation of Au-NPs between these two organs.
In summary, differences reported in phenol concentration might be one of the causes of the different reducing properties shown by the extracts in Au-NPs production. Furthermore, also the possible presence of metal clusters in the samples, formed in planta as a consequence of the treatment and the relative metal accumulation, could play a role by acting as nucleation grains for the synthesis of nanoparticles with different shapes.
4. Conclusions
This work demonstrates that a bio-catalyzed synthesis of AuNPs can be effectively performed by Cucurbita pepo extracts, prepared through a completely green hydrotropic route.
The nanoparticle yield was plant organ-dependent, in that root and especially shoot extracts from untreated plants produced a large number of small spheroidal particles. Root extracts from plants pretreated with metal ions, i.e. Ag(I), Au(III), and Cu(II), gave larger particles, showing an interesting treatment-dependent shape. Specifically, exposure to Ag ions induced abundant production of sharp-edged triangles, while in the presence of Cu ions larger polygons were formed. Both these shapes were found when extracts from plants treated with Au were used for AuNP preparation. Finally, extracts from Cu-treated plants were used also gave fractal-like nanoparticles (“raspberries”). These results were attributed to a different concentration of polyphenols in the extracts and, possibly, to metal clusters preformed in planta, which acted as nucleation seeds.
Our findings may open an interesting field of research to obtain nanoparticles with desired size and shape using Green Chemistry.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Anderson, C.W.N.; Bhatti, S.M; Gardea-Torresdey, J.; Parson, J. In Vivo Effect of Copper and Silver on Synthesis of Gold Nanoparticles inside Living Plants. Sust. Chem. Eng. 2013, 1, 640–648.
- Bali, R.; Harris, A.T. Biogenic Synthesis of Au Nanoparticles Using Vascular Plants. Ind. Eng. Chem. Res. 2010, 49, 12762–12772.
- Baruwati, B.; Varma, R.S. High Value Products from Waste: Grape Pomace Extract-A Three-in-one Package for the Synthesis of Metal Nanoparticles. Chem. Sus. Chem. 2009, 2, 1041–1044.
- Betts, K. A Greener Route to Gold Nanoparticles. Environ. Sci. Technol. 2005, 39, 104–105.
- Dahl, J.A.; Maddux, B.L.S.; Hutchison, J.E. Toward Greener Nanosynthesis. Chem. Rev. 2007, 107, 2228–2269.
- Daniel, M.C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-size-related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346.
- Del Bubba, M.; Ancillotti, C.; Checchini, L.; Ciofi, L.; Fibbi, D.; Gonnelli, C.; Mosti, S. Chromium Accumulation and Changes in Plant Growth, Selected Phenolics and Sugars of Wild Type and Genetically Modified Nicotiana langsdorffii. J. Haz. Mat. 2013, 262, 394–403.
- Dong, S.; Zeng, M.; Wang, D.; Liu, Z.; Zhao, Y.; Yang, H. Antioxidant and Biochemical Properties of Protein Hydrolysates Prepared from Silver Carp (Hypophthalmichthys molitrix). Food. Chem. 2008, 107, 1485–1493.
- Doumett, S.; Fibbi, D.; Cincinelli, A.; Giordani, E.; Nin, S.; Del Bubba, M. Comparison of Nutritional and Nutraceutical Properties in Cultivated Fruits of Fragaria vesca L. Produced in Italy. Food. Res. Int. 2011, 44, 1209–1216.
- Duressa, D.; Soliman, K.; Cebert, E. Protein and Polyphenol Profile Changes in Soybean Roots under Aluminum Stress. Int. J. Plant. Physiol. Biochem. 2010, 2, 38–45.
- Eckelman, M.J.; Zimmerman, J.B.; Anastas, P.T. Toward Green Nano. J. Ind. Ecol. 2008, 12, 316–328.
- Faramazi, M.A., Sadighi, A. Insights into Biogenic and Chemical Production of Inorganic Nanomaterials and Nanostructures. Adv. Colloide. Interface. Sci. 2013, 189–190, 1–20.
- Ghule, K.; Ghule, A.V.; Liu, J.Y.; Ling, Y.C. Microscale Size Triangular Gold Prisms Synthesized using Bengal Gram beans (cicer arietinum l.) Extract and HAuCl4·3H2O: A Green Biogenic Approach. J. Nanosci Nanotechnol. 2006, 6, 3746–3751.
- Giordani, E.; Doumett, S.; Nin, S.; Del Bubba, M. Selected Primary and Secondary Metabolites in Fresh Persimmon (Diospyros kaki Thunb.): A Review of Analytical Methods and Current Knowledge of Fruit Composition and Health Benefits. Food. Res. Int. 2011, 44, 1752–1767.
- Girotti, S.; Eremin, S.; Montoya, A.; Moreno, MJ; Caputo, P; D’Elia, M; Ripani, L; Romolo, FS; Maiolini, E. Development of a Chemiluminescent ELISA and a Colloidal Gold-based LFIA for TNT Detection. Anal Bioanal Chem. 2010, 396, 687–695.
- Gluhoi, A.C.; Lin, S.D.; Nieuwenhuys, B.E. The Beneficial Effect of the Addition of Base Metal Oxides to Gold Catalysts on Reactions Relevant to Air Pollution Abatement. Catal. Today. 2004, 90, 175.
- Grzelczak, M.; Perez-Juste, J.; Mulvaney, P.; Liz-Marzan, L.M. Shape Control in Gold Nanoparticle Synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791.
