Abstract
In total, 378 Shaver White layers were allocated into 7 treatments with 6 replicates, from 30 to 42 wk of age, to assess the effects dietary organic (ethylenediamine dihydroiodide [EDDI]) versus inorganic (calcium iodate [CIOD]) iodine in laying hens. A basal diet served as control while the remaining six diets were supplemented with either CIOD (CIOD2, CIOD4, and CIOD8) or EDDI (EDDI2, EDDI4, and EDDI8) to provide 2.0, 4.0, and 8.0 mg of added iodine/kg of diet, respectively. Performance and egg quality were not affected by adding 2.0 or 4.0 mg/kg of iodine to the diets. However, a progressive decline in egg performance and feed intake occurred with EDDI8 and CIOD8 diets. The EDDI8 diet also increased abnormal eggs in parallel with decreasing the eggshell strength and Haugh unit and disturbing the serum and egg yolk lipids. This trend was connected with increase in triiodothyronine (T3) and thyroxine (T4), which led to oxidative stress in serum and egg contents. The iodine levels of eggshell and egg contents were increased by dietary iodine in a dose and time-dependent manner, while the effect of EDDI was higher than CIOD at all levels. To summary, supplementation of diets with 2 or 4 mg/kg of iodine as CIOD and specifically EDDI increased the iodine content of eggs without adverse effect on hen performance and egg quality traits. However, considering the time-dependent nature of this increase, a 12-wk period of supplementation might be not sufficient to achieve a specified level of iodine in the eggs.
Diet supplementation with 2, 4, and 8 mg/kg of iodine as CIOD and EDDI increased iodine content of eggs.
The highest level of iodine, especially as EDDI, diminished egg performance and egg quality connected with an increase in serum T3 and T4, which led to oxidative stress.
A 12-wk period of supplementation appears to be not sufficient to achieve a specified level of iodine in the egg.
Highlights
Introduction
Iodine as a component of thyroid hormones, triiodothyronine (T3) and thyroxine (T4), plays multiple functions as a regulator of cell metabolism and growth and as an essential factor for brain development (Li et al. Citation2012). Goitre, diminished growth, skeletal deformations, and mental retardation are the major symptoms of a deficient supply of iodine (Kotwal et al. Citation2007), whereas excess iodine intake reflects in elevated thyroid volume, thyroid autoimmunity, and thyroid dysfunction (Michalaki et al. Citation2018). Moreover, both deficiency and excess intake of iodine seem to increase the risk of thyroid cancer (Li and Wang Citation2004; Gärtner et al. Citation2010).
With the aim of increasing iodine levels in human diets, the application of iodised salt has been widespread. However, the effectiveness of this approach depends largely on the socioeconomic and cultural status of the considered population (Caffagni et al. Citation2011). Based on the World Health Organisation (WHO Citation2019), about 8.5% of the global population has a daily iodine intake below the required level, which is between 65 and 290 µg, depending upon age, gender, and other factors (e.g. pregnancy and lactation). Therefore, alternative iodine delivery strategies are needed to complement the salt iodination programme.
One strategy is to supplement the diet of laying hens with iodine and thus to produce an egg with a higher content of iodine (Kaufmann and Rambeck Citation1998; Laurberg Citation2004). Egg is one of the few foods that are used widely worldwide and the per capita egg consumption is expected to touch three-digit marks by 2024 (Omid et al. Citation2013; Conway Citation2015). Furthermore, the rate of increase in egg consumption is predicted to be higher in developing countries than in the developed world (Ren Citation2009). Seising this opportunity, several recent studies have investigated the transfer of iodine from feed into eggs of laying hens (Dobrzański et al. Citation2001; Yalçin et al. Citation2004; Opaliński et al. Citation2012; Röttger et al. Citation2012; Saki et al. Citation2012; Słupczyńska et al. Citation2014; Bakhshalinejad et al. Citation2018). However, the reported iodine concentration of eggs varies significantly due to the variation in age of hens, laying rate, and chemical methods used for iodine determination. For example, whereas Dobrzański et al. (Citation2001) reported that supplemental dietary iodine at a level of 2.68 mg/kg increased the iodine content of the whole egg (yolk plus albumen) to about 1.05 µg/g, Słupczyńska et al. (Citation2014) and Bakhshalinejad et al. (Citation2018) reported that such a level of egg iodine was achieved only when the supplementary level of iodine increased to 5 and 13 mg/kg of diet, respectively. Therefore, the available data provide no support for any universal recommendation of a particular level of dietary iodine. This issue is further complicated by the fact that the excess of ingested iodine can cause an excessive amount of iodine in the egg and lead to thyroid dysfunction in human consumers (Flachowsky et al. Citation2006). It is necessary to note that the ratio of the tolerable upper limit of iodine intake for adults to the recommended level is lower than 5:1, which means that iodine can be toxic at daily intake levels as low as 750 µg (WHO Citation2019). Moreover, some studies have reported that high dietary iodine is associated with adverse effects upon productive performance, egg quality, and blood cholesterol in laying hens (Lichovnikova et al. Citation2003; Lewis Citation2004; Yalçin et al. Citation2004; Flachowsky et al. Citation2006; Opaliński et al. Citation2012; Röttger et al. Citation2012; Saki et al. Citation2012). Rodent studies have suggested that the association of excessive dietary iodine and elevated plasma cholesterol and triglycerides levels is mediated by oxidative stress pathways (Venditti et al. Citation1997; Joanta et al. Citation2006; Zhao et al. Citation2011). However, confirmation of this association in poultry studies is still pending.
