Abstract
Fresh sausages are not always thoroughly cooked before consumption and can support the growth of pathogenic microorganisms, especially when stored at incorrect temperatures. The aim of this study was to verify the growth of Listeria monocytogenes in Italian salsiccia stored at 2 °C, 7 °C and 12 °C for 9 days (t9) with (PC+) and without (PC–) a commercial protective culture (Lactobacillus sakei and Staphylococcus xylosus). L. monocytogenes PC + counts were statistically different from PC–, after 7 days (t7) at 7 °C and at 12 °C. At 2 °C, they increased in PC + by 0.03 and 0.36 log CFU/g vs. 0.25 and 0.91 in PC–, at t7 and t9. Moreover, quality characteristics (total aerobic counts, colour parameters, TBARS values, pH, sensory attributes) were assessed in fresh sausages stored at 7 °C. Significant differences were obtained in PC + samples at t7 for Pseudomonadaceae and Enterobacteriaceae (about 2 log CFU/g). Yeasts and moulds and Brochothrix thermosphacta were also significantly lower in PC + samples. PC + samples were more acidic than PC–, with statistically different colour parameters values particularly at the external surface; raw sausages resulted sensorially discernible at t7, whereas PC + and PC– cooked samples did not show any significant sensory difference. The studied protective culture proved to be a useful tool to increase safety and microbiological quality of salsiccia at abusive storage temperature, effectively limiting the growth of L. monocytogenes and Gram negative spoilage microorganisms, with some sensory drawbacks, especially at the end of the shelf life.
The tested culture improves the microbial safety of salsiccia.
It reduces the growth of spoilage microorganisms, especially Pseudomonadaceae and Enterobacteriaceae.
It mainly influences colour and acidity evolution of the raw product with no sensory drawbacks in the cooked product.
HIGHLIGHTS
Introduction
In Italy, pork sausages, labelled on the market as ‘salsiccia’, are a very popular food. The term ‘salsiccia’ refers to very different products, from fresh unripened sausages to dry fermented sausages (Consigliere et al. Citation2018). Fresh salsiccia is a little sausage (about 100 g), made by a ground mixture of pork lean meat and fat, with salt and variable spice mixes; it is stuffed in natural casings and commonly sold in strings, at refrigeration temperature. Fresh unripened sausages have an intrinsic perishable nature, due to several factors, such as high water and nutrient content of the ground matrix and absence of a lactic acid protective microflora. As a result, the shelf life of fresh sausages is usually short (7–10 days).
The habit of consuming fresh sausages without cooking or not thoroughly cooked, not uncommon in Italy as well as in other countries (Rossi et al. Citation2011), is a matter of concern for public health. In particular, the prevalence of Listeria monocytogenes in these products is not negligible (De Cesare et al. Citation2007). This can be related with their high pH and aw values and with the widespread diffusion of the pathogen in pork meat processing facilities (Meloni Citation2015). Listeriosis is an important food disease worldwide (Desai et al. Citation2019). A statistically significant increasing trend of confirmed listeriosis cases in the EU has been observed in 2009–2018 (EFSA Citation2019), and consumption of raw or undercooked fresh salsiccia can represent a vehicle for human listeriosis.
Protective cultures (PCs) are food-grade bacteria, mostly lactic acid bacteria (LAB), which represent an alternative method to food preservatives for the inhibition of undesired microorganisms, with the aim to increase the safety of food products and extend their storage (Lorenzo et al. Citation2014; Hammami et al. Citation2019). They are selected for their ability to actively grow in a product, inhibit pathogenic or spoilage microorganisms (Rodgers Citation2001) and change the sensory properties of products as little as possible (Lücke Citation2000).
Among LAB, Lactobacillus sakei has a technological use in the preservation and fermentation of various meat products mainly due to the production of organic acids, hydrogen peroxide and bacteriocins (Chaillou et al. Citation2005). L. sakei has been studied for its potential protective effect both on spoilage microorganisms, with the aim to extend the shelf life of meat products, and on pathogenic microorganisms (Zagorec and Champomier-Vergès Citation2017; Gelinski et al. Citation2019). Coagulase negative staphylococci are also commonly employed in meat products (Laranjo et al. Citation2017). Particularly, Staphylococcus xylosus is often used as a starter culture, but its use as a PC has also been studied (Laukovà et al. Citation2010).
