ABSTRACT
This paper presents a novel, green Knoevenagel procedure for the chemical transformation of benzaldehydes into their corresponding α,β-unsaturated acids. The essential part of this procedure is a solvent-free condensation which uses environmentally benign amines or ammonium salts as catalysts instead of pyridine and piperidine as used in the traditional Knoevenagel condensation. The condensation step is followed by a decarboxylation in the solid phase, resulting in high overall yields and purity. The influence of temperature and catalyst type on the yield of sinapinic acid was monitored for the reaction between syringaldehyde and malonic acid. After optimization of this reaction, its scope was explored for various types of benzaldehydes demonstrating a broad applicability of this procedure. The developed method provides good to an excellent conversion of various benzaldehydes and high selectivity to the respective α,β-unsaturated acids in an environmentally friendly and efficient way.
GRAPHICAL ABSTRACT
1. Introduction
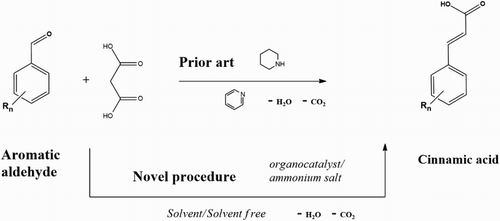
The Knoevenagel condensation is one of the most important methodologies for carbon–carbon double bond formation in synthetic organic chemistry and allows the production of various α,β-unsaturated acids, commonly referred to as cinnamic acids (Citation1). These cinnamic acids are key intermediates for the synthesis of natural and therapeutic drugs, polymer, cosmetic, and perfumes (Citation2–5). Generally, Knoevenagel reactions are carried out by the condensation of an active methylene compound with an aldehyde using large amounts of pyridine as a solvent and piperidine as an organocatalyst () (Citation6).
For a variety of reasons, the use of pyridine as a solvent is not desirable and should be significantly reduced or eliminated (Citation7, Citation8). Pyridine is toxic and represents a significant health risk above a vapor concentration of 3500 ppm (Citation9). The use of ionic liquids has been examined as an alternative because of their unique properties, e.g. good solubility, non-flammability, negligible vapor pressure, and recyclability (Citation10). However, their industrial application is limited due to their high cost (Citation11). Another possibility is to exclude the solvent completely from the reaction medium (Citation12). These so-called solvent-free conditions can lead to additional improvements such as the reduced amount of catalyst needed, increased reaction rate and higher yield (Citation13). It is believed that solvent-free organic transformations are industrially useful and fit well with the principles of Green Chemistry (Citation14, Citation15).
There are also various examples that limit the exposure to the catalyst piperidine by coupling piperidine to a solid support (Citation16) or by applying alternative organocatalysts such as dimethylamino pyridine and 1,2-diaminoethane (Citation17–19). Alternatively, Knoevenagel protocols have been developed that use Lewis acids such as LiOH, ZnCl2, InCl3, TiCl4, and NbCl5 (Citation20–22) and various heterogeneous solid bases as a catalyst (Citation23). However, the use of heavy metals for the synthesis of food and pharmaceutical products is not desirable because of their toxicity (Citation24).
The current procedure of the Knoevenagel reaction can be improved further, directed toward an environmentally friendly, efficient, and operationally simple condensation. Often, microwave reactions are used, but these also have their limitations on scalability, applicability, and energy use (Citation25–28). The present work, therefore, explores the possibilities to conduct the Knoevenagel reaction of syringaldehyde and malonic acid without pyridine and piperidine using a solvent-free condensation which employs environmentally benign amines and ammonium salts as catalysts (Citation29). After the reaction has been optimized for syringaldehyde, the scope of the method will be explored using various benzaldehydes with different electron-donating or electron-withdrawing groups, see .
2. Materials and methods
2.1 Materials and products
Syringaldehyde (1) and all other chemicals (99% purity) were purchased from Sigma-Aldrich and were used as received. DMSO-d6 was purchased from Cambridge Isotope with 99 atom% deuterated. For the spectral data of the products, see Supporting Information.