Difference in sources of iodine could also contribute to the heterogeneity of the study results. In poultry diets, iodine may be used in the form of inorganic salts such as potassium iodide (KI), potassium iodate (KIO3), and calcium iodate (Ca(IO3)2; CIOD), or organic salts like ethylenediamine dihydroiodide (EDDI) that are being mixed into the mineral mixtures (Miller and Ammerman Citation1995; European Commission Citation2016). Słupczyńska et al. (Citation2014) reported that KI was more effective than KIO3 in accumulation of iodine in the egg contents, whereas Röttger et al. (Citation2012) reported that calcium iodate and KI had the same effects on performance of laying hens and on deposition of iodine in the egg contents. However, since KI is very unstable and spoils quickly with moderate exposure to heat, light, and moisture, CIOD is preferred to KI (Leeson and Summers Citation2009). Ammerman and Miller (Citation1972) reported that the bioavailability of CIOD is approximately 95% in poultry. In a study with cows, Preston (Citation1994) found that iodine was absorbed equally well from KI and EDDI, while CIOD had a relative bioavailability of 88% when compared with the other two sources, and Herzig et al. (Citation2000) reported similar results in pigs. However, no scientific study has been published on the effect of EDDI in any type of poultry.
In view of these facts, the present study was carried out to evaluate the effects of various levels of CIOD and EDDI as supplemental iodine source in the diet of laying hens on productive performance, egg quality, serum and egg yolk lipids, antioxidant status, and iodine enrichment of eggs.
Materials and methods
Bird husbandry and experimental diets
A total of 378 Shaver White laying hens were used in this study. They were randomly allocated into 7 treatment groups of 54 hens split over 6 replicates of 9 birds each (3 adjacent cages containing 3 hens/40 × 50 × 40 cm). Hens were kept in cleaned and fumigated cages of wire floored batteries (Paravar Company, Sabzevar, Iran) in a closed system house. Each cage was equipped with a rectangular feeder outside the front wall, one nipple drinker on the back wall (36 cm above floor), and a manure collection pen underneath the wire-mesh floor. Feed and water were provided ad libitum and the room temperature was maintained at 21 + 2 °C. Daily lighting programme conditions were controlled to 16 h of light and 8 h of dark, as used in commercial operations (Kleyn Citation2013).
A basal diet was used as the control and the other six diets were supplemented with either calcium iodate (CIOD2, CIOD4, and CIOD8) or ethylenediamine dihydroiodide (EDDI2, EDDI4, and EDDI8) to provide 2.0, 4.0, and 8.0 mg of iodine/kg of diet. CIOD was supplied by Merck (Darmstadt, Germany), while EDDI came from Sigma-Aldrich (Steinheim, Germany). The experimental period started at 30 wk of age and lasted until 42 wk of age. From 28 to 30 wk of age, all hens were fed the control diet to allow them to adapt to their diet and environmental conditions as well as to obtain baseline data (wk 0). The control diet (Table ) was formulated (expected intake, 105 g/day) to meet or exceed the specifications for Shaver White commercial hen management guidelines (ISA Citation2011a). Chemical composition of the basal diet was determined in triplicate according to standard methods of AOAC (Citation1995) for dry matter (method 930.15), crude ash (method 942.05), crude protein (method 984.13), crude fibre (method 932.09), and ether extract (method 920.39). Total phosphorus content was analysed by the molybdovanadophosphoric acid procedure (method 965.17), and calcium concentration was analysed using atomic absorption spectroscopy (method 968.08). All experimental procedures were approved by the Animal Care Committee at the Islamic Azad University, Isfahan (Khorasgan) Branch.
Table 1. Ingredients and chemical composition of the basal diet.
Measurements, sampling, and analysis of the samples
Feed consumption was recorded at the end of each week of the experimental period. Daily egg production and egg weight were monitored during the trial. The egg production percentage was calculated as the total number of eggs produced divided by the number of birds in each replicate. The rate of production of abnormal eggs such as soft-shelled, cracked, and broken was also assessed daily. The egg mass was calculated by multiplying egg weight by daily egg production, while the feed to egg ratio was calculated by dividing the total feed consumed by total egg mass. Body weights of the birds were also recorded every week. Feed intake, egg production, egg weight, egg mass, feed to egg ratio, body weight changes, and abnormal egg percentage were calculated and analysed every 6 wk. Line graphs with weeks (wk 0–12) as X (abscissa axis) and performance data as Y (ordinate axis) were drawn.
Egg quality assessments were performed 1 wk before the start of the study and then every 3-wk during the treatment phase. All eggs laid in the last 3 d of each period were used for measurements. Weight of each egg sample and the albumen, yolk, and shell weights were measured with a sensitive weighing scale (Radwag PS.R1, Toruńska 5, 26-600 Radom, Poland) to the nearest 0.01 g. Relative yolk, albumen, and shell weights were expressed as the percentage of egg weight. Egg albumen quality (Haugh units) was evaluated by a P6085 spherometer (tripod micrometer) having an accuracy of 0.01 mm. The yolk index was determined as the ratio of yolk height to yolk width and yolk colour was compared to the Roche yolk colour fan, which ranges from a pale yellow at score 1 to a dark orange at score 15 (Vuilleumier Citation1969). Eggshell thickness was determined by a micrometer gauge (Ames, Waltham, MA, USA), while eggshell breaking strength was measured by using an eggshell force gauge (Robotmation Co. Ltd., Tokyo, Japan). The shells were ground to pass through a 20-mesh sieve for calcium and phosphorus analysis. The ground eggshell samples were ashed at 750 °C for 2 h and then cooled. The ashed samples were slightly moistened by water, and 10 mL 3 N HCL was added. The subsequent procedures were the same as the AOAC methods (AOAC Citation1995).
After measuring the egg quality, yolk samples from each replicate were separated from the broken eggs and extracted to determine yolk cholesterol and triglycerides. One gram of each egg yolk was homogenised with 19 mL of chloroform-methanol 2:1 (by volume), sonicated, and filtered as detailed elsewhere (Elkin and Rogler Citation1990). This solution was analysed to determine triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and total cholesterol using enzymatic colorimetric assay kits (Pars Azmun, Tehran, Iran) as per recommended protocols.