The aim of this study was primarily to evaluate the protective effect of a commercial culture of L. sakei and S. xylosus, routinely applied by the sausages producer who supplied the tested samples, on the growth of L. monocytogenes in Italian fresh pork sausages (salsiccia) stored at 2 °C and at abusive temperatures (7 °C and 12 °C). The effect of the added culture on the quality characteristics of salsiccia stored at 7 °C was also evaluated.
Materials and methods
Fresh sausages preparation and sampling
Six batches of salsiccia were prepared in 6 different days in a Tuscan meat products plant following the same recipe. Each batch was split into two halves, one with the tested protective culture (PC+) and one without the protective culture (PC–). The following ingredients were used: pork lean and fat (from shoulder and belly, 70% and 30% w/w, respectively), marine salt, spices (black pepper, coriander, anise, cloves, allspice, minced garlic), natural flavours, dextrose, additives (ascorbic acid, sodium ascorbate, citric acid). The mixture was formed in approximately 100 g sausages, in natural pork casings (4 cm diameter). A commercial PC of L. sakei and S. xylosus (SafePro® B-FM, Ch. Hansen, Hoersholm, Denmark) was used at a dosage of 5 g for every 30 kg of sausage mixture in PC + batches. L. sakei strain was described by the supplier’s technical sheet as glucose, fructose and saccharose fermenting, lactose not fermenting, L+-lactic acid producing, and S. xylosus strain as catalase and nitrate reductase positive. No bacteriocin activity was declared. The sausages were packaged in polystyrene trays and overwrapped in polyvinylchloride film; analyses were carried out on freshly opened packages. Three PC+/PC– batches were used for L. monocytogenes growth tests: experimental and control sausages were stored at 2 °C and at abusive temperatures (7 °C and 12 °C) and analysed at 0, 3, 7 (shelf life assigned by the producer at 2 °C) and 9 days after production (t0, t3, t7, t9). On the remaining three PC+/PC– batches (two for sensory evaluation) stored at 7 °C, quality traits analyses (microbial evolution, physico-chemical and sensory parameters) were performed at the same sampling times both on PC + and PC–.
Microbiological analyses
For each considered batch, determinations were carried out in duplicate on two sausages.
Evaluation of Listeria monocytogenes growth
Three strains of L. monocytogenes (two wild strains, DSV-10 and DSV-60, isolated from fresh salsiccia and a meat product, respectively, and strain ATCC 7644) were used to inoculate PC + and PC– sausages. The strains, previously stored at −80 °C, were subcultured twice (Beaufort et al. Citation2014) in Brain Hearth Infusion (Oxoid, Basingstoke, UK). An inoculum, made of equal quantities of the three strains, was prepared in the same medium. Bacterial cells were then pelleted and washed twice with sterile saline solution. The inoculum was adjusted using the same diluent to achieve a final concentration in the product of about 2 log CFU/g. In each sausage the total volume of the inoculum (1%) was injected with a syringe in three different spots (Sperandii et al. Citation2015). The absence of L. monocytogenes in the tested batches was also verified. The growth potential (δ), defined as the difference between the logarithmic medians of the counts of the tested batches at the end and at the beginning of the challenge test, was calculated to quantify the behaviour of L. monocytogenes in the tested conditions. The δ value was calculated for each batch, and the highest obtained δ value for PC + and PC– was retained, to identify the worst scenario (Beaufort et al. Citation2014). All the L. monocytogenes determinations were performed following ISO (ISO Citation2017a, 2017b).
Microbiological counts
Ten grams of salsiccia for each sample were aseptically removed and homogenised (400 Circulator stomacher, PBI International, Milan, Italy) with 90 mL of Maximum Recovery Diluent (MRD). Serial dilutions in MRD were used for standard plate enumerations. Total aerobic mesophilic (TMC) and psychrotrophic (TPC) counts were determined on Plate Count Agar (pour plates) after incubation at 30 °C for 3 days and at 7 °C for 10 days, respectively. LAB were determined on MRS Agar (pour plates) after incubation in anaerobiosis at 25 °C for 3 days. Brochothrix thermosphacta was determined on Streptomycin Thallous Acetate (STA) agar with STA selective supplement (spread plates) after incubation at 25 °C for 2 days. Enterobacteriaceae were determined on Violet Red Bile Glucose Agar (spread plates) after incubation at 25 °C for 1 day. Pseudomonas spp. were determined on Pseudomonas Agar base with CFC supplement (spread plates) after incubation at 25 °C for 3 days. Staphylococci were determined on Mannitol Salt Agar (spread plates) after incubation at 37 °C for 2 days. Yeasts and moulds were determined on Yeast Extract Glucose Chloramphenicol Agar (pour plates) after incubation at 25 °C for 5 days. All cultural media and supplements were purchased from Oxoid.