2.2 Methods
Instrumentation: 1H-NMR spectroscopy measurements were performed on an Agilent 400-MHz NMR system with DMSO-d6 as a solvent. Data were acquired using the VnmrJ3 software. Chemical shifts are reported in ppm, relative to tetramethylsilane (TMS).
HPLC analysis was carried out using a Liquid Chromatographic system (Agilent 1100 series) equipped with a diode array detector (Agilent 1200 series) and an autosampler injector with a 20-µL loop (Agilent 1100 series G1316A). The system was equipped with a Luna 5 µm C18 column (250 mm × 4.6 mm) using acetic acid:water:methanol (0.01:50:50, v/v/v) as the mobile phase. The flow rate was 1.0 mL/min at a temperature of 20°C. Agilent’s ChemStation Software was used for data analysis. The quantitative determination of components in the reaction mixtures was carried out by the external standard method and was based on the peak areas.
LC-MS analysis was performed using a Liquid Chromatographic system (Agilent 1200 series) equipped with a Luna 3 µm C18 column (250 × 2.0 mm) and an ion trap (Agilent 6300 series).
Melting points were determined with a Büchi Melting Point B-540 apparatus.
2.3 Synthetic procedure
Protocol for optimized Knoevenagel condensation: Malonic acid (1.0 g, 10 mmol) was dissolved in a minimum amount (e.g. 2.5 mL) of solvent (often ethyl acetate although ethanol and water were also used). Syringaldehyde (1a, 0.90 g, 5 mmol) and piperidine (0.20 mL, 2 mmol) were subsequently added. Then, the solvent (ethyl acetate) was removed by distillation under reduced pressure at 40°C.
The solid was kept for 2 h at 90°C for complete conversion. Samples were taken for reversed-phase HPLC analysis and diluted in 7.5 mL methanol, filtered, and analyzed using the method described in Section 2.2. The calculated percentage of the peak area of the component of interest is in relation to the total area of peaks.
In the workup to sinapinic acid, the residue was dissolved in 10 mL of a saturated aqueous NaHCO3 solution and subsequently acidified to a pH of 2 by using 6 M HCl. The resulting precipitate was separated by filtration and washed with (demineralized) water. After recrystallization in a mixture of water–ethanol (4:1, v/v), the crystals were separated by filtration, dried using a suction pump and dried at 60°C in a vacuum oven.
3. Results and discussion
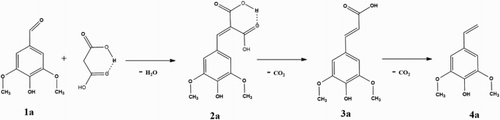
As stated before, the Knoevenagel–Doebner reaction is traditionally carried out using pyridine as a solvent and piperidine as an organocatalyst. In our initial efforts to develop an environmentally friendly procedure, we explored the possibility to perform a pyridine-free condensation between syringaldehyde (1a) and malonic acid using piperidine as a catalyst in refluxing water, ethanol, and ethyl acetate (). The concentration of syringaldehyde in solution at different times was monitored using HPLC analysis. It was observed that sinapinic dicarboxylic acid (2a) was initially formed to a limited extent, followed by a quick decarboxylation resulting in sinapinic acid (3a). As previously reported by our research group (Citation30), the formation of 4-vinylsyringol (4a) was avoided at this relatively low temperature. High conversion toward sinapinic acid (>97%) was obtained after 7 h ().
Figure 3. Conversion of syringaldehyde (1a) in time in solution and in the solid state. Reagents and conditions in solution: syringaldehyde, 2 eq. malonic acid and 0.4 eq. piperidine in ethyl acetate at 77°C. Reagents and conditions in solid state: above-mentioned mixture was concentrated in vacuo at 40°C; resulting solvent-free mixture was heated at 77°C. Reported conversions are based on the ratio of peaks of the HPLC chromatogram.
