Abstract
MicroRNA (miRNA) are single-stranded non-coding RNA of approximately 22 nucleotides estimated to regulate 60% of human and animal genes some of which are associated with cell differentiation, signal transduction and immune processes. Streptococcus agalactiae (S. agalactiae)-induced bovine mastitis is associated with up-regulation of miR-122 and down-regulation of erythropoietin (EPO) abundance in mammary gland tissue. TargetScan analysis revealed that EPO is a predicted target gene of miR-122 and this regulatory relationship was verified by dual-luciferase reporter assay. Overexpression of miR-122 in primary bovine mammary cells led to down-regulation of EPO and also the JAK-STAT signalling pathway genes EPOR, JAK2, STAT5A and STAT5B. Accordingly, the mRNA abundance of BCL-2, a downstream gene in the JAK-STAT signalling pathway was significantly down-regulated. Under conditions of miR-122 inhibition, EPO, EPOR, JAK2, STAT5A and STAT5B were up-regulated. These results demonstrated that, through its action on EPO, miR-122 may play an important role in S. agalactiae-induced mastitis by regulating the JAK-STAT signalling pathway.
Understanding the relationship among miR-122, EPO and the JAK-STAT pathway would be helpful in developing new strategies for controlling mastitis.
Highlights
Introduction
Mastitis is a common and serious disease in dairy cows resulting in enormous economic losses to the dairy industry worldwide (Thompson-Crispi et al. Citation2014; Reshi et al. Citation2015; Motaung et al. Citation2017). Over 137 pathogenic microorganisms induce intramammary infections (Watts Citation1988). However, this disease is mainly caused by three pathogenic microorganisms: Staphylococcus aureus (S. aureus), Streptococcus agalactiae (S. agalactiae) and Escherichia coli (E. coli) that invade the mammary gland and induce an inflammatory reaction (Thompson-Crispi et al. Citation2014; Ruegg Citation2017). S. agalactiae generally causes a chronic persistent infection and has a low rate of self-cure (Keefe Citation1997). Moreover, S. agalactiae deteriorated milk composition. In vitro study showed S. agalactiae bacterial degraded caseins, the most economically important proteins in milk, up to 75%, and degraded α-lactalbumin and β-lactoglobulin, the major whey proteins, up to 21% (Akerstedt et al. Citation2012). The immune response of the mammary gland is a very complex biological process that involves immune cells, mammary epithelial cells and endothelial cells. Therefore, to develop new strategies to control mastitis, it is essential to understand the pathophysiological process and the host immune response to mastitis in mammary gland tissue at a molecular level (Rinaldi et al. Citation2010).
MicroRNA (miRNA) are non-coding RNA of approximately 22 nucleotides. They regulate approximately 60% of human and animal genes expression during physiological and pathological processes, such as cell proliferation, signal transduction and immune responses and so on. A mature miRNA contains a seed sequence to pair the 3′ untranslated region (3′UTR) of its targeted mRNA and composes miRNA/mRNA duplex which may lead to mRNA degradation or suppress mRNA translation (Mollashahi et al. Citation2019). A number of studies have reported that miRNA may play roles in regulating inflammatory and immune responses (Bartel Citation2009). For example, miR-223, miR-146a and miR-155 were demonstrated to be associated with inflammation. These miRNA were up-regulated in bovine monocytes stimulated with lipopolysaccharide and S. aureus enterotoxin B (Dilda et al. Citation2012). MiR-16a, that targets IL6, IL8 and IL10, was down-regulated following an intramammary challenge with S. uberis in dairy cows (Naeem et al. Citation2012). In our previous research, up-regulation of miR-122 (Pu, Li, et al. Citation2017) in mammary tissue from cows challenged with S.. agalactiae was associated with down-regulation of EPO (Pu Citation2017, Supplemental Files 1 and 2). In mice, Rivkin has identified that EPO was a target gene of miR122 (Rivkin et al. Citation2016). According to the predicted result of the Target Scan database (http://www.targetscan.org), EPO was the predicted target gene of bta-miR-122. It is noteworthy that the seed region of miRNA is conserved among species and matches target gene sites. Therefore, bta-miR-122 may play a role in regulating EPO gene expression. Moreover, EPO was enrichment in the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway as we found in previous study (Pu Citation2017). It is also interesting that EPO has been reported as an inducer of signal transduction via JAK-STAT pathway (Ofir et al. Citation1997). Actually, JAK-STAT signalling pathway plays important role in mammary gland. It not only controls milk synthesis (Campo Verde Arbocco et al. Citation2017), but also participates in the regulation of the immune response (Villarino et al. Citation2017). Li reported that JAK/STAT pathway could be suppressed by 8-methoxypsoralen to protect bovine mammary epithelial cells against lipopolysaccharide-induced inflammatory injury (Li et al. Citation2019). Usman et al. indicated that mastitis in Chinese Holstein cows was associated with down-regulation of the JAK-STAT pathway genes JAK2, STAT5A and STAT5B (Usman et al. Citation2014). On the basis of the above facts and theoretical analysis, we hypothesised that the JAK-STAT pathway would be suppressed by miR-122 through down-regulating EPO.