Physico-chemical analyses
pH was determined in duplicate using a pH metre (XS Instruments, pH 7 Portable metre, Bormac S.r.l, Modena, Italy) equipped with a glass electrode for meat penetration (Hamilton Double Pore, Hamilton Bonaduz AG, Bonaduz, Switzerland) and an automatic temperature compensator.
TBARS were measured by the determination of malondialdehyde (MDA) levels (Ke et al. Citation2007, as modified by Dal Bosco et al. Citation2009). A 5 g sausage sample was homogenised for 45 s at 9,000 xg (Polytron PT 3000, Kinematica AG, Eschbach, Germany) with 10 mL of 7.5% trichloroacetic acid (TCA) and 0.1% diethylenetriaminepentaacetic acid (DTPA) in distilled water.
The homogenised sample was centrifuged (10,000 xg for 10 min) (4235 A CWS, ALC International, Milan, Italy) and filtered, and 5 mL of the filtrate were mixed with 2.5 mL of 2-thiobarbituric acid solution (0.288% in distilled water) in capped test tubes. The tubes were vortexed and placed in a water bath at 95 °C for 45 min. After cooling, the absorbance was determined at 532 nm (V-530 Jasco International, Milan, Italy) against a blank containing TCA/DTPA solution instead of a sample extract. A calibration curve was plotted with 1,1,3,3-tetraethoxypropane (0–15 μM, final concentrations) to obtain the MDA concentration, and the results were expressed as mg equivalent of MDA per kg of fresh sausage. All determinations were performed in triplicate.
Salsiccia colour was measured using a Minolta CR300 chroma metre (Minolta, Osaka, Japan) and expressed as L* (lightness), a* (redness), and b* (yellowness) according to the CIElab system (CIE (Commission Internationale de l’Eclairage) Citation1976). The illuminant was D65, and an incidence angle of 0° was used. For each analysed sausage, colour was measured on the surface after removal of the casing and in the interior. For each determination, the measured value was the average of three replications at randomly selected locations. Prior to each session, the chroma metre was calibrated using a white tile (L* = 98.14, a* = −0.23 and b* = 1.89). The L* value indicates lightness (0 = darkness, 100 = lightness), the a* value indicates redness (+60 = red, −60 = green) and the b* value indicates yellowness (+60 = yellow, −60 = blue).
Sensory analysis
On two PC+/PC– batches, sensory differences were explored with discriminative and descriptive analyses. Specifically, triangle test was carried out, using a semi-trained panel, between raw PC + and PC– samples at t3, t7 and t9. Quantitative descriptive analysis (QDA) was carried out at all sampling times on raw sausages, and at t3 and t7 for cooked samples. For the QDA, a panel of 8 experienced assessors was preliminary formed and trained on the specific product. To allow the evaluation of internal and external characteristics, analyses of raw sausages were carried out on samples composed of a terminal portion approximately 4 cm long and of a 1.5 cm thick slice, coming from two different sausages. Eight sensory parameters were evaluated using a five points structured scale. For cooked samples analyses, sausages were cooked in a clam-shell electrical grill until an inner temperature of 70 °C was reached. Then, 3 cm long non-terminal portions were wrapped in aluminium foil and kept warm in a water bath, until served. Nine sensory parameters were evaluated using a five points structured scale. Evaluated sensory parameters, and the respective used scales, are detailed in Table . In all cases samples were codified anonymously with a three-digit number and served to panellists following a balanced design (Macfie et al. Citation1989).
Table 1. Description of attributes and associated scales’ anchor points used for sensory evaluation of sausage samples.
