ABSTRACT
An eco-friendly and efficient method was developed for the preparation of a new series of sulfur-containing quinolinthiones. Compounds 6a–o were synthesized from 4-methyl-2-thiocoumarin and arylhydrazides using water as a solvent under microwave irradiation. Some noteworthy features of our novel method are its cleanliness, short reaction time and high conversion rate, and the reaction proceeds (profiles) using a simple procedure. All of the prepared compounds were screened for their antibacterial efficacy in vitro using the disc diffusion method against bacterial strains. Compound (6j) showed the greatest potency with a 16 and 19 mm inhibition zone against Klebsiella pneumonia and Staphylococcus aureus, respectively.
GRAPHICAL ABSTRACT
1. Introduction
The Quinoline skeleton is a key structural element in a variety of bioactive natural compounds, pharmaceuticals and other functional materials ( Citation1). They are also one of the important groups of heterocyclic scaffolds as synthetic precursors ( Citation2). Only a few ways have been reported to access these structures ( Citation3).
Quinoline rings containing a thioxo group are of interest and biologically active owing to the potential chemical and physical properties of this heterocycle ( Citation4–6). Among these are quinoline-2-thiones are an important skeleton, which has been used as NS ligands ( Citation7), synthetic intermediates ( Citation8) and drug candidates ( Citation9). Despite these attributes, a few general protocols have been developed for the preparation of quinoline-2-thiones which show great biological efficiency ( Citation10). A typical synthesis of this group of compounds involves sulfurizing quinoline-2-ones using P2S5, also based on reacting 2-haloquinolines with thiourea or sodium sulfide, which requires tedious multistep transformations ( Citation11). Saito and Otani recently developed a new method of constructing the quinoline-2-thiones entity by the Friedel–Crafts alkenylation-cyclization of 2-alkynylphenyl isothiocyanates promoted by indium at a higher temperature ( Citation12). Recently, chromenes with a thioxo group increased antibacterial activity ( Citation13). In view of all these facts, two entities, namely, arylated hydrazides and 2-thiocoumarin, were combined in a single molecule to synthesize a series of new quinoline-2-thiones as antibacterial agents.
This was as a continuation of our previous research based on the synthesis of therapeutic and heterocyclic agents ( Citation14) and the development of more new eco-friendly synthesis and methods ( Citation15). Thus, we decided to optimize the synthesis of novel substituted quinoline-2-thiones derivatives and in vitro anti-bacterial (Scheme 1).
2. Results and discussion
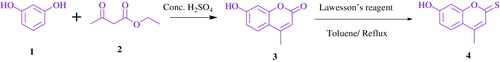
To synthesize the target molecules, the key molecule 7-hydroxy-4-methyl-2-thiocoumarin 4 was obtained by Pechmann condensation of ethyl acetoacetate 2 with resorcinol 1 in the presence of sulfuric acid ( Citation16). Then, the resulting compound was successfully transferred to the corresponding 2-thiocoumarin 4 using Lawesson’s reagent in toluene under reflux (Scheme 2) ( Citation17).
2.1. Optimizing the conditions for the preparation of the target molecule
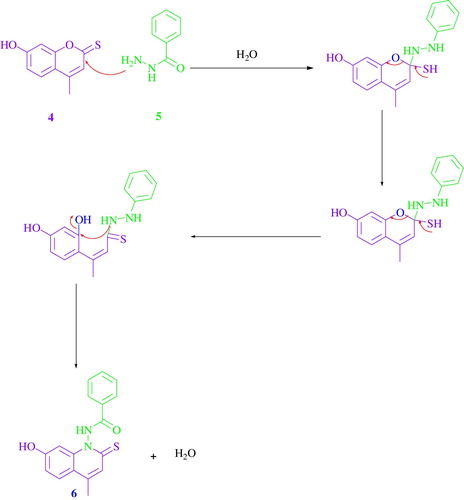
Initially, optimizing the reaction conditions started by coupling of 7-hydroxy-4-methylcoumarin 4 with 4-chloro-benzoic acid hydrazide 5a, which was chosen as the reaction model (). As indicated in , the effects of several solvents were studied (entries 1–7). Low yields were obtained using CH3CN or toluene as a solvent (entry 1 and 2). Using MeOH or dioxane led to an increase of the yields with 44% and 48%, respectively (entries 3 and 4). Moreover, using either DMF, THF or EtOH gave the desired compound with good yields. Interestingly, we noted that a high yield (92%) was obtained in just 20 min when water was used as the most efficient solvent under microwave irradiation (entry 9). In addition, irradiating the reaction under microwaves gave a better result than the classical reflux (entries 8, 9).