The aim of this study was to identify whether miR-122 regulates the JAK-STAT signalling pathway by down-regulating EPO. Understanding the relationship among miR-122, EPO and the JAK-STAT pathway would provide more sights into the intervene of bovine mastitis in a molecular way.
Materials and methods
Ethics statement
This project was approved by the Animal Care and Use Committee from College of Animal Science and Technology, Yangzhou University, China. All experiments carried out according to the Regulations for the Administration of Affairs Concerning Experimental Animals published by the Ministry of Science and Technology, China in 2004.
Materials
The following is a list of materials used in this study: Dulbecco’s modified Eagle medium (DMEM)/F12 medium (A4192001, Gibaco, Invitrogen, Carlsbad, CA) , Opti-MEM™ (31985062, Gibaco, Invitrogen, Carlsbad, CA), Medium foetal bovine serum (FBS, 10091, Gibaco, Invitrogen), 0.25% trypsin-EDTA (25200056, Gibaco, Invitrogen), TRIzol reagent (15596026, Invitrogen), LipofectamineTM 2000 reagent (11668019, Invitrogen-Gibco, Carlsbad, CA); 6- and 96-well culture plates (3516,3599, Corning, NY), cell culture flasks (707003, Corning, NY), Dual-Luciferase Reporter Assay System (E2920, Promega, Madison, WI); miR-122 mimic (synthesis according to bta-miR-122 mature sequence), miR-122 inhibitor (the chemical modified mature bta-miR-122 complementary single chain) and miR-122 negative control (NC) (RiboBio Co., Ltd, Guangzhou, China); tryptone and yeast extract (LP0043B, LP0021B, OXIOD, UK); XhoI, NotI (ER0692, ER0595, ThermoFisher Scientific, Waltham, MA); pmiR-RB-ReportTM vecter plasmid (PMIR1001, RiboBio Co., Ltd, Guangzhou, China); TIANprep Mini Plasmid Kit and DH5α (DP105, CB101, Tiangen Biotech Bejing Co., Ltd, Bejing, China); MiR-X miRNA First-Strand Synthesis Kit, PrimeScript™ RT reagent Kit with gDNA Eraser, and TB Green® Premix Ex Taq™ II (Tli RNaseH Plus) (638313, RR047A and RR820A, Takara Biotech Co., Ltd, Beijing, China); Phusion DNA (F530L, ThermoFisher Scientific, Waltham, MA); and Ampicillin (A5354, Sigma, St. Louis, MO).