At the same week intervals, 5 ml of blood was drawn from the brachial vein of 18 birds per treatment (3 per replicate) into clean sterilised tubes. The serum was obtained by centrifugation of blood at 1500 ×g for 15 min at room temperature and stored at −20 °C until analysis. The serum was analysed for triglycerides, total cholesterol, and lipoprotein cholesterol fractions using the same kits described above for the egg yolk analysis. Serum T3 and T4 concentrations were analysed using radioimmunoassay kits (Byk-Sangtec Diagnostica, Dietzenbach, Germany). Intra-assay coefficients of variations were 5.4 and 4.0% for T3 and T4, respectively.
The serum and egg contents (yolk and albumen) were assessed for antioxidant status using the spectrophotometric methods. The egg contents were homogenised (1:10) in medium containing 120 mM potassium chloride and 30 mM phosphate buffer (pH 7.4) and were centrifuged (800 ×g, 10 min, −4 °C) and then the supernatant fractions and sera were measured for malondialdehyde (MDA) and protein carbonyl (PC) levels and activity of superoxide dismutase (SOD). The MDA levels were measured by the thiobarbital technique (Buege and Aust Citation1978), whereas the PC levels were determined by measurement of 2,4-dinitrophenylhydrazine derivatives of protein carbonyls (Parissis et al. Citation2009). The SOD activity was examined by the xanthine oxidase method (Sun et al. Citation1988), which monitors the inhibition of the reduction of nitroblue tetrazolium and the change of absorbance at 560 nm.
Iodine concentrations in feed and egg compartments (yolk, albumen, and eggshell) were assessed using inductively coupled plasma mass spectrometry (Perkin Elmer, Elan 6000, Toronto, Canada) after digestion with HNO3-HClO4 (Benkhedda et al. Citation2009).
Statistical analysis
Data analysis was performed by SAS 9.1 (SAS Citation2003). Assumptions of normal distribution (Shapiro-Wilk’s test) and homogeneity of variance (Bartlett’s test) were checked before analysis. Differences among the treatment groups over time were first tested using multivariate ANOVA (MANOVA) with repeated measures. The MANOVA revealed that either time effects or a time × treatment interaction or both existed in almost all measures, so data were then analysed using the GLM procedure for variance based on each time point of the measurements. Least squares means (LSmeans) were computed for the dietary treatments, and the SLICE and PDIFF options within the GLM procedure were used to accurately compare untreated controls to iodine-fed birds, iodine sources (CIOD and EDDI), iodine inclusion levels (2.0, 4.0, and 8.0 mg/kg), and the source × inclusion level interactions within each time period. This analysis was similarly conducted to assess the effect of time on each treatment separately. Differences between treatment and control were assessed by Dunnett’s test, whereas other differences were compared by Tukey’s test. Statistical significance level was set at p < .05. The pre-treatment values of each variable were used for covariate adjustment where appropriate. For performance characteristics, the mean of each pen was used as the experimental unit, whereas individual hen data were used for other variables, and the pen was considered a random effect.
Results
Iodine content in the diets
Feed iodine analyses indicated values slightly above the expected levels (Table ), which can be explained based on iodine present naturally in the feed matrix, as determined in the control diet. The results did not indicate significant differences between treatments of the same targeted iodine level for different iodine sources.
Table 2. Feed iodine source, level and analysis for the different dietsTable Footnote*.
Hen productivity
A summary of performance data is depicted in Figure , and the results of the statistical analysis of the data are given in Table . As Figure illustrates, all performance variables were similar at the beginning of the experiment. Egg production in the control group showed a slight increase up to 5 wk, but then decreased slowly during the rest of the treatment period (Figure ). No change was recorded in egg production when supplemental iodine amounts of 2.0 and 4.0 mg/kg were added to the diet as either CIOD (CIOD2 and CIOD4) or EDDI (EDDI2 and EDDI4). Hens receiving the CIOD8 diet also gave an egg production pattern similar to the control until 5 wk after treatment, but then showed a decrease below the control afterward, so that a marked reduction (about 4.5%) in egg production was observed in the second 6-wk of the study (p < .05). However, when the EDDI8 diet was offered to the birds, the egg production decreased more rapidly, starting at wk 2, and was consistently lower than the control by about 4 and 10% during the first and second 6-wk periods, respectively (p < .05). These differences were reflected in significant interactions between level and source of iodine at both the first and second 6-wk periods (p < .05). The production of abnormal eggs increased during the second 6-wk period (p < .05), but this increase was about twice as great in birds fed the EDDI8 diet as in those fed the control or other iodine-supplemented diets (p < .05).
Figure 1. Weekly performance as affected by dietary iodine source and level in laying hens (29–42 wk of age). CIOD: calcium iodate; EDDI: ethylenediamine dihydroiodide.
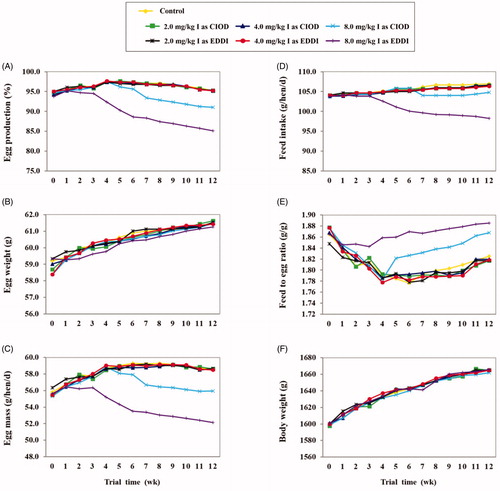
Table 3. Effects of dietary treatments on performance variables in laying hens (30–42 weeks of age).
Egg weight increased gradually over time (p < .05; Figure ), which was the same in all treatments. Thus, the egg mass followed the same trend as the egg production (Figure ). Feed intake increased as birds grow older (p < .05; Figure ), except when birds provided with the highest level of iodine. However, there was an interaction between iodine level and source on feed intake (p < .05). Hens receiving the EDDI8 diet showed an increasing decline in their consumption from wk 3 until the end of the study, with less than a 2% drop than the control in the first 6-wk as compared with a greater than 7% drop in the second 6-wk of the treatment (p < .05). In birds receiving the CIOD8 diet feed intake was relatively constant in the second 6-wk and remained less (2.3%) than those of the control (p < .05). Feed to egg ratio showed an opposite tendency to those observed for egg production and feed intake in different treatment groups (Figure ). Regardless of dietary iodine, the mean body weight showed an age‐related increase, but this increase was less pronounced in the second 6-wk period (p < .05; Figure ).