Statistical analyses
All statistical analyses were performed with the software R v. 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria). Microbial counts were previously converted in log CFU/g. L. monocytogenes counts were subjected to ANOVA, with presence of PC (PC + and PC–) and temperature (2, 7, 12 °C) as fixed effects and their interaction, with time as a nested factor. For the other microbial and physico-chemical parameters, a two-way ANOVA, with PC presence (PC + and PC–) and time as factors, and their interaction, was used to evaluate sample differences. For sensory parameters, at each sampling time, the effect of the presence of PC (PC + and PC–) was tested with t-test, while the effect of time was evaluated with a one-way ANOVA within each type of samples (PC + and PC–). Tukey test was used for all post-hoc comparisons. For the sensory triangle test, the binomial distribution was used to calculate the significance level associated with the number of observed correct answers. Statistical differences were considered significant if associated with a p-value < .05.
Results
Microbiological analyses
Listeria monocytogenes
The results of the L. monocytogenes growth test showed a significant interaction of PC and temperature, as reported in Table . The initial inoculum resulted in a L. monocytogenes load of approximately 2 log CFU/g both in PC– and PC + samples. PC had only a moderated effect, not statistically significant (except for t9), at 2 °C due to a limited growth of L. monocytogenes in both PC– and PC + samples at the correct storage temperature. Nonetheless, L. monocytogenes counts increased in PC + by 0.03 and 0.36 log CFU/g vs. 0.25 and 0.91 in PC–, at t7 and t9. The protective effect of the culture was more relevant in samples stored at abusive temperatures. Particularly, already at t3 at 12 °C, L. monocytogenes loads of PC + samples were 0.86 log CFU/g lower than the corresponding PC– samples. The effect of the PC was more evident at the subsequent sampling times, when statistically significant differences of 1.60 and 2.22 log CFU/g in L. monocytogenes counts at 7 °C, and of 2.15 and 1.97 log CFU/g at 12 °C, were observed at t7 and t9, respectively. We used the δ calculation to determine whether the increase in L. monocytogenes counts would exceed the limit of 0.50 log CFU/g. This value is the limit of growth beyond which a food is considered to support the growth of L. monocytogenes during storage conditions. PC was able to keep the growth potential within the limit of 0.50 log CFU/g both at 2 °C and 7 °C for the whole shelf life period (7 days). At the same temperatures, the δ of PC + samples showed a very limited increase, also after a more prolonged storage time (within 0.60 log CFU/g at t9). In case of strongly abusive temperature (12 °C), at t7, the limit of 0.50 log CFU/g was exceeded both with and without PC, but there was a δ difference between PC– and PC + samples of 2.78 and 2.35 log CFU/g, at t7 and t9, respectively.
Table 2. Growth potential (δ, log CFU/g) and significative interaction (p < .001) of protective culture presence (PC + and PC–) and temperature (abusive temperatures and control refrigerated conditions) at different sampling times on Listeria monocytogenes mean counts (log CFU/g) of experimental sausages.
Microbial evolution
The results of the microbial analyses are reported in Figure . For all microbial groups, except for B. thermosphacta and yeasts and moulds, there was significant interaction between PC presence and time. As expected, TMC and TPC counts were significantly higher in PC + sausages starting from t0 due to the presence of the PC itself. In PC– sausages, instead, total counts were moderated at t0, but they increased during the storage due to the spoilage microorganisms growth reaching at t7 (end of the commercial shelf life) a considerable microbial load, higher than 8 log CFU/g. The high LAB and staphylococci loads in PC + samples at t0 reflect the proper use of the PC. In PC + samples, LAB counts increased during storage, statistically from t0 to t7 and t9, whereas staphylococci loads remained approximately constant during storage at 7 °C. This is related to the different minimum growth temperature of the two protective strains (2 °C for L. sakei and 10 °C for S. xylosus). Enterobacteriaceae counts were limited at t0, attesting an adequate level of manufacturing hygiene. During storage, they increased only slightly in PC + samples, even at t9, while they reached in PC– samples considerably higher loads (more than 5 log CFU/g at t9), significantly different from PC + counts at t7 and t9.
Figure 1. Evolution of bacterial counts (log CFU/g, mean ± SD) at different sampling times (t0, t3, t7 and t9, days) of experimental sausages, with (PC+) and without (PC–) protective culture, stored at 7 °C. In case of significant interaction (PC × Time), different letters show statistically significant differences among samples.