Generation of the wild-type and mutated vectors
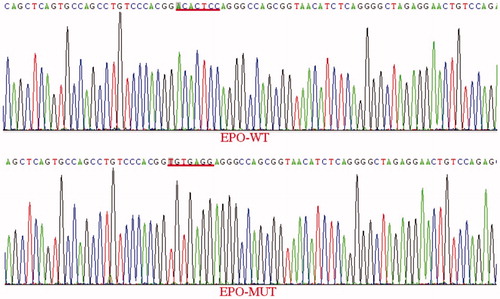
Total RNA was isolated from 270 d of the second lactation period mammary glands of one Chinese Holstein cow, using TRIzol reagent following the manufacturer’s instructions and reverse transcribed into cDNA. On the basis of the bovine EPO 3′UTR sequence (XM_015460412.1) from the NCBI database, full-length sequences were amplified by PCR to construct the wild-type vector. Primers were then designed to mutate ACACTCC into TGTGAGG and disrupt the binding target sites of bta-miR-122 on bovine EPO 3′UTR predicted by TargetScan (www.targetscan.org) (Table ). Primers are listed in Table , and the underlined sequences are the XhoI and NotI restriction enzyme sites. Following conventional protocols, including use XhoI and NotI to double – enzyme digest amplified DNA production and pmiR-RB-ReportTM vector plasmid, respectively; purify recovered enzyme cut products; link the DNA fragment (2 μL, about 150 ng) and the vector plasmid (0.5 μL, about 50 ng) together with junction solution (5 μL) and sterilised deionised water (2 μL), 16 °C for 30 min. The constructed wild-type vector and mutated vector were transformed into competent DH5α cells. Positive clones with wild-type vector were selected for sequencing, and a single positive clone with mutated vector was inoculated in liquid culture medium for 12 h and extracted plasmids (TIANprep Mini Plasmid Kit)for sequencing. The operation of extracting plasmids was processed according to the kit specification: first, take 1–4 mL overnight culture liquid into centrifuge tube for depositing bacteria at 12,000 rpm (∼13,400×g), 1 min; next, add Buffer P1, P2, P5, PWT in turn to crack the bacteria fully and purify plasmid; finally, add Buffer TB to collect plasmid. Primers were synthesised by Suzhou Jin Weizhi. Biotechnology Co., Ltd., Suzhou, China and cloning verification and plasmid sequencing were performed by Guangzhou Ribo Biotech Co., Ltd, Guangzhou, China.
Table 1. Predicted location of the miR-122 target site on the EPO 3′UTR.
Table 2. EPO 3′UTR PCR primer sequences.
Dual-luciferase reporter assay
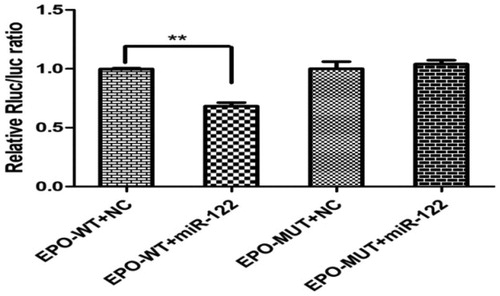
The EPO 3′UTR vector with the miR-122 mimic was transfected into 293 T cells, and the experiment performed with the following groups: miR-122 mimic NC+EPO wild-type vector (WT), miR-122 mimic+EPO WT, miR-122 mimic NC+EPO mutant vector (MUT), and miR-122 mimic+EPO MUT. Each group included three replicates. The 293 T cells were seeded in 96-well dishes at a density of 2 × 105 cells/well for 24 h. Prepared the premix of 9.5 μL Opti-MEMTM with 0.5 μL,10μM miR-122 NC or miR-122 mimic; 12.5 μL Opti-MEMTM with 2.5 μL, 10 μg EPO WT or EPO MUT and 24.75 μL Opti-MEMTM with 0.25 μL Lipo-fectamineTM 2000, respectively. After 5 min, mix softly the three premix solutions together and store for 20 min at room temperature, then remove 50 μL culture liquid from the wells and transfect the four group mixture solutions into 293 T cells, respectively. The final transfection content of miR-122 NC or miR-122 mimic was 50 nM/well and of EPO WT or EPO MUT was 250 ng/well. After 48 h, the firefly fluorescence signal and Renilla fluorescence signal were measured with a microplate reader. Renilla fluorescence was used as a normalisation control. The operation was performed following the manufacturer’s instructions of the Dual Luciferase Assay System.