Egg quality
Data on external egg quality traits are shown in Table , while Table summarises the results of internal egg quality traits. The values of all measured variables were similar for all treatment groups at the beginning of the experiment. Eggshell thickness decreased parallel with the reduction of the shell as a percentage of egg weight as the birds advanced in age (p < .05), while percentage contents of calcium and phosphorus remained relatively constant, without any difference among the experimental treatments. Eggshell strength showed a steady state in all groups (p < .05), except in group receiving the EDDI8 diet, which revealed a decreasing trend in eggshell strength from the 6 wk (p < .05). This trend led to significant differences between the controls versus the EDDI8 treatment as well as significant differences between the EDDI8 and other iodine-supplemented treatments at the 9 and 12 wk stages (p < .05).
Table 4. Effects of dietary treatments on external egg quality characteristics in laying hens (29–42 wk of age).
Table 5. Effects of dietary treatments on internal egg quality characteristics in laying hens (29–42 wk of age).
Yolk weight percentage increased steadily, while albumen weight percentage dropped considerably as the hens aged (p < .05), whereas no change from the control levels occurred in the other groups. No statistically significant difference in the yolk index or yolk colour resulted from the addition of iodine to the diet. All treatment groups showed a declining trend in Haugh unit as the birds became older, but the most reduction occurred with the EDDI8 diet that reached a statistical significance than all other groups at the 12 wk stage (p < .05; EDDI8 versus control and iodine source × iodine level interaction).
Serum and egg yolk lipids
The serum and egg yolk lipid contents are listed in Tables and , respectively. The serum and egg yolk lipids (triglycerides, total, HDL, and LDL cholesterol) were not different at the beginning of the experiment. However, up to the 6 wk sampling, the increasing trend of each variable was greatly affected by its initial level (p < .05). Hens treated with the EDDI8 diet exhibited much more increase in the levels of triglycerides, total cholesterol, and LDL-cholesterol that accompanied by a much less increase in the level of HDL cholesterol, though the pattern of treatment differences indicated significance only at the 12 wk stage (p < .05; EDDI8 versus control and iodine source × iodine level interaction).
Table 6. Effects of dietary treatments on serum triglyceride and cholesterol levels (29–42 wk of age).
Table 7. Effects of dietary treatments on egg yolk triglyceride and cholesterol levels (29–42 wk of age).
T3 and T4 levels and antioxidant status
Table shows the serum concentrations of T3 and T4 and the results of antioxidant activity can be seen in Table . The values of these parameters were not different at the baseline. The serum T4 concentration increased with age (p < .05) and tended to increase with increasing iodine level from the 6 wk sampling date. However, when compared with the control, the observed increase was only statistically significant in groups receiving the CIOD8 and EDDI8 diets that showed, respectively, about 17 and 30% increase in T4 concentrations during this period (p < .05). This was confirmed by significant interactions between source and level of iodine (p < .05). The serum concentration of T3 showed a minor decrease at the 6 wk in all groups, but then recovered to its steady state, except in groups that provided with the CIOD8 and EDDI8 diets that had increased T3 concentration when compared with both the initial and the control values at the 9 and 12 wk stages (p < .05). These increases were about 12 and 71% and 24 and 108% in the former and latter groups, respectively, and reflected in an interaction between the source and level of iodine as well (p < .05). Both groups also represented a parallel increase in serum and egg SOD activities (p < .05), while an increase in MDA and PC contents could only be found in that receiving the EDDI8 diet (p < .05).
Table 8. Effects of dietary treatments on serum triiodothyronine (T3) and thyroxine (T4) concentrations (29–42 wk of age).
Table 9. Effects of dietary treatments on antioxidant measurements in serum and egg contents (29–42 wk of age).
Iodine accumulation in eggs
The concentrations of iodine in egg yolk, egg albumen, and eggshells are shown in Table . The initial iodine levels were not different at the beginning of the study. The control group revealed a slight (non-significant) decrease in iodine levels as progressed in age, while all egg compartments showed gradually increases in iodine contents as the level of iodine increased in the diet (p < .05). The effect of EDDI, however, was superior to that of CIOD at all tested levels, as indicated by significant source × level interactions during the treatment period (p < .05).
Table 10. Effects of dietary treatments on iodine contents in egg compartments (29–42 wk of age).
Discussion
The productivity of the control hens was acceptable for this age (ISA Citation2011b), and none of the production traits were influenced by dietary supplementation of 2 or 4 mg/kg of iodine, indicating that the iodine content in the basal diet (0.87 mg/kg) might be to meet the iodine requirement for laying hens. Röttger et al. (Citation2012) also reported that dietary iodine levels of 0.4–5.0 mg/kg (as CIOD or KI) had no significant effect on egg production, egg weight, and egg mass. This result is consistent with the recommendation given by the Gesellschaft für Ernährungsphysiologie (GfE 1999) that a 0.5 mg/kg of dietary iodine is adequate for maximal productivity in laying hens. In contrast, Damaziak et al. (Citation2018) found that the dietary addition of 10 mg/kg of iodine (as CIOD) caused beneficial effect on laying performance of hens in terms of egg production, egg weight, and feed conversion efficiency. This is while, in the present study, a progressive decline in egg production was noticed when the supplementation level of iodine increased to 8 mg/kg. Also, this reduction began earlier and proceeded to a greater extent with the EDDI8 diet than with the CIOD8 diet. A corresponding decrease in egg mass was also evident, because egg weight did not respond to dietary treatments. Such a difference is rather surprising taking into account the similarity of our findings to those of previously published reports. Lichovnikova et al. (Citation2003) reported an age-dependent decrease in egg production and egg mass when the iodine level of diet increased from 3.57 to 6.07 mg/kg by using a higher level of KI. Likewise, in his review of literature, Lewis (Citation2004) found that birds tend generally, but not always, to adjust their egg production rather than egg weight when excessive levels of dietary iodine are utilised. Arrington et al. (Citation1967) indicated that the reason for the suppressive effect of excess iodine on the laying rate is a progressive increase in the number of birds that stop laying, and not a lower rate of egg production for all birds, and further studies revealed that this effect is caused by an inhibition of ovulation as the prevention of follicular development (Marcilese et al. Citation1968; Perry et al. Citation1990). This decrease in egg production then results in a lower nutrient requirement, which reflects in decreasing feed intake and an inferior feed to egg ratio (Perry et al. Citation1989; Citation1990), as was also the case in this study. Since thyroid hormones can exert marked stimulatory and retardational effects on ovarian function (Dawson et al. Citation2001), and because of the major accumulation of iodine in the thyroid gland and its indispensable role in T3 and T4 synthesis, it is highly probable that the effects of excess iodine on egg production are mediated by changes in thyroid hormone release (Travnicek et al. Citation1999). Sechman (Citation2013) showed that T3 treatment resulted in lower concentrations of LH (luteinizing hormone) and sex-steroid hormone in blood circulation, which in turn, led to atresia of preovulatory follicles and stoppage of egg-laying. In support of this view is our observation that the serum concentrations of T3 and T4 increased by passing from the control to the CIOD8 and then the EDDI8 diet.