- Hall, J.L. Cellular Mechanisms for Heavy Metal Detoxification and Tolerance. J. Exp. Bot. 2002, 53, 1–11.
- Hoagland, D.R.; Arnon, D.I. The Water-culture Method for Growing Plants without Soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 1–39.
- Hughes, M.D.; Xu, Y.J.; Jenkins, P.; Mcmorn, P.; Landon, P.; Enache, D.L.; Carley, A.F.; Attard, G.A.; Hutchings, G.J.; King, F.; Stitt, E.H.; Johnston, P.; Griffin, K.; Kiely, C.J. Tunable gold catalysts for selective hydrocarbon oxidation under mild conditions. Nature 2005, 437, 1132.
- Iravani, S. Green Synthesis of Metal Nanoparticles using Plants. Green. Chem. 2011, 13, 2638–2650.
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, 2001.
- Kharissova, O.V.; Dias, H.V.; Kharisov, B.I.; Perez, B.O.; Perez, V.M. The Greener Synthesis of Nanoparticles. Trends. In. Biotechnol. 2013, 31, 240–248.
- Kumar, V.; Kumar Yadav, S. Plant-mediated Synthesis of Silver and Gold Nanoparticles and Their Applications. J. Chem. Technol. Biotechnol. 2009, 84, 151–157.
- Lavid, N.; Schwartz, A.; Yarden, O.; Tel-Or, E. The Involvement of Polyphenols and Peroxidase Activities in Heavy-metal Accumulation by Epidermal Glands of the Waterlily (Nymphaeaceae). Planta. 2001, 212, 323–331.
- Li, M.; Gou, H.L.; Al-Ogaidi, I.; Wu, N.Q. Nanostructured Sensors for Detection of Heavy Metals: A Review. ACS. Sus. Chem. Eng. 2013, 1, 713–723.
- Liu, Z.P.; Jenkins, S.J.; King, D.A. Origin and Activity of Oxidized Gold in Water-gas-shift Catalysis. Phys. Rev. Lett. 2005, 94, 196102.
- Marschner, H. Mineral Nutrition of Higher Plants; Oxford University Press: London, 1995.
- Meharg, A. Mechanisms of Plant Resistance to Metal and Metalloid Ions and Potential Biotechnological Applications. Plant. Soil. 2005, 274, 163–174.
- Mohan Kumar, K.; Mandal, B.K.; Kiran Kumar, H.A.; Maddinedi, S.B. Green Synthesis of Size Controllable Gold Nanoparticles. Spectrochim. Acta. Part. A: Mol. Biomol. Spectr. 2013, 116, 539–545.
- Montes, M.O.; Mayoral, A.; Deepak, F.L.; Parsons, J.G; Jose-Yacaman, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Anisotropic Gold Nanoparticles and Gold Plates Biosynthesis using Alfalfa Extracts. J. Nanop. Res. 2011, 13, 311–3121.
- Nadagouda, M.N.; Varma, R.S. Green Synthesis of Silver and Palladium Nanoparticles at Room Temperature using Coffee and Tea Extract. Green. Chem. 2008, 10, 859–862.
- Narayanan, K.B.; Sakthivel, N. Green Synthesis of Biogenic Metal Nanoparticles by Terrestrial and Aquatic Phototrophic and Heterotrophic Eukaryotes and Biocompatible Agents. Adv. Colloid. Interface. Sci. 2011, 169, 59–79.
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant Properties of Phenolic Compounds. Trends. In. Plant. Sci. 1997, 2, 152–159.
- Roduner, E. Nanoscopic Material: Size Dependent Phenomena; RSC Publishing: Cambridge, UK, 2006.
- Shabnam, N.; Pardha-Saradhi, P.; Sharmila, P. Phenolics Impart Au3+-Stress Tolerance to Cowpea by Generating Nanoparticles. PlosOne. 2014, 9, e85242.
- Song, J.Y.; Yang, H.K.; Kim, B.S. Biological Synthesis of Gold Nanoparticles using MAGNOLIA kobus and Diopyros kaki Leaf Extracts. Process Biochem 2009, 44, 1133–1138.
- Sujitha, M.V.; Kannan, S. Green Synthesis of Gold Nanoparticles using Citrus Fruits (Citrus limon, Citrus reticulate and Citrus sinensis) Aqueous Extract and Its Characterization. Spectrochim. Acta. Part A: Mol. Biomol. Spectr. 2013, 102, 15–23.
- The Royal Society & The Royal Academy of Engineering. Nanoscience and Nanotechnologies: Opportunities and Uncertainties. 2004. http://www.nanotec.org.uk/finalReport.htm (accessed Mar 20, 2015).
- Thompson, D.T. Using Gold Nanoparticles for Catalysis. Nanotoday. 2007, 2, 40–43.
- Varma, R.S. Greener Approach to Nanomaterials and Their Sustainable Applications. Curr. Opin. Chem. Eng. 2012, 1, 123–128.
- Xia, T.; Li, N.; Nel, A.E. Potential Health Impact of Nanoparticles. Annu. Rev. Public. Health. 2009, 30, 137–150.
- Yan, Z.Z.; Tam, N.F. Temporal Changes of Polyphenols and Enzyme Activities in Seedlings of Kandelia obovata Under Lead and manganese Stresses. Mar. Pollut. Bull. 2011, 63, 438–444.