MiR-122 mimic and inhibitor transfection into primary bovine mammary epithelial cells
Primary mammary epithelial cells were isolated from mammary gland tissues of the Chinese Holstein cow, in the mid-lactation and identified their morphology and function for transfection trial with approaches as previous described (Pu, Zhu, et al. Citation2017). The fifth passage mammary epithelial cells were seeded in 6-well plates 24 h before transfection to achieve 50–60% density at transfection. The 100 nM miR-122 mimic or inhibitor with 3.0 μL transfection reagent was transfected into primary mammary epithelial cells, according to the manufacturer’s instructions, repeated three times. After transfection for 48 h, the transcript abundance of miR-122, EPO and other genes in the JAK-STAT pathway were detected.
RNA extraction and reverse-transcription
Total RNA was isolated from epithelial cells using TRIzol reagent according to the manufacturer’s instructions. The average ratio (OD260/OD280) of total RNA purity of samples was 1.95 (range: 1.87–2.06) and two bands of 18 and 28 s of the RNA samples were demonstrated in the 1% agarose gel (Figure S1). miRNA was reverse-transcribed using the MiR-X™ miRNA First-Strand Synthesis Kit, and mRNA was reverse-transcribed into cDNA using PrimeScript™ RT reagent Kit with gDNA Eraser.
Analysis of real-time PCR data
The miR-122 upstream primer sequence (TGGAGTGTGACAATGGTGTTTG) was designed using the bta-miR-122 sequence in the miRBase database. The downstream primer was the universal primer U6 included in the One Step PrimeScript miRNA cDNA Synthesis Kit. The primer sequences for genes of interest are listed in Table . According to GenBank accession number of EPO, EPOR, JAK2, STAT5A, STAT5B and BCL-2, we designed these genes primers on line (https://www.ncbi.nlm.nih.gov/tools/primer-blast). All primers were synthesised by Shanghai Biotechnology, Co., Ltd. Eight reference genes (ACTB (β-actin), GAPDH, UBC, HPRT1, B2M, HMBS, YWHAZ, RPS23) were selected from the Affymetrix gene chip data and were calculated their stability values by NormFinder software (https://moma.dk/normfinder- software). The result showed that best gene was ACTB and its stability value was 0.065 (Supplemental File 3). According to this result, we used a single reference gene: β-actin as the internal reference gene to normalise the target genes expression level. Qualitative PCR-based analysis was performed according to the manufacturer’s instructions. Before analysing the RT-qPCR data, we used LinRegPCR software (https://www.medischebiologie.nl/files/?main=files&fileName=LinRegPCR.zip&description=LinRegPCR:%20qPCR%20data% 20analysis&sub = LinRegPCR) to calculate the amplification efficiency of reference gene and target genes. The results showed that the average PCR efficiency of β-actin, EPO, EPOR, JAK2, STAT5A, STAT5B and BCL-2 were 1.911, 1.922, 1.925, 1.869, 1.877, 1.913 and 1.898 (Supplemental File 4; Figure S2). According to these results, the target genes expression levels were calculated by using the (target gene amplification efficiency)−ΔΔCt method.
Table 3. Primers for amplification of β-actin, EPO, EPOR, JAK2, STAT5A, STAT5B and BCL-2.
Data analysis
A t-test was performed using the biostatistical software SPSS version16.0 (SPSS, Chicago, IL), and the results are expressed as the mean ± standard error (mean ± SE). GraphPad Prism version 5.0 (GraphPad Inc., La Jolla, CA) software was used for graphical presentation of the data.
Results
Identification of a positive clone and sequencing of the mutated vector
The EPO 3′UTR wild-type vector sequence is listed below with the underlined sequence corresponding to the EPO 3′UTR obtained from GenBank and the rest of the sequence corresponding to the vector. Figure depicts differences between EPO 3′UTR WT and MUT sequences. The sequence underlined with red was mutated from ACACTCC to TGTGAGG, and underscores that the EPO-3′UTR MUT vector was constructed successfully.
miR-122 targets the EPO 3′UTR
According to the bioinformatics analysis, a match for the seed region of miR-122 was identified in the 3′UTR of EPO mRNA. The dual-luciferase reporter assay results revealed that in 293 T cells co-transfected with bta-miR-122 and the reporter plasmid containing the WT 3′UTR of EPO, luciferase activity was markedly decreased by the miR-122 mimic to 68 ± 0.07% of the NC (Figure ). However, compared with the NC, luciferase activity did not change in cells co-transfected with the reporter plasmid containing the MUT 3′UTR of EPO. These results validated that miR-122 inhibits EPO expression through the miR-122 binding sequence in the 3’UTR of the gene.