Moreover, during the second 6-wk period of the present study, an increase in the percentage of abnormal (soft-shelled, cracked, and broken) eggs occurred, which was about twice as high in birds fed the EDDI8 diet. This increase well corresponded with the decreasing of eggshell strength in the latter group. Also, in line with this result is the finding that irrespective of dietary iodine, the thickness and percentage of eggshell decreased as the birds aged. Interestingly, these changes occurred while the percentage of calcium and phosphorus remained constant in eggshells. Our findings are supported by those of Saki et al. (Citation2012), who found that eggshell weight and thickness did not respond to dietary inorganic iodine (as CIOD) in a range of 0.8–5.6 mg/kg of diet. However, using the same source of supplement, Bakhshalinejad et al. (Citation2018) reported that a level of 10.7 mg/kg of dietary iodine resulted in a reduction of eggshell strength that was independent of weight and thickness of the eggshell. Therefore, the detrimental effect of the EDDI8 diet on the incidence of abnormal eggs and eggshell strength seems likely to result from a higher level of bioavailable iodine. Nevertheless, this information does not specify how the excess iodine affects eggshell structure and strength. Previous studies (Christensen and Ort Citation1990; Christensen et al. Citation1991) have shown that dietary T4 elevates plasma T4 fivefold with no effect on functional characteristics of eggshell. Thus, the data suggest that the mechanism may not involve only the plasma concentrations of thyroid hormones.
The EDDI8 diet also exacerbated the age-dependent decrease of Haugh unit, while no changes in yolk index or yolk colour could be noticed according to dietary iodine or age. Besides, with increasing age, there tended to be an increase in the percentage of yolk weight and a decrease in the percentage of albumen weight, which was not altered by dietary iodine. In agreement with our results, Christensen and Ort (Citation1990), Bakhshalinejad et al. (Citation2018), and Damaziak et al. (Citation2018) reported that dietary inorganic iodine (2.1–12.9 mg/kg as CIOD or KI) had no significant effects on egg yolk and albumen percentages, yolk colour, or Haugh unit. However, Yalçin et al. (Citation2004) found a linear decrease in albumen index and Haugh unit when the level of dietary iodine increased to 24 mg/kg by using CIOD as the additive, and Lichovnikova et al. (Citation2003) reported that even a level of 6.1 mg/kg of iodine could have deleterious effect on Haugh unit in long‐term period of treatment (30 wk). Therefore, our results indicate that EDDI exerts its harmful impact on Haugh unit at a much lower level and in a shorter period than have been obtained for inorganic sources of iodine. The mechanism of these findings remain to be more precisely examined, but might be linked to the inducing effect of excess iodine on oxidative stress, which has been reported to correlate negatively with Haugh unit score (Seven Citation2008; Zhang et al. Citation2012; Liu et al. Citation2014; Ma et al. Citation2014; Wang et al. Citation2016).
As the study progressed, we recognised that the EDDI8 diet increased the serum and egg contents of MDA and PC, which are products of lipid and protein oxidation, respectively (Wang et al. Citation2018). The phenomenon in which excess iodine could exert oxidative stress in some target tissues of the thyroid hormones has been reported by Joanta et al. (Citation2006), and they found that Wistar rats supplemented with a high-iodine diet (1 μg/100 g body weight/daily) for 10 d showed a marked increase in MDA levels in the liver as compared with the control group. These increases are probably related to the increase of thyroid hormone production. Indeed, the synthesis of thyroid hormones crucially depends on H2O2, which works as a donor of oxidative equivalents for thyroperoxidase (Corvilain et al. Citation1991). Because of its high toxicity, H2O2 synthesis must always remain in adequation with the hormonal synthesis and strictly contained at the apical pole of the cell. Thyrocytes possess various enzymatic systems, such as SOD, glutathione peroxidase, catalase, and peroxiredoxins that contribute to limit cellular injuries when H2O2 or other reactive oxygen species are produced in excess (Kim et al. Citation2000; Mutaku et al. Citation2002; Poncin et al. Citation2008). These results are confirmed by our findings, which showed a parallel increase in serum and egg yolk SOD activities as the level of serum T3 and T4 increased in birds by feeding the CIOD8 and EDDI8 diets. However, in the latter group, this increase was not sufficient to prevent the oxidative damage. Along with this modification, this group of hens also exhibited a much more increase in serum and egg yolk levels of triglycerides, total cholesterol, and LDL cholesterol that accompanied by a much less increase in contents of HDL cholesterol as the study progressed. These findings support the results of previous studies which showed that T3 increased lipogenesis and lipogenic enzyme activities in relation or secondary to oxidative stress (Venditti et al. Citation1997; Rosebrough Citation1999; Rosebrough and McMurtry Citation2000). Rodent studies have confirmed that excessive iodine can induce similar effects (Zhao et al. Citation2011; Sarkar et al. Citation2018). Some studies reported that iodine supplementation had no significant effect on lipid indices such as triglycerides or cholesterol fractions (Rys et al. Citation1997; Yalçin et al. Citation2004; Saki et al. Citation2012; Słupczyńska et al. Citation2014). A regression of data from Perry et al. (Citation1989, Citation1990) showed that plasma cholesterol concentration increases by 0.3 mM/L for each 100 mg/kg increase in dietary iodine concentration (Lewis Citation2004). However, our data suggest that this estimation cannot be extended to organic sources of iodine.