Table 1. Optimizing the reaction conditions.a
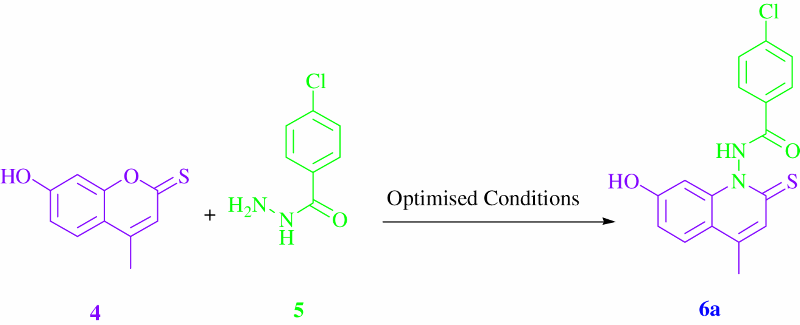
2.2. Typical procedure for preparing 4-chloro-N-(7-hydroxy-4-methyl-2-thioxo-2H-quinolin-1-yl)-benzamide 6a
A microwave glass vial (10 mL) was charged with an equimolar mixture of 7-hydroxy-4-methyl-2-thiocoumarin 4 (0.25 mmol) and 4-chloro-benzoic acid hydrazide 5a (0.25 mmol) along with 5 mL of water. The reaction was irradiated under microwaves for 20 min (160 W, 120°C). The reaction mixture was cooled to rt, then filtered to produce the crude compound. The compound was purified by recrystallization from water/ethanol (1/3) to produce 4-chloro-N-(7-hydroxy-4-methyl-2-thioxo-2H-quinolin-1-yl)-benzamide 6a.
2.3. Methodology extension
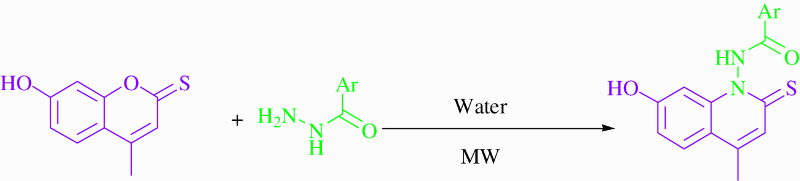
With the optimal conditions to hand, the scope of the reaction was developed using 7-hydroxy-4-methyl-2-thiocoumarin 4 (0.25 mmol) and a series of aryl hydrazides 5. The aryl hydrazides were readily synthesized according to the literature ( Citation18).
The presence of an electron donating group (CH3 or OCH3) on the phenyl ring decreased the aryl hydrazines’ reactivity toward 7-hydroxy-4-methyl-2-thiocoumarin (, entries 3–6, 11). On the other hand, the presence of an electron withdrawing a group like (F, Cl, NO2) increased the reactivity of the corresponding aryl hydrazides and led to the corresponding products having higher yields (entries 1, 2, 7,8, 9, 10, 12, 13). Using aryl hydrazide bearing a furyl or thienyl (, entries 14, 15) gave a lower yield compared with the other results. Using microwaves as an energy source increased the yields compared with the classical reflux (Scheme 3).
