ABSTRACT
In this investigation, magnetite nanoparticles (Fe3O4 NPs) with a diameter of 5–10 nm were synthesized by coprecipitation from an alkaline solution of ferrous/ferric mixed salt-solution in the presence of copolymers. In this process, linear–dendritic copolymers containing a linear poly (ethylene glycol) core and dendritic poly (citric acid) arms were used as the stabilizer. The Fe3O4 NPs were characterized by transmission electron microscopy, energy-dispersive spectra, X-ray powder diffraction, field emission scanning electron microscopy, Fourier transform infrared spectrometer, and vibrating sample magnetometer. Compared to classical coprecipitation, the results showed that uniform, ultrafine, nearly spherical, and high purity nano-magnetite particles with better magnetic properties could be prepared by this method.
GRAPHICAL ABSTRACT
Introduction
Magnetic nanoparticles (NPs), especially magnetite (Fe3O4), can be widely used in science, technology, and healthcare in applications such as cell separation (Citation1), microwave absorption (Citation2), magnetic resonance imaging (MRI) techniques (Citation3), recording materials (Citation3), ferrofluids (Citation4), biological sensing (Citation5), bioactive molecule separation (Citation3), catalysis (Citation6–8), and magnetic targeted therapy (Citation9). For future highly sensitive magnetic nanostructures and biological and pharmacological applications, Fe3O4 NPs with controlled size, shape, and a narrow size distribution are needed. Recently, research on the synthesis and application of Fe3O4 NPs in chemistry and pharmaceuticals has received particular attention (Citation10). Several methods have been developed for the synthesis of magnetic Fe3O4 NPs, including chemical coprecipitation (Citation10–14), the hydrothermal process (Citation15), the solvothermal method (Citation16), the sol–gel technique (Citation17, Citation18), microemulsion (Citation19, Citation20), etc. Among these, the chemical coprecipitation is considered as the simplest, most economical, and most efficient method (Citation21).
However, the classical coprecipitation method has certain limitations, including insufficient dispersion, broad and uncontrollable particle size distribution, difficulty of mass production, and phase control of NPs. Therefore, achieving uniform-sized Fe3O4 particles and high magnetic response is hard; especially when the Fe3O4 particle size should be in the nanoscale (Citation12). To overcome these limitations, several coprecipitation techniques were used such as interfacial coprecipitation (Citation12), ultrasonic coprecipitation (Citation11), microwave coprecipitation (Citation22), mechanochemical coprecipitation (Citation13), and coprecipitation with fast stirring (Citation14). Furthermore, other research has utilized surfactants (Citation23), macromolecules (Citation24), or dilute acids (Citation25) to improve the dispersibility of Fe3O4 NPs in water. Although several methods have been reported for the synthesis of magnetic NPs, the development of a template and stabilizer in water for the synthesis of Fe3O4 NPs is an active, ongoing research area and thus, there is potential for further improvement toward greener conditions, uniform-sized Fe3O4 particles and high magnetic response.
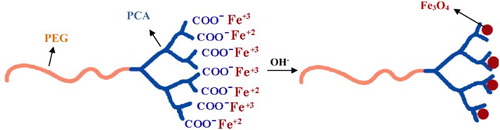
Linear–dendritic copolymers are hybrid macromolecules containing linear polymers and dendrimers (Citation26, Citation27). Poly (citric acid) (PCA)–poly (ethylene glycol) (PEG)–PCA copolymers containing hyperbranched dendritic citric acid parts (with a large number of OH functional groups in their structures) and linear PEG as flexible part (which enhances the solubility) could be effective as a template and stabilizer in water for the synthesis of NPs (Citation28, Citation29). In this study, PCA–PEG–PCA dendritic macromolecules have been used to synthesize magnetic NPs. Therefore, this work elucidates the decreased particle size and increased saturation magnetization of Fe3O4 NPs prepared via a modified coprecipitation reaction by PCA–PEG–PCA copolymers.