In this study, the transfer of iodine into the egg contents and eggshells increased significantly as the level of iodine increased in the diet, which is consistent with the other studies (Dobrzański et al. Citation2001; Yalçin et al. Citation2004; Röttger et al. Citation2012; Saki et al. Citation2012; Słupczyńska et al. Citation2014; Bakhshalinejad et al. Citation2018). Moreover, in agreement with the literature (Opaliński et al. Citation2012), EDDI as the organic form of iodine was found to be more effective than CIOD in increasing the level of iodine in the egg compartments. However, whereas in most of the previous studies the iodine level of the eggs has been reported at the end of the treatment period (Dobrzański et al. Citation2001; Yalçin et al. Citation2004; Opaliński et al. Citation2012; Röttger et al. Citation2012; Saki et al. Citation2012; Bakhshalinejad et al. Citation2018), the most interesting feature revealed by the present study was that the iodine levels continued to increase over the time course of the experiment. This means that a 12-wk period of iodine supplementation in laying hens might not be sufficient to achieve a specified level of iodine in the eggs. These findings add to the complexities that are involved in the production of iodine-enriched eggs. Iodine content in eggs depends on many factors, including the source and level of iodine supplements (Marcilese et al. Citation1968; Perry et al. Citation1989; Röttger et al. Citation2012; Saki et al. Citation2012), the type of diet (Słupczyńska et al. Citation2014), genetic characteristics of birds (Knapp et al. Citation1998), bird age, laying rate, as well as the chemical methods used for the determination of iodine content in the biological matter (Gąsior and Szczypuła Citation2010). Determining the appropriate level of iodine from a given source, thus, still requires further investigation in more specifically designed studies. The presence of variable amounts of iodine in egg contents makes it difficult to control the dose of mineral that the consumer receives and represents a risk factor, especially in those countries, populations, and individuals that are already at risk of excessive iodine consumption. Moreover, considering the fact that eggshells are a potential substrate for producing food supplements (Opaliński et al. Citation2012), the variable iodine concentration in eggshells should also be taken into account.
Conclusions
The results of this study indicate that dietary supplementation of 2 or 4 mg/kg of iodine as CIOD or especially EDDI could be used to increase the iodine content of eggs without any adverse effect on performance, egg quality, and health of laying hens. However, due to the increasing accumulation of iodine in the eggs, further studies are necessary to evaluate the safety of egg fortification with iodine and to determine the optimal iodine intake of laying hens for the enrichment of their eggs.
Ethical approval
All experimental procedures were approved by the Animal Care Committee at the Islamic Azad University, Isfahan (Khorasgan) Branch.
Acknowledgments
We give special thanks to the laboratory personnel of the Department of Animal Science, Islamic Azad University, Isfahan (Khorasgan) Branch, for performing the chemical analyses.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ammerman C, Miller SM. 1972. Biological availability of minor mineral ions: a review. J Anim Sci. 35(3):681–694.
- AOAC 1995. Official methods of analysis of AOAC international. 16th ed. Arlington (VA): Association of Official Analytical Chemists.
- Arrington LR, Santa Cruz RA, Harms RH, Wilson HR. 1967. Effects of excess dietary iodine upon pullets and laying hens. J Nutr. 92(3):325–330.
- Bakhshalinejad R, Hassanabadi A, Nassiri‐Moghaddam H, Zarghi H. 2018. The effects of dietary calcium iodate on productive performance, egg quality and iodine accumulation in eggs of laying hens. J Anim Physiol Anim Nutr (Berl). 102(3):746–754.
- Benkhedda K, Robichaud A, Turcotte S, Béraldin FJ, Cockell KA. 2009. Determination of total iodine in food samples using inductively coupled plasma-mass spectrometry. J AOAC Int. 92(6):1720–1727.
- Buege JA, Aust SD. 1978. Microsomal lipid peroxidation. Meth Enzymol. 52:302–310.
- Caffagni A, Arru L, Meriggi P, Milc J, Perata P, Pecchioni N. 2011. Iodine fortification plant screening process and accumulation in tomato fruits and potato tubers. Commun Soil Sci Plan. 42(6):706–718.
- Christensen VL, Donaldson WE, Ort JF. 1991. The effect of dietary iodine on the hatchability of eggs from two commercial strains of turkeys. Poult Sci. 70(12):2529–2537.
- Christensen VL, Ort JF. 1990. Influence of diet-mediated maternal thyroid alterations on functional properties of turkey eggs. Poult Sci. 69(9):1576–1581.
- Conway A. 2015. Global egg consumption to rise worldwide through 2024 [Internet]. Rockford (IL, USA) WATT Global Media; [accessed 2018 Mar 22]. https://www.wattagnet.com.
- Corvilain B, Van SJ, Laurent E, Dumont JE. 1991. The H2O2-generating system modulates protein iodination and the activity of the pentose phosphate pathway in dog thyroid. Endocrinology. 128(2):779–785.
- Damaziak K, Marzec A, Riedel J, Szeliga J, Koczywas E, Cisneros F, Michalczuk M, Lukasiewicz M, Gozdowski D, Siennicka A, et al. 2018. Effect of dietary canthaxanthin and iodine on the production performance and egg quality of laying hens. Poult Sci. 97(11):4008–4019.