Table 2. Reactions of 7-hydroxy-4-methylcoumarin with various arylhydrazides.a
The in vitro antibacterial activities of compounds (6a– 6n) were performed against three bacterial strains, namely, Escherichia coli (Ec), Klebsiella pneumoniae (Kp) and Staphylococcus aureus (Sa); the results are summarized in . Of the 15 newly prepared compounds, only 6 compounds were found to be active against Kp or/and Sa at a concentration of 250 μg/ml. However, 7-hydroxy-4-methylquinoline-2-thiones (6a, 6i and 6j) carrying a 4-chlorophenyl substituent was found to be significantly active with an average inhibition zone of 11–19 and 12–16 mm against Kp and Sa, respectively, at a concentration of 250 μg/ml. In addition, 7-hydroxy-4-methylquinoline-2-thiones (6b and 6 l) with a 2-chlorophenyl substituent exhibited moderate activity with an average inhibition zone of 13–18 and 12–16 mm against Kp and Sa, respectively, at a concentration of 250 μg/ml. In contrast, compound 6 g with the 4-fluorophenyl group showed poor activity (9 mm average inhibition zone) specifically against a Gram-negative Sa bacterial strain. Surprisingly, all of the obtained products (6a– 6o) were found to be inactive against E. coli.
Table 3. The in vitro activity of 7-hydroxy-4-methylquinoline-2-thiones (6a–o) at a concentration of 250 μg/ml.a
In conclusion, we have developed a fast, simple, eco-friendly and efficient method for preparing substituted 7-hydroxy-4-methylquinoline-2-thiones, from 7-hydroxy-4-methyl-2-coumarin and aryl hydrazides in water as a non-toxic, eco-friendly and green benign solvent in mild conditions under microwave irradiation in good yields. The synthesized products were performed against three bacterial strains showing good activity except for the case of E.coli. This method is extremely advantageous and attractive and could contribute to reducing environmental problems.
Supplemental_Material
Download MS Word (20.3 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Mohammed H. Geesi received his Ph.D. in organic chemistry from the University of Southampton in the UK in 2014 under the supervision of Professor Richard C. Brown. Dr Geesi is currently a researcher and assistant professor at the College of Science and Humanities at Prince Sattam Bin Abdulaziz University. His research focuses on the synthesis of natural products, the stereocontrolled synthesis of organic compounds, the development and synthesis of new heterocyclic compounds using green, simple and low-cost processing methods.
Azzam Ahmed Mohammed Al-Hadedi received his Ph.D. in organic chemistry from the University of Southampton, UK. Dr Azzam is a lecturer and researcher at the Department of Chemistry at the College of Science at the University of Mosul in Iraq. His research focuses on the discovery and optimization of new chemical transformations, the synthesis of natural products, the stereocontrolled synthesis of organic compounds, the synthesis of heterocyclic compounds, NMR studies of organic compounds and the use of electrochemistry as an environmentally friendly method to drive chemical reactions.
Mohammed A. Bakht received his Ph.D. in pharmaceutical chemistry in 2009. He is currently working as an associate professor at the College of Science and Humanities at Prince Sattam Bin Abdulaziz University, KSA. His research interest is in the synthesis of potential organic scaffold utilizing new green chemistry technics and studying their pharmacological activities. He has few novel green solvents and catalysts to his name. He has also used ultrasound technology for the synthesis purposes.
Abdellah Kaiba received his Ph.D. in organic physical chemistry from the University of Bordeaux in France. Dr Abdellah is an Assistant Professor and researcher at the Department of Physics at the College of Science and Humanities Studies at Prince Sattam bin Abdulaziz University in Saudi Arabia. His research focuses on the development of new green catalysts.
Mohammed B. Alshammari was born in Saudi Arabia. He received his B.Sc. and M.Sc. degrees in Chemistry from King Saud University in Saudi Arabia. He received his Ph.D. degree from Cardiff University in the UK in 2013 under the supervision of Professor Keith Smith. His research focuses on the use of organolithium reagents as intermediates in organic synthesis. He is currently working as Assistant Professor of Organic Chemistry at Prince Sattam Bin Abdulaziz University in Saudi Arabia.
Meryem Boukili is preparing her Ph.D. in biology, specialty of food microbiology at Moulay Ismail University, Morocco. She is a Ph.D. student and researcher at the group of microbiology and health, laboratory of chemistry–biology applied to the environment. Her research topic concerns the descriptive and epidemiological study of foodborne diseases, and especially those caused by red meat in Morocco, as well as she is working on the hygienic quality of red meats and more specifically those of cattle, to fight against food poisoning, and to highlight the factors influencing its contamination at the city of Meknes in Morocco. In addition to that, she contributes in the isolation of Listeria monocytogenes (pathogenic microorganism that is responsible for the listeriosis infection) and study its genetic and resistive property.