Experimental
Chemical and apparatus
Chemical reagents were purchased from the Merck, Aldrich, and Sigma Chemical Companies. Fourier transform infrared spectrometer (FT-IR) spectra were recorded with KBr pellets on a Shimadzu Perkin-Elmer 550 spectrometer. The magnetic measurements of samples were obtained using a vibrating sample magnetometer (VSM) (4 inch, NDKF, Kashan, Iran) at room temperature. Morphological characteristics of the nanostructures were analyzed using field emission scanning electron microscopy (FE-SEM) (MAIA) and transmission electron microscopy (TEM) (Ziess EM10C). Nanostructures were characterized using a Holland Philips Xpert X-ray diffraction (XRD) diffractometer (CuK, radiation, λ = 0.154056 nm), at a scanning speed of 2°/min from 10° to 100° (2θ).
Procedure for preparation of Fe3O4 NPs by PCA–PEG–PCA
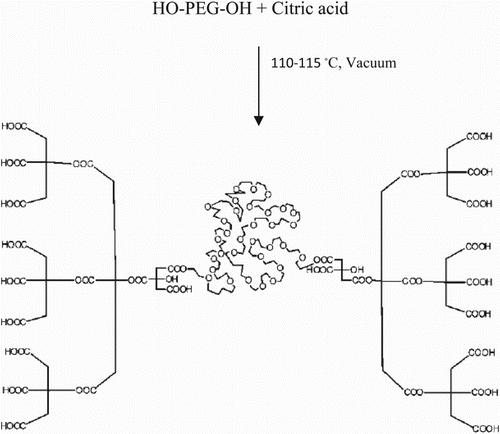
PCA–PEG–PCA macromolecules (Mw ≈ 2000) were prepared according to the reported method in literature (Citation29). Copolymers were synthesized following the procedure shown in Scheme 1. Fe3O4 NPs were prepared via the Massart’s processes as a simple chemical coprecipitation technique (Citation11) in the presence of PCA–PEG–PCA. Typically, 20 mmol of FeCl3·6H2O, 10 mmol of FeCl2·4H2O and 0.1 g of PCA–PEG–PCA were dissolved in 80 ml of distilled water in a three-necked bottom (250 ml) under Ar atmosphere for 1 h. Next, a certain amount of NH4OH (28% (w/w)) was added into the solution within 30 min until the pH value was titrated to 11.0 under continuous Ar atmosphere bubbling. Once the NH4OH solution was added into the reaction flask, the color of the mixture turned from orange to black immediately. The reaction mixture was rapidly stirred for 1 h. Then, the resultant black dispersion was heated to 85°C for 1 h. Fe3O4 NPs were isolated by magnetic decantation, exhaustively washed with double-distilled water until neutrality, and dried at 60°C overnight under vacuum tray drying.
Results and discussion
The chemical coprecipitation of Fe (III) and Fe (II) salts in alkaline solutions was used as a traditional method of preparing Fe3O4 NPs:However, the Fe3O4 NPs synthesized by classical procedure shared some flaws and deficiencies such as poor dispersity, impurity of production, nonuniform particle size, and so on. To overcome these limitations, in this paper, the PCA–PEG–PCA copolymers were used to modify the classical coprecipitation technique to synthesize the uniform-sized Fe3O4 particles and high magnetic response of Fe3O4 NPs.
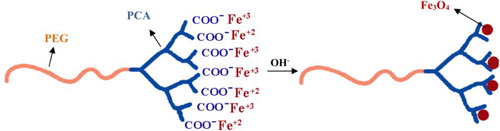
The proposed mechanism for the modified preparation of Fe3O4 NPs was shown in Scheme 2. In the first stage, the Fe2+ and Fe3+ ions and the carboxylic acid functional groups of PCA–PEG–PCA copolymers formed a complex structure. After the NH4OH solution was added, carboxylic acid groups on the surface of PCA–PEG–PCA promoted the nucleation of Fe3O4 NPs. Because the PCA–PEG–PCA macromolecules acted as the support for Fe3O4 nucleation, the growth of NPs was hindered by the copolymers and the size of the Fe3O4 NPs was about 5–10 nm, which was smaller than the traditional method. The addition of the PCA–PEG–PCA with a hyperbranch structure during the reaction process provided steric repulsion to improve the quality of the dispersion of particles (Citation4). These effects of polymeric stabilizers in the synthesis of metallic nanoparticle were also discussed in previous research (Citation30–33). Thus, the PCA–PEG–PCA copolymers acted as templates and steric stabilizers that reduced the intrinsic particle size of Fe3O4 NPs and aggregation of particles.