Abundance of miR-122, EPO, EPOR, JAK2, STAT5A, STAT5B and BCL-2 after transfection of mammary gland epithelial cells with miR-122 mimics or inhibitor
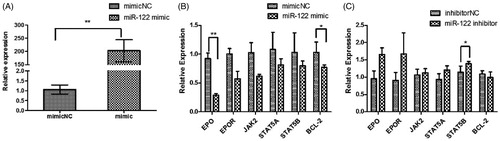
Compared with the NC, miR-122 abundance increased by more than 200-fold after the 48 h transfection with 100 nM miR-122 mimic (Figure ). This result indicated that miR-122 was successfully transfected into mammary cells. Furthermore, abundance of EPO, EPOR, JAK2, STAT5A, STAT5B and BCL-2 decreased (Figure ). However, abundance of EPO, EPOR, JAK2, STAT5A and STAT5B increased after transfection with miR-122 inhibitor (Figure ).
Figure 3. Abundance of miR-122 and genes after transfection with miR-122 mimic or inhibitor into mammary cells. (A: miR-122 abundance; B: EPO, EPOR, JAK2, STAT5A, STAT5B and BCL-2 abundance after transfection with miR-122 mimic into mammary epithelial cells; C: EPO, EPOR, JAK2, STAT5A, STAT5B and BCL-2 abundance after transfection with miR-122 inhibitor into mammary epithelial cells).
Note: EPO: erythropoietin; EPOR: erythropoietin receptor; JAK2: janus kinase 2; STAT5A: signal transducer and activator of transcription 5A; STAT5B: signal transducer and activator of transcription 5B; BCL-2: B-cell lymphoma-2.
Discussion
The JAK-STAT pathway, and specially STAT5 action, is central for milk synthesis by mammary cells (Reichenstein et al. Citation2011). Recent studies have also revealed that the JAK-STAT pathway is closely related to cellular immunity (Stabile et al. Citation2018; Li et al. Citation2019). When STAT5 was abnormally activated, it regulated the expression level of BCL-2 to change cell cycle progression and block apoptosis in several cancer cell lines (Xi et al. Citation2003; Li et al. Citation2005). Xiong et al. reported that STAT5 was depleted by a small interference RNA (siRNA), which induced CRC Cell Growth suppression and induced G1 Cell Cycle arrest in Colorectal cancer cells (Xiong et al. Citation2009).In addition, in the early stages of S. aureus sepsis in rats, JAK-STAT pathway activation was suppressed to reduce the inflammatory response in local tissues and to protect the function of the tissues (Yao et al. Citation2002).
Several studies have shown that the JAK-STAT pathway plays an important role in mastitis. For instance, STAT5A mRNA and protein abundance were down-regulated in mammary tissue during S. Aureus-induced mastitis (Yuan Citation2011). Usman et al. reported a decrease in abundance of JAK2, STAT5A and STAT5B in cows with mastitis relative to controls. Correlation analysis indicated that JAK2, STAT5A and STAT5B abundance was significantly correlated with serum cytokine concentration, suggesting it could serve as a candidate gene for resistance to mastitis (Usman et al. Citation2014). A comprehensive analysis of five sets of publicly available published microarray data with expression profiles from bovine mammary epithelial cells infected with E. coli revealed that significantly down-regulated pathways including JAK-STAT were mainly related to the immune system, infectious diseases and signal transduction (Zhao et al. Citation2016). It was proposed that the JAK-STAT pathway was down-regulated during the immune response process to help mitigate inflammation (Quintas-Cardama and Verstovsek Citation2013).