Oussama Dehbi received his Ph.D. in organic chemistry from the University of Orleans in France in December 2012. Actually, he is an assistant professor and researcher in the College of Science and Arts, Al Qurayyat, Jouf University, KSA. His research focuses on the design and synthesis of new drug and studies of their structure–activity relationship and also the development of a new eco-friendly method for the synthesis of organic compounds.
Yassine Riadi received his Ph.D. in organic pharmaceutical chemistry from the University of Orleans in France in 2013. Dr Riadi is an assistant professor and researcher at the College of Pharmacy at Prince Sattam Bin Abdulaziz University. His research focuses on the development of new green catalysts using simple and low-cost processing methods and their use in the synthesis of new drugs and compounds.
Additional information
Funding
References
- (a) Alhaider, A.A.; Abdelkader, M.A.; Lien, E.J. J Med Chem 1985, 28, 1394. (b) Labaudiniere, R.; Hendel, W.; Terlain, B.; Cavy, F.; Marquis O. and Dereu, N. J. Med. Chem. 1992, 35, 4306; (c) Kumar, G.; Parasuraman, P.; Sharma, S.K.; Banerjee, T.; Karmodiya, K.; Surolia, N.; Surolia, A.J. Med. Chem. 2007, 50, 2665; (d) Cheng, P.; Zhang, Q.; Ma, Y.B.; Jiang, Z.Y.; Zhang, X.M.; Zhang, F.X.; Chen, J.J. Bioorg. Med. Chem. Lett. 2008, 18, 3787; (e) Miyashiro, J.; Woods, K.W.; Parkb, C.H.; Liu, X.; Shi, Y.; Johnson, E.F.; Bouska, J.J.; Olson, A.M.; Luo, Y.; Fry, E.H.; Girand, V.L. Bioorg. Med. Chem. Lett. 2009, 19, 4050. doi: 10.1021/jm00148a004
- (a) Hino, K.; Furukawa, K.; Nagai, Y.; Uno, H. Chem Pharm Bull 1980, 28, 2618. (b) Angibaud, P. R.; Venet, M.G.; Filliers, W.; Broeckx, R.; Ligny, Y.A.; Muller, P.; Poncelet, V.S.; End, D.W. Eur. J. Org. Chem. 2004, 479; (c) Zhao, P.; Yan, X.; Yin, H.; Xi, C. Org. Lett. 2014, 16, 1120. doi: 10.1248/cpb.28.2618
- (a) Zhang, X.; Han, X.; Chen, J.; Lu, X. Tetrahedron 2017, 73, 1541. (b) Vacala, T.; Bejcek, L.P.; Williams, C.G.; Williamson, A.C.; Vadola, P.A. J. Org. Chem. 2017, 82, 2558; (c) Zhang, Z.; Liao, L.-L.; Yan, S.-S.; Wang, L.; He, Y.-Q.; Ye, J.-H.; Li, J.; Zhi, Y.-G.; Yu, D.-G. Angew. Chem., Int. Ed. 2016, 55, 7068; (d) Iwai, T.; Fujihara, T.; Terao, J.; Tsuji, Y. J. Am. Chem. Soc., 2010, 132, 9602; (e) Chen, J.; Natte, K.; Spannenberg, A.; Neumann, H.; Beller, M.; Wu, X.-F. Chem. – Eur. J. 2014, 20, 14189; (f) Deng, Y.; Gong, W.; He, J.; Yu, J.-Q. Angew. Chem., Int. Ed. 2014, 53, 6692; (g) Li, X.; Li, X.; Jiao, N. J. Am. Chem. Soc. 2015, 137, 9246. doi: 10.1016/j.tet.2017.01.053
- Vengurlekar, S.; Sharma, R.; Trivedi, P. Lett. Drug Des. Discov. 2012, 9, 549–555. doi: 10.2174/157018012800389322
- Petrlíková, E.; Waisser, K.; Doležal, R.; Holý, P.; Gregor, J.; Kunes, J.; Kaustova, J. Chem. Papers 2011, 65, 352–366.