The properties of Fe3O4 NPs with an average size of 5–10 nm were investigated. (a) shows the FE-SEM image of Fe3O4 NPs prepared by modified coprecipitation. The average size of Fe3O4 NPs was approximately 10 nm, and the particles are spherical shaped, uniform, and monodispersed. (b) shows a SEM image of magnetic NPs under classical conditions. It is worthwhile to note that the size distribution is 15–30 nm.
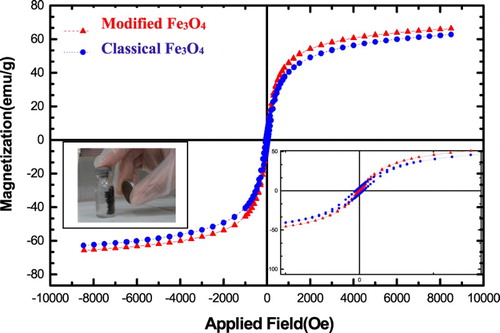
The magnetization curves of Fe3O4 NPs are shown in . As demonstrated in , magnetic performance of the modified NPs is better than NPs without modification. Because the remanence (Mr) value of unmodified Fe3O4 is about 1.25 emu/g; while, that of the modified Fe3O4 NPs is less than 0.65 emu/g that this could be attributed to the fact that, the NPs with modification were so small that they might be considered to have a single magnetic domain (Citation10). From the magnetization curve, we can also see that the saturation magnetization (Ms) of the NPs increase from 62.76 to 66.54 emu/g when the sizes of Fe3O4 increase from 15–30 to 5–10 nm, which can be attributed to the increase of crystallinity of the modified NPs.
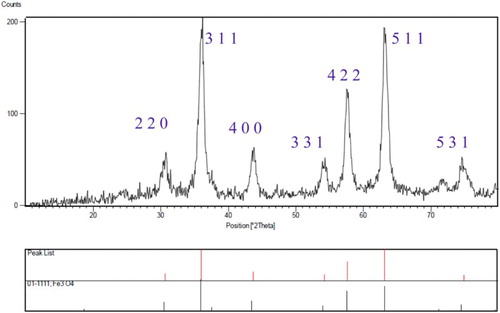
presents the XRD powder diffraction patterns of the magnetic modified Fe3O4. The position and relative intensities of all peaks confirm well with the standard XRD pattern of Fe3O4 (JCPDS card No. 01-1111). The data for the Fe3O4 NPs at 2θ = 30.66, 36.06, 43.72, 54.22, 57.68, 63.18, 74.85 corresponded to (2 2 0), (3 1 1), (4 0 0), (3 3 1), (4 2 2), (5 1 1), and (5 3 1) respectively. The crystallographic parameters calculated using software provided by Panalytical company (Holland) are: a = b = c = 8.3941 Å, α = β = γ = 90.0000°, they are indexed to the crystalline cubic spinel structure of modified Fe3O4 NPs.
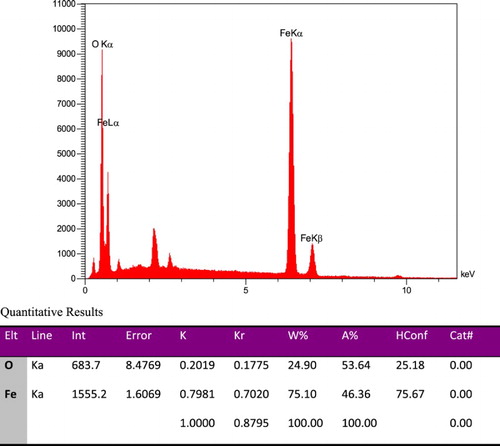
Elemental components of Fe3O4 are calculated from energy-dispersive spectra (EDS) in . The elemental compositions of magnetic iron oxide particles are 24.90% and 75.10% for Fe and O, respectively. According to this analysis, it could be concluded Fe3O4 have been successfully synthesized.