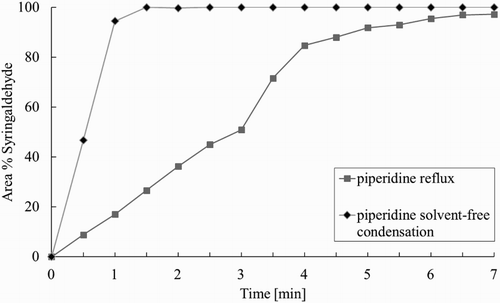
When sinapinic acid was subsequently isolated from the different HPLC samples by evaporation of the solvent much higher yields were obtained compared to the conversions from the HPLC measurements in solution. This observation gave rise to the idea that the evaporation of the solvent itself might promote the conversion of syringaldehyde toward sinapinic acid or that the reaction might proceed after the solvent has been removed.
In order to test this hypothesis, a new protocol was performed where syringaldehyde, malonic acid, and piperidine were dissolved in ethyl acetate and the mixture was immediately concentrated in vacuo at 40°C to obtain an effective mixing of the solids. After ethyl acetate had been removed, the resulting solvent-free mixture was placed at 77°C and the conversion of syringaldehyde was measured using HPLC (). Immediately after removal of the solvent (t = 0 h), the conversion of syringaldehyde was negligible. Once the solvent-free mixture was placed at 77°C, however, we observed a much faster conversion of syringaldehyde toward sinapinic acid than what was observed in solution. The step in which the reactants are mixed in solution preceding the solvent-free reaction can be skipped when working on a small scale. When working with larger badges, however, sufficient mixing in the solid state could not be accomplished, resulting in varying conversions and yields. Therefore, all subsequent reactions are first mixed properly in ethyl acetate prior to the solvent-free reaction step.
To investigate the influence of the temperature after the removal of the solvent, the temperature at which the solvent-free mixture was kept was varied between 40°C and 130°C (). Higher temperatures increased the conversion rate of syringaldehyde (1a). At temperatures below 50°C, a low syringaldehyde conversion resulted only in sinapinic dicarboxylic acid (2a) (see supplemental information). At 90°C, complete conversion of syringaldehyde is reached after 120 min with almost 100% selectivity toward sinapinic acid (3a). At higher temperatures, the yield of sinapinic acid decreases because of a second decarboxylation reaction resulting in 4-vinylsyringol (4a), which in turn is followed by the formation of its dimers and trimers (see Supporting Information).
Figure 4. Conversion of syringaldehyde (1a) and yield of sinapinic acid (3a) as a function of time at various reaction temperatures. Reagents and conditions: syringaldehyde, 2 eq. malonic acid and 0.4 eq. piperidine in ethyl acetate; concentrated in vacuo at 40°C; resulting solvent-free mixture was heated at specified temperatures. Reported conversions and yields are based on the ratio of peaks of the HPLC chromatogram.
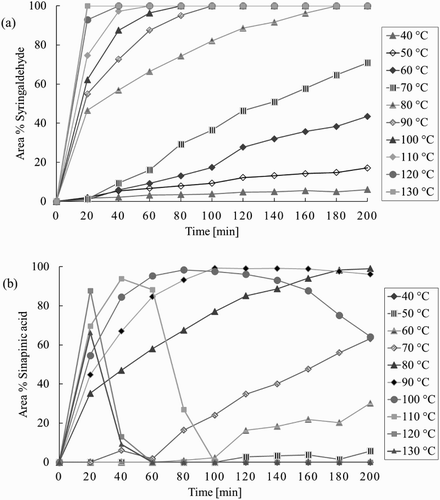
This simple and fast solvent-free procedure demonstrates that pyridine is no longer necessary as a solvent in this novel Knoevenagel condensation. Subsequently, we investigated the possibility to replace piperidine as the catalyst while using the optimized conditions from , i.e. 90°C and 2 h. Outright omission of piperidine resulted in a much lower reaction rate and yield of sinapinic acid () demonstrating the role of piperidine as the catalyst. Next, a variety of amines and ammonium salts were tested as an alternative catalyst.
Table 1. Conversion of syringaldehyde (1a) and yield of sinapinic acid (3a) with different catalysts.