JAK-STAT pathway activation or inhibition can be regulated by miRNA or cytokines. Kong et al. discovered that miR-122 was over-expressed in human osteoma cells and suppressed the PI3K/AKT, JAK/STAT and Notch pathways by down-regulating the protein expression levels of p-PI3K, p-AKT, p-JAK1, p-STAT3, Jagged-1 and Notch1 (Kong and Wang Citation2018). MiR-9, secreted by tumour cells, promoted endothelial cell migration and angiogenesis by activating the JAK-STAT pathway (Zhuang et al. Citation2012). By demonstrating that over-expression of miR-122 in mammary gland epithelial cells reduced EPO abundance along with that of EPOR, JAK2, STAT5A and STAT5B, this study provides strong indication that the JAK-STAT pathway was inhibited. The EPO 3′UTR mRNA clearly is a target of miR-122.
Abundance of BCL-2, a downstream target of the JAK-STAT pathway and inhibitor of apoptosis, also decreased significantly. The inhibition of BCL-2 abundance increased cell apoptosis, which agreed with the pathological changes in mammary tissue during S. agalactiae-induced mastitis in which mammary cells were loosely connected, and intercellular gaps increased (Pu, Li, et al. Citation2017). The decline in BCL-2 expression was the host immune response to S. agalactiae invasion into mammary gland tissue. During the early stages of inflammation in the mammary gland, polymorphonuclear leukocytes (PMN) are attracted from blood into milk to exert a proinflammatory effect. These cells are considered one of the first lines of defence against pathogen invasion of the mammary gland. After activation of the inflammatory response, PMN clearance in the mammary gland is necessary for dampening the inflammatory response. However, when PMN apoptosis was delayed and PMN not cleared from the mammary gland, endotoxin release into epithelial cells increased and aggravated local damage of mammary tissue (Yao et al. Citation2010). During mastitis, PMN apoptosis plays an important role in the resolution of inflammation, and it might serve to control accumulation of PMN in mammary tissue (Boutet et al. Citation2004). As such, we speculate that a key role of miR-122 is to suppress BCL-2 abundance to accelerate PMN apoptosis, clearance and regression of the inflammatory response within mammary gland.
Limitations to the approach
Some housekeeping genes, such as β-actin, GAPDH, UBC, B2M and so on, are often used as reference gene. Different reference genes are suitable for different tissue samples or cells, which are related to the expression level and transcriptional stability of these reference genes in tissues or cells. In this article, we chose β-actin as the reference gene to normalise target genes expression levels for its stability value was 0.065. Verbeke et al. reported that among eight reference genes (B2M, H2A, HPRT1, SDHA, YWHAZ, UBC, RPS15A and ACTB), UBC, RPS15A and ACTB were the most stable genes for their low inter- and intra-group variation in expression, which demonstrated that their expression levels were not significantly affected during the experimental intramamary challenged with S. chromogenes (Verbeke et al. Citation2015). However, Vandesompele et al. put forward a normalisation strategy by choosing two or more internal reference genes to normalise target genes expression levels (Vandesompele et al. Citation2002). Based on the above evidence, there were two limitations in this article. The first was that we used the Affymetrix data instead of RT-qPCR data of eight chosen genes to calculate their stability value. The second was although β-actin was suitable for the normalisation of target gene expression level in this study, it may be more accurate to normalise the expression levels of target genes by choosing two or more internal reference genes.
Conclusions
In conclusion, miR-122 overexpression inhibited EPO expression and led to the suppression of the JAK-STAT pathway and its downstream genes in bovine mammary epithelial cells. This might represent a mechanism to regulate immune response of bovine mammary epithelial cells during S. agalactiae-induced mastitis.
Supplemental Material
Download MS Excel (47 KB)Supplemental Material
Download MS Excel (17 KB)Supplemental Material
Download MS Excel (90.5 KB)Supplemental Material
Download PDF (17.6 MB)Supplemental Material
Download MS Word (145.6 KB)Disclosure statement
Authors have declared that no competing interest exists.