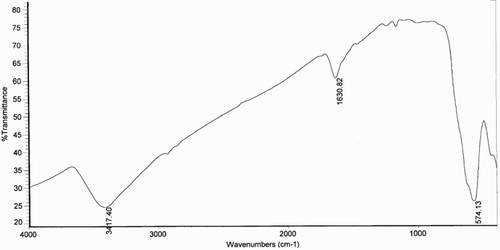
shows the FT-IR spectrum of Fe3O4 NPs synthesized with PCA–PEG–PCA. This sample exhibits a strong absorption peak around 3417 cm−1, corresponding to the stretching mode of OH group adsorbed on the surface of the Fe3O4 NP while the peak appearing at approximately 1630 cm−1 was associated with the bending vibration of the O–H group. These peaks were owing to the adsorbed water on the surface of Fe3O4. The peak at 574 cm−1 corresponds to the Fe–O bond NPs (Citation7, Citation8).
Conclusion
We have reported an efficient coprecipitation for the synthesis of Fe3O4 NPs in the presence of dendritic macromolecules as the stabilizer. The obtained iron oxide NPs can be promising as a potentially suitable magnetic support to be employed in different branches of magnetic technology with good biocompatibility, good economic aspects, and good magnetic quality.
Supplement_Material.zip
Download Zip (6.3 MB)Acknowledgement
The authors are grateful from the Research Council of the University of Kashan for supporting this research by Grant NO.256722/20.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on Contributors
Zohre Zarnegar received her BS (2002) in Pure Chemistry from University of Kashan (Iran). Next, she studied her MS (2008) in Organic Chemistry at the Lorestan University. She performed her MS research in the field of nanochemistry which included synthesis of novel dendrimers under the supervision of Prof. Mohsen Adeli. She obtained her PhD degree in Organic Chemistry at University of Kashan (2014) which included development of magnetic nanoparticles and their application in host-guest systems and some of the organic reactions under the supervision of Dr Javad Safari. She added a postdoctoral stage from the Iran Nanotechnology Initiative Council (INIC) in 2016.
Javad Safari was born in Kashan, Iran in 1965. He obtained his B.Sc. degree in Chemistry at the University of Kashan in 1989. He received his M.Sc and Ph.D. degrees in Organic Chemistry at the University of Isfahan in 1992 and 2002. He is teaching as associate professor of Chemistry at the University of Kashan. His research interests focus on heterocyclic chemistry, spectroscopy, catalysis and organic methodology.
Additional information
Funding
References
- Zhu, X.; Zhang, L.; Fu, A.; Yuan, H. Efficient Purification of Lysozyme from Egg White by 2-Mercapto-5-benzimidazolesulfonic Acid Modified Fe3O4/Au Nanoparticles. Mater. Sci. Eng. C. 2016, 59, 213–217. doi: 10.1016/j.msec.2015.10.009
- Hekmatara, H.; Seifi, M.; Forooraghi, K. Microwave Absorption Property of Aligned MWCNT/Fe3O4. J. Magn. Magn. Mater. 2013, 346, 186–191. doi: 10.1016/j.jmmm.2013.06.032
- Hola, K.; Markova, Z.; Zoppellaro, G.; Tucek, J.; Zboril, R. Tailored Functionalization of Iron Oxide Nanoparticles for MRI, Drug Delivery, Magnetic Separation and Immobilization of Biosubstances. Biotechnol. Adv. 2015, 33, 1162–1176. doi: 10.1016/j.biotechadv.2015.02.003
- Lin, C.L.; Lee, C.F.; Chiu, W.Y. Preparation and Properties of Poly(Acrylic Acid) Oligomer Stabilized Superparamagnetic Ferrofluid. J. Colloid Interface Sci. 2005, 291, 411–420. doi: 10.1016/j.jcis.2005.05.023