The first replacements for piperidine which were tested were other aliphatic amines. No apparent differences were observed between primary (butyl amine), secondary (dibutylamine), and tertiary amines (triethyl amine). The diamine 1,2-diaminoethane gave very high conversion and yields of sinapinic acid, but is very toxic. However, it can successfully be replaced by the less toxic 2-amino-ethanol. The commonly used hydroxylamine TRIS (2-amino-2-hydroxy-methyl-propane-1,3-diol), on the other hand, performed relatively poor.
There are several claims in the literature (Citation1, Citation9, Citation18, Citation28) that pyridine is not only a solvent in the traditional Knoevenagel–Doebner condensation but also acts as a catalyst. In the case of the solvent-free procedure, this was confirmed. Besides pyridine, benzylamine was taken as an alternative aromatic amine, which gave better results compared to pyridine. Urea and glycine were also screened as benign alternatives to piperidine, but showed lower activity than the preceding amines.
Because these results suggested that the presence of nitrogen is the only essential prerequisite for the catalyst in the condensation reaction of the Knoevenagel reaction, several ammonium salts were screened. It was striking that these benign ammonium salts also gave high conversion and yields of sinapinic acid. We expect that the dissociation of the ammonium salts at higher temperature results in the formation of ammonia which serves as a nitrogen-based catalyst. Based on these observations, ammonium bicarbonate (NH4HCO3) was selected as the catalyst for further optimization of the reaction.
The traditional Knoevenagel–Doebner condensation in solution requires a large molar excess of malonic acid to obtain high yields. As a final step in the optimization of this new solvent-free procedure, a reduction of the amount of malonic acid used was attempted. Reducing the amount of malonic acid from 2.0 equivalents to 1.2 equivalents had no negative effect on the reaction rate, conversion, or yield. When the amount of malonic acid was reduced further to 1.0 equivalent, a drop in the reaction rate was observed. Therefore, the molar ratio of syringaldehyde and malonic acid was set at 1.2 for larger scale experiments and the application of the solvent-free condensation reaction on other benzaldehydes.
Solvent-free reactions can be hard to scale-up because of poor heat transfer and insufficient mixing. However, this novel solvent-free Knoevenagel condensation did not show a drop in performance on 20 g of syringaldehyde. Furthermore, we were able to obtain sinapinic acid with a yield of 80% after recrystallization.
To investigate the scope of the solvent-free Knoevenagel condensation, various derivatives of benzaldehydes were screened using the optimized reaction conditions of syringaldehyde (). Overall, high conversion of the aromatic aldehydes was achieved at 90°C after 2 h and there was no obvious difference between electron-withdrawing and electron-donating groups at the para-position of the benzaldehyde. In most cases, the corresponding cinnamic dicarboxylic acid (2) was the major product. The temperature of the reaction was therefore raised to 140°C, promoting the required decarboxylation leading to the respective cinnamic acids. For entries 1b–1i, this resulted in the formation of the proper cinnamic acids (3). For the entries with a hydroxyl group on the para-position (3a, 3j, and 3k), this resulted in a second decarboxylation leading to the corresponding styrene analogues (4) (and their dimers and trimers). The second decarboxylation (and polymerization) is probably accelerated by the formation of the transient quinone-like structures of 3a, 3j, and 3k and seems to depend on the number of methoxy groups on the aromatic ring (Citation31).
Table 2. Conversion of various benzaldehydes.
Furthermore, it was remarkable that 3l was already fully converted to the styrene variant at 90°C. The second decarboxylation, in this case, is probably accelerated by the formation of the transient iminium ion-like structure of 3l.
In summary, it was found that a wide array of benzaldehydes can be used in this solvent-free Knoevenagel condensation with ammonium bicarbonate, although differences in reactivity are observed. However, the selectivity of the reaction can be controlled by optimizing the reaction time and temperature. The benzaldehydes missing a hydroxyl or amine group on the para-position are less susceptible for a second decarboxylation, and are therefore very suitable to be converted by this procedure to their corresponding cinnamic acids. Investigations into the role of ammonium bicarbonate in these catalytic reactions are ongoing in our laboratory.