- Dawson A, King VM, Bentley GE, Ball GF. 2001. Photoperiodic control of seasonality in birds. J Biol Rhythms. 16(4):365–380.
- Dobrzański Z, Strzelbicka G, Szczypel J, Trziszka T. 2001. Study on enrichment of hen eggs with selenium and iodine. Electr J Polish Agric Univ. 4:e01.
- Elkin RG, Rogler JC. 1990. Reduction of the cholesterol content of eggs by the oral administration of lovastatin to laying hens. J Agric Food Chem. 38(8):1635–1641.
- European Commission 2016. Summary report of the standing committee on plants, animals, food and feed held in Brussels, on 12 November 2015–13 November 2015 (Section Animal Nutrition). Brussels (Belgium): Directorate-General for Health and Food Safety.
- Flachowsky G, Schöne F, Jahreis G. 2006. Zur jodanreicherung in lebensmitteln tierischer herkunft. Ernahrungs Umschau. 53:17–21.
- Gärtner R, Rank P, Ander B. 2010. The role of iodine and delta-iodolactone in growth and apoptosis of malignant thyroid epithelial cells and breast cancer cells . Hormones (Athens). 9(1):60–66.
- Gąsior R, Szczypuła M. 2010. Validation of a method for determination of iodine in food and biological material. Rocz Nauk Zoot. 37:63–73.
- Gesellschaft Für E. 1999. Empfehlungen zur Energie-und Nährstoffversorgung der Legehennen und Masthühner (Broiler). 1st ed. Frankfurt am Main (Germany): DLG Verlag.
- Herzig I, Písaríková B, Kursa J, Suchý P. 2000. Utilisation of iodine from different sources in pigs. Arch Anim Nutr. 53(2):179–189.
- ISA 2011a. Shaver white nutrition management guide [Internet]. Boxmeer (Netherlands), Hendrix genetics; [accessed 2018 Apr 14]. https://www.shaver-poultry.com/en/.
- ISA 2011b. Shaver product guide [Internet]. Boxmeer (Netherlands), Hendrix genetics; [accessed 2018 Apr 14]. https://www.shaver-poultry.com/en/.
- Joanta AE, Filip A, Clichici S, Andrei S, Daicoviciu D. 2006. Iodide excess exerts oxidative stress in some target tissues of the thyroid hormones. Acta Physiol Hung. 93(4):347–359.
- Kaufmann S, Rambeck WA. 1998. Iodine supplementation in chicken, pig and cow feed. J Anim Physiol Anim Nutr. 80(1–5):147–152.
- Kim H, Lee TH, Park ES, Suh JM, Park SJ, Chung HK, Kwon OY, Kim YK, Ro HK, Shong M. 2000. Role of peroxiredoxins in regulating intracellular hydrogen peroxide and hydrogen peroxide-induced apoptosis in thyroid cells. J Biol Chem. 275(24):18266–18270.
- Kleyn R. 2013. Chicken nutrition: a guide for nutritionists and poultry professionals. Leicestershire (UK): Context Products.
- Knapp G, Maichin B, Fecher P, Hasse S, Schramel P. 1998. Iodine determination in biological materials options for sample preparation and final determination. Fresen J Anal Chem. 362(6):508–513.
- Kotwal A, Priya R, Qadeer I. 2007. Goiter and other iodine deficiency disorders: a systematic review of epidemiological studies to deconstruct the complex web. Arch Med Res. 38(1):1–14.
- Laurberg P. 2004. Victories and challenges in optimizing iodine intake. Thyroid. 14(8):589.
- Leeson S, Summers JD. 2009. Commercial poultry nutrition. 3rd ed. Nottingham (UK) Nottingham University Press; p. 72–73.
- Lewis PD. 2004. Responses of domestic fowl to excess iodine: a review. Br J Nutr. 91(1):29–39.
- Li Q, Mair C, Schedle K, Hammerl S, Schodl K, Windisch W. 2012. Effect of iodine source and dose on growth and iodine content in tissue and plasma thyroid hormones in fattening pigs. Eur J Nutr. 51(6):685–691.
- Li Y, Wang D. 2004. Effect of iodine deficiency and excess on thyroid apoptosis in rats. Chin J Endemiol. 23:201–203.
- Lichovnikova M, Zeman L, Cermakova M. 2003. The long-term effects of using a higher amount of iodine supplement on the efficiency of laying hens. Br Poult Sci. 44(5):732–734.
- Liu HN, Liu Y, Hu LL, Suo YL, Zhang L, Jin F, Feng XA, Teng N, Li Y. 2014. Effects of dietary supplementation of quercetin on performance, egg quality, cecal microflora populations, and antioxidant status in laying hens. Poult Sci. 93(2):347–353.
- Ma X, Lin Y, Zhang H, Chen W, Wang S, Ruan D, Jiang Z. 2014. Heat stress impairs the nutritional metabolism and reduces the productivity of egg-laying ducks. Anim Reprod Sci. 145(3–4):182–190.
- Marcilese NA, Harms RH, Valsecchi RM, Arrington LR. 1968. Iodine uptake by ova of hens given excess iodine and effect upon ova development. J Nutr. 94(2):117–120.
- Michalaki MA, Mamali I, Tsekouras A, Vlassopoulou B, Anastasiou E, Koukkou EG, Vagenakis AG, Sakellaropoulos G, Georgopoulos NA, Rashitov M, et al. 2018. Thyroid-stimulating hormone is not the primary regulator of thyroid development in euthyroid children and adolescents living in an iodine-replete area. Hormones. 17(3):391–396.
- Miller ER, Ammerman CB. 1995. Iodine bioavailability. In: Ammerman CB, Baker DH, Lewis AJ, editors. Bioavailability of nutrients for animals. San Diego (CA): Academic Press; p. 157–167.
- Mutaku JF, Poma JF, Many MC, Denef JF, van Den Hove MF. 2002. Cell necrosis and apoptosis are differentially regulated during goitre development and iodine-induced involution. J Endocrinol. 172(2):375–386.