- Li, N.; Hao, X.; Kang, B.H.; Xu, Z.; Shi, Y.; Li, N.B.; Luo, H.Q. Enzyme-free Fluorescent Biosensor for the Detection of DNA Based on Core-Shell Fe3O4 Polydopamine Nanoparticles and Hybridization Chain Reaction Amplification. Biosens. Bioelectron. 2016, 77, 525–529. doi: 10.1016/j.bios.2015.10.004
- Safari, J.; Zarnegar, Z. Sulfamic Acid-functionalized Magnetic Fe3O4 Nanoparticles as Recyclable Catalyst for Synthesis of Imidazoles Under Microwave Irradiation. J. Chem. Sci. 2013, 125, 835–841. doi: 10.1007/s12039-013-0462-2
- Safari, J.; Zarnegar, Z.; Heydarian, M. Magnetic Fe3O4 Nanoparticles as Efficient and Reusable Catalyst for the Green Synthesis of 2-Amino-4H-chromene in Aqueous Media. Bull. Chem. Soc. Jan. 2012, 85, 1332–1338. doi: 10.1246/bcsj.20120209
- Safari, J.; Zarnegar, Z. A Highly Efficient Magnetic Solid Acid Catalyst for Synthesis of 2,4,5-Trisubstituted Imidazoles Under Ultrasound Irradiation. Ultrasonic. Sonochem. 2013, 20, 740–746. doi: 10.1016/j.ultsonch.2012.10.004
- Wang, C.; Huang, L.; Song, S.; Saif, B.; Zhou, Y.; Dong, C.; Shuang, S. Targeted Delivery and pH-Responsive Release of Stereoisomeric Anti-cancer Drugs Using β-Cyclodextrin Assemblied Fe3O4 Nanoparticles. Appl. Surf. Sci. 2015, 357, 2077–2086. doi: 10.1016/j.apsusc.2015.09.189
- Feng, J.; Mao, J.; Wen, X.; Tu, M. Ultrasonic-Assisted in Situ Synthesis and Characterization of Superparamagnetic Fe3O4 Nanoparticles. J. Alloys Compd. 2011, 509, 9093–9097. doi: 10.1016/j.jallcom.2011.06.053
- Massart, R. Preparation of Aqueous Magnetic Liquids in Alkaline and Acidic Media. IEEE Trans. Magn. 1981, 17, 1247–1248. doi: 10.1109/TMAG.1981.1061188
- Tao, K.; Dou, H.; Sun, K. Interfacial Coprecipitation to Prepare Magnetite Nanoparticles: Concentration and Temperature Dependence. Colloids Surf. A Physicochem. Eng. Asp. 2008, 320, 15–122. doi: 10.1016/j.colsurfa.2008.01.051
- Iwasaki, T.; Kosaka, K.; Yabuuchi, T.; Watano, S.; Yanagida, T.; Kawai, T. Novel Mechanochemical Process for Synthesis of Magnetite Nanoparticles Using Coprecipitation Method. Adv. Powder Technol. 2009, 20, 521–528. doi: 10.1016/j.apt.2009.06.002
- Valenzuela, R.; Fuentes, M.C.; Parra, C.; Baeza, J.; Duran, N.; Sharma, S.K.; Knobel, M.; Freer, J. Influence of Stirring Velocity on the Synthesis of Magnetite Nanoparticles (Fe3O4) by the Coprecipitation Method. J. Alloys Compd. 2009, 488, 227–231. doi: 10.1016/j.jallcom.2009.08.087
- Ahmadi, S.; Chia, C.-H.; Zakaria, S.; Saeedfar, K.; Asim, N. Synthesis of Fe3O4 Nanocrystals Using Hydrothermal Approach. J. Magn. Magn. Mater. 2012, 324, 4147–4150. doi: 10.1016/j.jmmm.2012.07.023
- Gao, G.H.; Shi, R.R.; Qin, W.Q.; Shi, Y.G.; Xu, G.F.; Qiu, G.Z.; Liu, X.H. Solvothermal Synthesis and Characterization of Size-Controlled Monodisperse Fe3O4 Nanopartilces. J. Mater. Sci. 2010, 45, 3483–3489. doi: 10.1007/s10853-010-4378-7
- Raja, K.; Verma, S.; Karmakar, S.; Kar, S.; Jerome Das, S.; Bartwal, K.S. Synthesis and Characterization of Magnetite Nanocrystals. Cryst. Res. Technol. 2011, 46, 497–500. doi: 10.1002/crat.201100105
- Itoh, H.; Sugimoto, T. Systematic Control of Size, Shape, Structure, and Magnetic Properties of Uniform Magnetite and Maghemite Particles. J. Colloid Interface Sci. 2003, 265, 283–295. doi: 10.1016/S0021-9797(03)00511-3