Additional information
Funding
References
- Akerstedt M, Wredle E, Lam V, Johansson M. 2012. Protein degradation in bovine milk caused by Streptococcus agalactiae. J Dairy Res. 79(3):297–303.
- Bartel DP. 2009. MicroRNAs: target recognition and regulatory functions. Cell. 136(2):215–233.
- Boutet P, Boulanger D, Gillet L, Vanderplasschen A, Closset R, Bureau F, Lekeux P. 2004. Delayed neutrophil apoptosis in bovine subclinical mastitis. J Dairy Sci. 87(12):4104–4114.
- Campo Verde Arbocco F, Persia FA, Hapon MB, Jahn GA. 2017. Hypothyroidism decreases JAK/STAT signaling pathway in lactating rat mammary gland. Mol Cell Endocrinol. 450:14–23.
- Dilda F, Gioia G, Pisani L, Restelli L, Lecchi C, Albonico F, Bronzo V, Mortarino M, Ceciliani F. 2012. Escherichia coli lipopolysaccharides and Staphylococcus aureus enterotoxin B differentially modulate inflammatory microRNAs in bovine monocytes. Vet J. 192(3):514–516.
- Keefe GP. 1997. Streptococcus agalactiae mastitis: a review. Can Vet J. 38(7):429–437.
- Kong D, Wang Y. 2018. Knockdown of lncRNA HULC inhibits proliferation, migration, invasion, and promotes apoptosis by sponging miR-122 in osteosarcoma. J Cell Biochem. 119(1):1050–1061.
- Li H, Zhang Y, Glass A, Zellweger T, Gehan E, Bubendorf L, Gelmann EP, Nevalainen MT. 2005. Activation of signal transducer and activator of transcription-5 in prostate cancer predicts early recurrence. Clin Cancer Res. 11(16):5863–5868.
- Li J, Yin P, Gong P, Lv A, Zhang Z, Liu F. 2019. 8-Methoxypsoralen protects bovine mammary epithelial cells against lipopolysaccharide-induced inflammatory injury via suppressing JAK/STAT and NF-kappaB pathway. Microbiol Immunol. 63(10):427–437.
- Mollashahi B, Aghamaleki FS, Movafagh A. 2019. The roles of miRNAs in medulloblastoma: a systematic review. J Cancer Prev. 24(2):79–90.
- Motaung TE, Petrovski KR, Petzer IM, Thekisoe O, Tsilo TJ. 2017. Importance of bovine mastitis in Africa. Anim Health Res Rev. 18(1):58–69.
- Naeem A, Zhong K, Moisá SJ, Drackley JK, Moyes KM, Loor JJ. 2012. Bioinformatics analysis of microRNA and putative target genes in bovine mammary tissue infected with Streptococcus uberis. J Dairy Sci. 95(11):6397–6408.
- Ofir R, Qing W, Krup M, Weinstein Y. 1997. Identification of genes induced by interleukin-3 and erythropoietin via the Jak-Stat5 pathway using enhanced differential display-reverse southern. J Interferon Cytokine Res. 17(5):279–286.
- Pu JH, Li R, Zhang CL, Chen D, Liao XX, Zhu YH, Geng XH, Ji DJ, Mao YJ, Gong YC, et al. 2017. Expression profiles of miRNAs from bovine mammary glands in response to Streptococcus agalactiae-induced mastitis. J Dairy Res. 84(3):300–308.
- Pu JH. 2017. Expression profiles of genes and microRNAs from bovine mammary glands in response to streptococcus agalatiae-induced mastitis and regulation of miR-122 on EPO and JAK-STAT pathway [D]. Yangzhou, China: Yangzhou University; p. 39–54.
- Pu JH, Zhu XR, Xu X, Lu XB, Mao YJ, Yang ZP. 2017. Culture of dairy cow primary mammary epithelial cells and expression of β-casein mRNA. Chin Anim Husband Vet Med. 44(7):1975–1981.
- Quintas-Cardama A, Verstovsek S. 2013. Molecular pathways: jak/STAT pathway: mutations, inhibitors, and resistance. Clin Cancer Res. 19(8):1933–1940.