- Omid M, Soltani M, Dehrouyeh MH, Mohtasebi SS, Ahmadi H. 2013. An expert egg grading system based on machine vision and artificial intelligence techniques. J Food Eng. 118(1):70–77.
- Opaliński S, Dolińska B, Korczyński M, Chojnacka K, Dobrzański Z, Ryszka F. 2012. Effect of iodine-enriched yeast supplementation of diet on performance of laying hens, egg traits, and egg iodine content. Poult Sci. 91(7):1627–1632.
- Parissis JT, Kourea K, Andreadou I, Ikonomidis I, Markantonis S, Ioannidis K, Paraskevaidis I, Iliodromitis E, Filippatos G, Kremastinos DT. 2009. Effects of Darbepoetin Alfa on plasma mediators of oxidative and nitrosative stress in anemic patients with chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 103(8):1134–1138.
- Perry GC, Lewis PD, Hannagan MJ. 1989. Iodine supplementation from two sources and its effect on egg output. Br Poult Sci. 30:973–974.
- Perry GC, Lewis PD, Hannagan MJ. 1990. Responses of the laying hen to dietary iodine supplementation. In: Proceedings of the 8th European Poultry Conference; Jun 25–28; Barcelona (Spain); p. 384–387.
- Poncin S, Gérard AC, Boucquey M, Senou M, Calderon PB, Knoops B, Lengele B, Many MC, Colin IM. 2008. Oxidative stress in the thyroid gland: from harmlessness to hazard depending on the iodine content. Endocrinology. 149(1):424–433.
- Preston RC. 1994. Serum inorganic iodine dynamics in cattle following a single oral dose of several iodine sources. Faseb J. 8:A431.
- Ren Y. 2009. Oxidative stability of omega-3 polyunsaturated fatty acids enriched eggs [MS Thesis]. Alberta (Canada): University of Alberta.
- Rosebrough RW. 1999. Dietary fat and triiodothyronine (T3) interactions in the broiler chicken. Int J Vitam Nutr Res. 69(4):292–298.
- Rosebrough RW, McMurtry JP. 2000. Supplemental triiodothyronine, feeding regimens, and metabolic responses by the broiler chicken. Domest Anim Endocrinol. 19(1):15–24.
- Röttger AS, Halle I, Wagner H, Breves G, Dänicke S, Flachowsky G. 2012. The effects of iodine level and source on iodine carry-over in eggs and body tissues of laying hens. Arch Anim Nutr. 66(5):385–401.
- Rys R, Wir-Konas E, Pyska H, Kuchta M, Pietras M. 1997. Changes in egg iodine concentration in three hen strains in relation to iodine level in diets. Rocz Nauk Zoot. 24:229–242.
- Saki AA, Farisar MA, Aliarabi H, Zamani P, Abbasinezhad M. 2012. Iodine-enriched egg production in response to dietary iodine in laying hens. J Agric Technol. 8:1255–1267.
- Sarkar D, Chakraborty A, Saha A, Chandra AK. 2018. Iodine in excess in the alterations of carbohydrate and lipid metabolic pattern as well as histomorphometric changes in associated organs. J Basic Clin Physiol Pharmacol. 29(6):631–643.
- SAS 2003. SAS 9.1. Cary (NC): SAS Institute Inc.
- Sechman A. 2013. The role of thyroid hormones in regulation of chicken ovarian steroidogenesis. Gen Comp Endocrinol. 190:68–75.
- Seven PT. 2008. The effects of dietary Turkish propolis and vitamin C on performance, digestibility, egg production and egg quality in laying hens under different environmental temperatures. Asian Australas J Anim Sci. 21(8):1164–1170.
- Słupczyńska M, Jamroz D, Orda J, Wiliczkiewicz A. 2014. Effect of various sources and levels of iodine, as well as the kind of diet, on the performance of young laying hens, iodine accumulation in eggs, egg characteristics, and morphotic and biochemical indices in blood. Poult Sci. 93(10):2536–2547.
- Sun YI, Oberley LW, Li Y. 1988. A simple method for clinical assay of superoxide dismutase. Clin Chem. 34(3):497–500.
- Travnicek J, Kroupova V, Kratochvil P, Krabacova I. 1999. The effect of excessive iodine intake on the histology of the thyroid gland in layers. Vet Med (Praha). 6:177–182.
- Venditti P, Balestrieri M, Di Meo S, De Leo T. 1997. Effect of thyroid state on lipid peroxidation, antioxidant defences, and susceptibility to oxidative stress in rat tissues. J Endocrinol. 155(1):151–157.
- Vuilleumier JP. 1969. The “Roche yolk colour fan” – an instrument for measuring yolk colour. Poult Sci. 48(3):767–779.
- Wang JP, He KR, Ding XM, Luo YH, Bai SP, Zeng QF, Su ZW, Xuan Y, Zhang KY. 2016. Effect of dietary vanadium and vitamin C on egg quality and antioxidant status in laying hens. J Anim Physiol Anim Nutr. 100(3):440–447.
- Wang XC, Wang XH, Wang J, Wang H, Zhang HJ, Wu SG, Qi GH. 2018. Dietary tea polyphenol supplementation improved egg production performance, albumen quality, and magnum morphology of Hy-Line Brown hens during the late laying period. J Anim Sci. 96(1):225–235.
- WHO. 2019. Global Scorecard: 30 years of iodine status monitoring. IDD Newsletter. 48:5–8.
- Yalçin S, Kahraman Z, Yalçin S, Yalçin SS, Dedeoğlu HE. 2004. Effects of supplementary iodine on the performance and egg traits of laying hens. Br Poult Sci. 45(4):499–503.
- Zhang M, Zou XT, Li H, Dong XY, Zhao W. 2012. Effect of dietary γ‐aminobutyric acid on laying performance, egg quality, immune activity and endocrine hormone in heat‐stressed Roman hens. Anim Sci J. 83(2):141–147.
- Zhao SJ, Ye Y, Sun FJ, Tian EJ, Chen ZP. 2011. The impact of dietary iodine intake on lipid metabolism in mice. Biol Trace Elem Res. 142(3):581–588.