- Ang, B.C.; Iskandar, I.Y. Synthesis and Characterization of Magnetic Iron Oxide Nanoparticles via w/o Microemulsion and Massart’s Procedure. J. Mater. Process. Technol. 2007, 191, 235–237. doi: 10.1016/j.jmatprotec.2007.03.011
- Zhou, Z.H.; Wang, J.; Liu, X. Synthesis of Fe3O4 Nanoparticles from Emulsions.J. Mater. Chem. 2001, 11, 1704–1709. doi: 10.1039/b100758k
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic Iron Oxide Nanoparticles (SPIONs): Development, Surface Modification and Applications in Chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. doi: 10.1016/j.addr.2010.05.006
- Hong, R.Y.; Pan, T.T.; Li, H.Z. Microwave Synthesis of Magnetic Fe3O4 Nanoparticles Used as a precursor of Nanocomposites and Ferrofluids. J. Magn. Magn. Mater. 2006, 303, 60–68. doi: 10.1016/j.jmmm.2005.10.230
- Sahoo, Y.; Pizem, H.; Fried, T.; Golodnitsky, D.; Burstein, L.; Sukenik, C.N.; Markovich, G. Alkyl Phosphonate/Phosphate Coating on Magnetite Nanoparticles: A Comparison with Fatty Acids. Langmuir 2001, 17, 7907–7911. doi: 10.1021/la010703+
- Zaitsev, V.S.; Filimonov, D.S.; Presnyakov, I.A.; Gambino, R.J.; Chu, B. Physical and Chemical Properties of Magnetite and Magnetite-Polymer Nanoparticles and their Colloidal Dispersions. J. Colloid Interface Sci. 1999, 212, 49–57. doi: 10.1006/jcis.1998.5993
- Kang, Y.S.; Risbud, S.; Rabolt, J.F.; Stroeve, P. Synthesis and Characterization of Nanometer-Size Fe3O4 and γ-Fe2O3 Particles. Chem. Mater. 1996, 8, 2209–2211. doi: 10.1021/cm960157j
- Wurm, F.; Frey, H. Linear–dendritic Block Copolymers: The State of the art and Exciting Perspectives. Prog. Polym. Sci. 2011, 36, 1–52. doi: 10.1016/j.progpolymsci.2010.07.009
- Adeli, M.; Zarnegar, Z.; Dadkhah, A.; Hossieni, R.; Salimi, F.; Kanani, A. Linear-dendritic ABA Triblock Copolymers as Nanocarriers. J. Appl. Polym. Sci. 2007, 104, 267–272. doi: 10.1002/app.25583
- Namazi, H.; Adeli, M. Novel Linear–globular Thermoreversible Hydrogel ABA Type Copolymers from Dendritic Citric Acid as the A Blocks and Poly(Ethyleneglycol) as the B Block. Eur. Polym. J. 2003, 39, 1491–1500. doi: 10.1016/S0014-3057(02)00385-3
- Tavakoli Naeini, A.; Adeli, M.; Vossoughi, M. Poly(Citric Acid)-Block-Poly(Ethylene Glycol) Copolymers—New Biocompatible Hybrid Materials for Nanomedicine. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 556–562. doi: 10.1016/j.nano.2009.11.008
- Pardoe, H.; Chua-Anusorn, W.; St. Pierre, T.G.; Dobson, J. Structural and Magnetic Properties of Nanoscale Iron Oxide Particles Synthesized in the Presence of Dextran or Polyvinyl Alcohol. J. Magn. Magn. Mater. 2001, 225, 41–46. doi: 10.1016/S0304-8853(00)01226-9
- Mendenhall, G.D.; Geng, Y.; Hwang, J. Optimization of Long-Term Stability of Magnetic Fluids from Magnetite and Synthetic Polyelectrolytes. J. Colloid Interface Sci. 1996, 184, 519–526. doi: 10.1006/jcis.1996.0647
- Lee, J.; Isobe, T.; Senna, M. Preparation of Ultrafine Fe3O4 Particles by Precipitation in the Presence of PVA at High pH. J. Colloid Interface Sci. 1996, 177, 490–494. doi: 10.1006/jcis.1996.0062
- Ding, X.B.; Sun, Z.H.; Wan, G.X.; Jiang, Y.Y. Preparation of Thermosensitive Magnetic Particles by Dispersion Polymerization. React. Funct. Polym. 1998, 38, 11–15. doi: 10.1016/S1381-5148(97)00154-5