4. Conclusion
In conclusion, we have developed a greener version of the Knoevenagel condensation using benign amines or ammonium salts as the catalysts. The use of pyridine and piperidine is avoided and the required excess of malonic acid is limited. The developed method with ammonium bicarbonate as a catalyst provides good to an excellent conversion of various benzaldehydes and high yields of the corresponding cinnamic acids in an efficient, rapid, and environmentally friendly way.
Supplement_Material.pdf
Download PDF (3 MB)Acknowledgements
We further thank Edward Knaven, Avans University of Applied Science, for expert help with the LC/MS measurements and Sebastian van Schijndel for his help on the graphical abstract. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Jack van Schijndel studied chemistry at the State University of Utrecht with a major in the field of structural chemistry. He has been teaching Chemistry at the tertiary level for over 26 years and has supervised both undergraduate and postgraduate research projects in this field at the Avans University of Applied Sciences, Breda. His latest work focusses on the sustainable use of renewable resources for the polymer industry. The synthetic routes developed are furthermore designed along the lines of the Green Chemistry principles. He supervised as a senior scientist more than 50 projects with SME's in the field of biopolymers. His main areas interest are green chemistry, polymer synthesis but also teaching and learning innovations.
Luiz Alberto Canalle received the M.Sc. degree in chemistry from the Radboud University Nijmegen, The Netherlands, in 2006. He received his PhD. in bio-organic chemistry from the same university in 2010. Since 2012, he has been with Avans University of Applied Sciences, Breda, The Netherlands as a lecturer in organic chemistry and head researcher of the BioPolymer Research Group. His main areas interest are polymer synthesis; green chemistry and the research/teaching nexus.
Dennis Molendijk received the B.Sc. degree in chemistry at Avans University of Applied Sciences, Breda, The Netherlands, and is working under the supervision of Jack van Schijndel. He is involved in all ongoing projects in the research laboratory and also guides younger students with their research projects. His current research is focused on the green synthesis of biobased blocks of biopolymers and polymerization of these.
Jan Meuldijk obtained his M.Sc. degree in chemistry at the State University of Utrecht in 1975. His PhD. work was in the field of inorganic and theoretical chemistry at the Free University of Amsterdam, graduation in 1979. After post-doctoral fellowships in solid state physics and chemical engineering at the State University of Groningen, he was appointed assistant professor in the field of Industrial Catalysis at the Eindhoven University of Technology in 1985. In 1988, he became associate professor in chemical reactor engineering. His work in the Chemical Reaction Engineering Group is focused on process development of complex chemical products. In the department of Chemical Engineering and Chemistry there is a close cooperation with the Polymer Chemistry Group and the Macromolecular and Organic Chemistry Group. As of September 1, 2010 he is full professor in Polymer Reaction Engineering and Scientific Director of the Post M.Sc. Program “Process and Product Design”.