- Jayanthi, P.; Lalitha, P. Pharma Sci. Monitor 2011, 2, 210–215.
- Leeaphon, M.; Ondracek, A.L.; Thomas, R.J.; Fanwick, P.E.; Walton, R.A. J Am Chem Soc 1995, 117, 9715. doi: 10.1021/ja00143a015
- (a) Zhang, X.-Z.; Gan, K.-J.; Liu, X.-X.; Deng, Y.-H.; Wang, F.-X.; Yu, K.-Y.; Zhang, J.; Fan, C.-A. Org Lett 2017, 19, 3207. (b) Zhang, L.; Qureshi, Z.; Sonaglia, L.; Lautens, M. Angew. Chem., Int. Ed. 2014, 53, 13850. doi: 10.1021/acs.orglett.7b01331
- Joseph, B.; Darro, F.; Behard, A.; Lesur, B.; Collignon, F.; Decaestecker, C.; Frydman, A.; Guillaumet, G.; Kiss, R. J Med Chem 2002, 45, 2543. doi: 10.1021/jm010978m
- Nithyadevi, V.; Sampathkumar, N.; Rajendran, S.P. Asian J Chem 2004, 16, 1594.
- Ismail, M.M.; Abass, M.; Hassan, M.M. Molecules 2000, 5, 1224. doi: 10.3390/51201224
- Otani, T.; Kunimatsu, S.; Takahashi, T.; Nihei, H.; Saito, T. Tetrahedron Lett 2009, 50, 3853. doi: 10.1016/j.tetlet.2009.04.045
- Mathew, B.P.; Aggarwal, N.; Kumar, R.; Natha, M. J Sulfur Chem 2014, 35, 31–41. doi: 10.1080/17415993.2013.769543
- (a) Riadi, Y.; Geesi, M. Chem Pap 2018, 72, 697–701. (b) Riadi, Y.; Massip, S.; Leger, J. M.; Jarry, C.; Lazar, S.; Guillaumet, G. Tetrahedron 2012, 68, 5018. doi: 10.1007/s11696-017-0325-2
- (a) Riadi, Y.; Mamouni, R.; Azzalou, R.; El Haddad, M.; Routier, S.; Guillaumet, G.; Lazar, S. Tetrahedron Lett 2011, 52, 3492. (b) Riadi, Y.; Mamouni, R.; Routier, S.; Guillaumet, G.; Lazar, S. Environ. Chem. Lett. 2014, 12, 523. (c) Riadi, Y.; Geesi, M.; Dehbi, O.; Bakht, M.A.; Alshammari, M.; Viaud-Massuarde, M-C. Green Chem. Lett. Rev. 2017, 10, 101. (d) Dehbi, O.; Ishak, E.A.; Bakht, M.A.; Geesi, M.H.; Alshammari, M.B.; Chagnault, V.; Kaiba, A.; Lazar, S.; Altamimi, A.M.S.; Riadi, Y. Green Chem. Lett. Rev. 2018, 11, 62. doi: 10.1016/j.tetlet.2011.04.121
- (a) Negoro, N.; Sasaki, S.; Mikami, S.; Ito, M.; Suzuki, M.; Tsujihata, Y.; Ito, R.; Harada, A.; Takeuchi, K.; Suzuki, N.; Miyazaki, J.; Santou, T.; Odani, T.; Kanzaki, N.; Funami, M.; Tanaka, T.; Kogame, A.; Matsunaga, S.; Yasuma, T.; Momose, Y. ACS Med. Chem. Lett. 2010, 1, 290–294. (b) Raju, B.C.; Tiwari, A.K.; Kumar, J.A.; Ali, A.Z.; Agawane, S.B.; Saidachary, G.; Madhusudana, K. Bioorg. Med. Chem. 2010, 18, 358–365. doi: 10.1021/ml1000855
- Gadre, J.N.; Audi, A.A.; Karambelkar, N.P. Ind. J. Chem. Section B: Org. Chem. Med. Chem. 1996, 35B, 60–62.
- Abdel Hamid, H.M.; Ramadan, E.; Hagar, M.; El Ashry, E.S.H. Synth. Comm. 2004, 34, 377–382. doi: 10.1081/SCC-120027275