- Reichenstein M, Rauner G, Barash I. 2011. Conditional repression of STAT5 expression during lactation reveals its exclusive roles in mammary gland morphology, milk-protein gene expression, and neonate growth. Mol Reprod Dev. 78(8):585–596.
- Reshi AA, Husain I, Bhat SA, Rehman MU, Razak R, Bilal S, Mir MR. 2015. Bovine mastitis as an evolving disease and its impact on the dariy industry. Int J Cur Res Rev. 7(5):48–55.
- Rinaldi M, Li RW, Capuco AV. 2010. Mastitis associated transcriptomic disruptions in cattle. Vet Immunol Immunopathol. 138(4):267–279.
- Rivkin M, Simerzin A, Zorde-Khvalevsky E, Chai C, Yuval JB, Rosenberg N, Harari-Steinfeld R, Schneider R, Amir G, Condiotti R, et al. 2016. Inflammation-induced expression and secretion of microRNA 122 leads to reduced blood levels of kidney-derived erythropoietin and anemia. Gastroenterology. 151(5):999–1010 e1013.
- Ruegg PL. 2017. A 100-year review: mastitis detection, management, and prevention. J Dairy Sci. 100(12):10381–10397.
- Stabile H, Scarno G, Fionda C, Gismondi A, Santoni A, Gadina M, Sciume G. 2018. JAK/STAT signaling in regulation of innate lymphoid cells: the gods before the guardians. Immunol Rev. 286(1):148–159.
- Thompson-Crispi K, Atalla H, Miglior F, Mallard BA. 2014. Bovine mastitis: frontiers in immunogenetics. Front Immunol. 5:493.
- Usman T, Yu Y, Liu C, Wang X, Zhang Q, Wang Y. 2014. Genetic effects of single nucleotide polymorphisms in JAK2 and STAT5A genes on susceptibility of Chinese Holsteins to mastitis. Mol Biol Rep. 41(12):8293–8301.
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3(7):RESEARCH0034.
- Verbeke J, Van Poucke M, Peelman L, De Vliegher S. 2015. Differential expression of CXCR1 and commonly used reference genes in bovine milk somatic cells following experimental intramammary challenge. BMC Genet. 16(1):402.
- Villarino AV, Kanno Y, O’Shea JJ. 2017. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 18(4):374–384.
- Watts JL. 1988. Etiological agents of bovine mastitis. Vet Microbiol. 16(1):41–66.
- Xi S, Zhang Q, Gooding WE, Smithgall TE, Grandis JR. 2003. Constitutive activation of Stat5b contributes to carcinogenesis in vivo. Cancer Res. 63(20):6763–6771.
- Xiong H, Su WY, Liang QC, Zhang ZG, Chen HM, Du W, Chen YX, Fang JY. 2009. Inhibition of STAT5 induces G1 cell cycle arrest and reduces tumor cell invasion in human colorectal cancer cells. Lab Invest. 89(6):717–725.
- Yao S, Yao Y, Hongyun LI, Dong N, Yan YU. 2002. Effect of inhibiting janus kinase/signal transducer and activator of transcription pathway on acute liver injury in rats with postburn Staphylococcus aureus sepsis. Chin Critic Care Med. 14(6):336–339.
- Yao XP, Cao SZ, Yang DY, Liu ZP. 2010. Molecular regulation of bovine polymorphonuclear neutrophil leukocytes (PMN) apoptosis and immunity of mammary gland. Chin Anim Husband Vet Med. 37(1):120–122.
- Yuan ZR. 2011. Study on experimental mastitis induced by Staphylococcus aureus in dairy cattle [D]. Beijing, China: Chinese Academy of Agricultural Science.
- Zhao J, Chen ZL, Wang Z, Zhang Z, Xiao Q, Guan YY, Sun H, Liu GL, Zhang XZ, Pan YC. 2016. Study on the critical pathways of dairy cow mastitis based on microarray dataset and integration analysis. Chin Anim Husband Vet Med. 43(4):862–869.
- Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, Oeh J, Modrusan Z, Bais C, Sampath D, et al. 2012. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 31(17):3513–3523.