ORCID
Jack van Schijndel http://orcid.org/0000-0002-2806-7536
Additional information
Funding
References
- List, B. Emil Knoevenagel and the Roots of Aminocatalysis. Angew. Chem. (Int. ed. in English). 2010, 49, 1730–1734. doi: 10.1002/anie.200906900
- Cho, H.; Ueda, M.; Tamaoka, M.; Hamaguchi, M.; Aisaka, K.; Kiso, Y.; Inoue, T.; Ogino, R.; Tatsuoka, T.; Ishihara, T. Novel Caffeic Acid Derivatives: Extremely Potent Inhibitors of 12-Lipoxygenase. J. Med. Chem. 1991, 34, 1503–1505. doi: 10.1021/jm00108a039
- Kwak, G.; Fujiki, M. Colored and Luminous Aliphatic Polyester via One-Pot Intra- and Intermolecular Knoevenagel Reactions. Macromolecules . 2004, 37, 2021–2025. doi: 10.1021/ma035679g
- Nokami, J.; Kataoka, K.; Shiraishi, K.; Osafune, M.; Hussain, I.; Sumida, S. Convenient Formation of 4-Hydroxyalk-2-en-1-One Functionality Via A Knoevenagel-Type Carbon Chain Elongation Reaction of Aldehyde With 1-Arylsulfinylalkan-2-One. J. Org. Chem. 2001, 66, 1228–1232. doi: 10.1021/jo001323g
- De, P.; Koumba Yoya, G.; Constant, P.; Bedos-Belval, F.; Duran, H.; Saffon, N.; Daffé, M.; Baltas, M. Design, Synthesis, and Biological Evaluation of New Cinnamic Derivatives as Antituberculosis Agents. J. Med. Chem. 2011, 54, 1449–1461. doi: 10.1021/jm101510d
- Knoevenagel, E. Ueber eine Darstellungsweise der Glutarsäure. Berichte der deutschen chemischen Gesellschaft 1894, 27, 2345–2346. doi: 10.1002/cber.189402702229
- Alfonsi, K.; Colberg, J.; Dunn, P.J.; Fevig, T.; Jennings, S.; Johnson, T.A.; Kleine, H.P.; Knight, C.; Nagy, M.A.; Perry, D.A.; et al . Green Chemistry Tools to Influence a Medicinal Chemistry and Research Chemistry Based Organisation. Green Chem. 2008, 10, 31–36. doi: 10.1039/B711717E
- Kerton, F.; Marriott, R.; Clark, J.H.; Seidl, P.; Clark, J.H. Alternative Solvents for Green Chemistry; Green Chemistry Series;Royal Society of Chemistry: Cambridge, 2013.
- Pawar, H.S.; Wagh, A.S.; Lali, A.M. Triethylamine: A Potential N-Base Surrogate for Pyridine in Knoevenagel Condensation of Aromatic Aldehydes and Malonic Acid. New J. Chem. 2016, 40, 4962–4968. doi: 10.1039/C5NJ03125G
- Ranu, B.C.; Jana, R. Ionic Liquid as Catalyst and Reaction Medium – A Simple, Efficient and Green Procedure for Knoevenagel Condensation of Aliphatic and Aromatic Carbonyl Compounds Using a Task-Specific Basic Ionic Liquid. Eur. J. Org. Chem. 2006, 2006, 3767–3770. doi: 10.1002/ejoc.200600335
- Hangarge, R.V.; Jarikote, D.V.; Shingare, M.S. Knoevenagel Condensation Reactions in an Ionic Liquid. Green Chem. 2002, 4, 266–268. doi: 10.1039/b111634g
- Kaupp, G.; Reza Naimi-Jamal, M.; Schmeyers, J. Solvent-Free Knoevenagel Condensations and Michael Additions in the Solid State and in the Melt with Quantitative Yield. Tetrahedron. 2003, 59, 3753–3760. doi: 10.1016/S0040-4020(03)00554-4
- Pinxterhuis, E.B.; Giannerini, M.; Hornillos, V.; Feringa, B.L. Fast, Greener and Scalable Direct Coupling of Organolithium Compounds with No Additional Solvents. Nat. Commun. 2016, 7, 11698. doi: 10.1038/ncomms11698
- Martins, M.A.P.; Frizzo, C.P.; Moreira, D.N.; Buriol, L.; Machado, P. Solvent-Free Heterocyclic Synthesis. Chem. Rev. 2009, 109, 4140–4182. doi: 10.1021/cr9001098
- Jawor, M.L.; Ahmed, B.M.; Mezei, G. Solvent- and Catalyst-Free, Quantitative Protection of Hydroxyl, Thiol, Carboxylic Acid, Amide and Heterocyclic Amino Functional Groups. Green Chem. 2016, 18, 6209–6214. doi: 10.1039/C6GC02562E
- Lu, J.; Toy, P. Organocatalytic Decarboxylative Doebner-Knoevenagel Reactions Between Arylaldehydes and Monoethyl Malonate Mediated by a Bifunctional Polymeric Catalyst. Synlett. 2011, 2011, 1723–1726. doi: 10.1055/s-0030-1260808
- Kasinathan, P.; Seo, Y.; Shim, K.; Hwang, Y.; Lee, U.; Hwang, D.; Hong, D.; Halligudi, S.B.; Chang, J. Effect of Diamine in Amine-Functionalized MIL-101 for Knoevenagel Condensation. Bull. Korean Chem. Soc. 2011, 32, 2073–2075. doi: 10.5012/bkcs.2011.32.6.2073
- Gu, X.; Tang, Y.; Zhang, X.; Luo, Z.; Lu, H. Organocatalytic Knoevenagel Condensation by Chiral C2-Symmetric Tertiary Diamines. New J. Chem. 2016, 40, 6580–6583. doi: 10.1039/C6NJ00613B
- Hoskins, T.J.C. Carbon-Carbon Bond Forming Reactions of Biomass Derived Aldehydes, Georgia Institute of Technology: Atlanta, 2008.
- Pasha, M.A.; Manjula, K. Lithium Hydroxide: A Simple and an Efficient Catalyst for Knoevenagel Condensation Under Solvent-Free Grindstone Method. J. Saudi Chem. Soc. 2011, 15, 283–286. doi: 10.1016/j.jscs.2010.10.010
- Leelavathi, P.; Kumar, SR. Niobium (V) Chloride Catalyzed Knoevenagel Condensation: An Efficient Protocol for the Preparation of Electrophilic Alkenes. J. Mol. Catal. A Chem. 2005, 240, 99–102.
- Ogiwara, Y.; Takahashi, K.; Kitazawa, T.; Sakai, N. Indium(III)-Catalyzed Knoevenagel Condensation of Aldehydes and Activated Methylenes Using Acetic Anhydride as a Promoter. J. Org. Chem. 2015, 80, 3101–3110. doi: 10.1021/acs.joc.5b00011
- Jackson, T.; Clark, J.H.; Macquarrie, D.J.; Brophy, J.H. Base Catalysts Immobilised on Silica Coated Reactor Walls for Use in Continuous Flow Systems. Green Chem. 2004, 6, 193–195. doi: 10.1039/b315764b
- Nageswara Rao, R.; Kumar Talluri, M.V.N. An Overview of Recent Applications of Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) in Determination of Inorganic Impurities in Drugs and Pharmaceuticals. J. Pharmaceut. Biomed. 2007, 43, 1–13. doi: 10.1016/j.jpba.2006.07.004
- Gupta, M.; Wakhloo, B.P. Tetrabutylammoniumbromide Mediated Knoevenagel Condensation in Water: Synthesis of Cinnamic Acids. Arkivoc. 2007, 2007, 94.
- Bhuiyan, M.M.H.; Hossain, M.I.; Ashraful Alam, M.; Mahmud, M.M. Microwave Assisted Knoevenagel Condensation: Synthesis and Antimicrobial Activities of Some Arylidene-malononitriles, 02.
- Moseley, J.D.; Kappe, CO. A Critical Assessment of the Greenness and Energy Efficiency of Microwave-Assisted Organic Synthesis. Green Chem. 2011, 13, 794. doi: 10.1039/c0gc00823k
- Trotzki, R.; Hoffmann, M.M.; Ondruschka, B. Studies on the Solvent-Free and Waste-Free Knoevenagel Condensation. Green Chem. 2008, 1, 767–772. doi: 10.1039/b801661e
- Mase, N.; Horibe, T. Organocatalytic Knoevenagel Condensations by Means of Carbamic Acid Ammonium Salts. Org. Lett. 2013, 15, 1854–1857. doi: 10.1021/ol400462d
- van Schijndel, J.; Canalle, L.A.; Smid, J.; Meuldijk, J. Conversion of Syringaldehyde to Sinapinic Acid through Knoevenagel-Doebner Condensation. Open J. Phys. Chem. 2016, 6, 101–108. doi: 10.4236/ojpc.2016.64010
- Aldabalde, V. Organocatalyzed Decarboxylation of Naturally Occurring Cinnamic Acids: Potential Role in Flavoring Chemicals Production. Open J. Phys. Chem. 2011, 1, 85–93. doi: 10.4236/ojpc.2011.13012