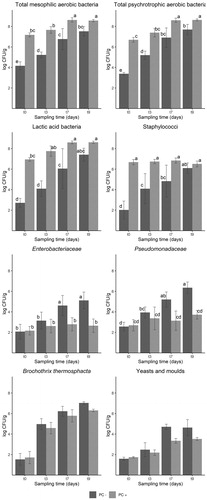
Pseudomonadaceae counts showed a similar trend, with PC– samples reaching loads higher than 5 log CFU/g already at t7, while PC+ Pseudomonadaceae loads were lower than 4 log CFU/g at t7 and t9. B. thermosphacta counts reached considerably high levels during storage, with quite similar values between PC + and PC– at the beginning, but with higher counts in PC– samples at the end of the storage time. Indeed, both PC presence (p = .020) and time (p < .001) were significant as factors, with B. thermosphacta loads constantly growing during storage and reaching at t9 a difference of 0.72 log CFU/g between PC + and PC– samples. Similarly, for yeasts and moulds counts PC and time were both significant factors (p = .003 and p < .001, respectively), with higher counts at t7 and t9 compared to t0 and t3 and reaching a difference of more than 1 log CFU/g, both at t7 and t9.
Physico-chemical parameters
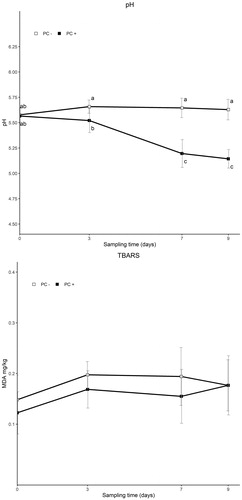
Recorded pH and TBARS values of the analysed samples are reported in Figure . PC + samples had significantly lower pH values compared to PC– samples, due to the active multiplication of L. sakei inoculated strain, which determined pH values of about 5.50 at t3 and lesser at t7 and t9. Autochthonous LAB were not able to support an equivalent growth in control sausages, where pH values, after an initial increase of 0.08 at t3, remained almost constant up to the end of the trial. As for TBARS, despite similar values were recorded for both PC– (0.15 MDA mg/kg at t0 and 0.18 MDA mg/kg at t9) and PC + samples (0.12 MDA mg/kg at t0 and 0.18 MDA mg/kg at t9), PC presence and time were significant, but not their interaction. Particularly, higher values were observed starting at 3 days of storage (p = .006), and PC + samples had lower values than PC– (p = .031).
Figure 2. Evolution of pH values and TBARS content (mg/kg of malondialdehyde, MDA, mean ± SD) at different sampling times of experimental sausages, with (PC+) and without (PC–) protective culture, stored at 7 °C. In case of significant interaction (PC × Time), different letters show statistically significant differences among samples.
The results of the colour determinations of sausages are reported in Table .
Table 3. External and internal mean colour parameters at different sampling times (t0, t3, t7 and t9, days) of experimental sausages, with (PC+) and without (PC–) protective culture, stored at 7 °C.
The a* parameter, related to the red colour of the tested sausages, had the greatest modification during storage, either at the external and at the internal surface. This was particularly evident on the surface of PC + samples that showed significantly different values from PC– at t7 and t9. L* and b* parameters showed to be less influenced by PC, with brighter surface in PC + and yellower PC– samples (external and internal).
Sensory analysis
The results of the discriminative analysis between PC + and PC– samples showed that PC + and PC– sausages were not discernible at t3 (17 correct out of 38 total answers), while they were discernible at t7 and t9 (43 correct out of 52 total answers, and 57 out of 64, for t7 and t9, respectively).
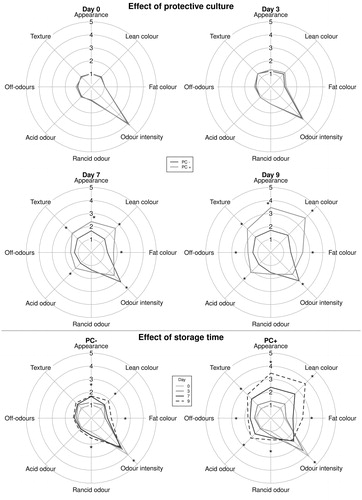
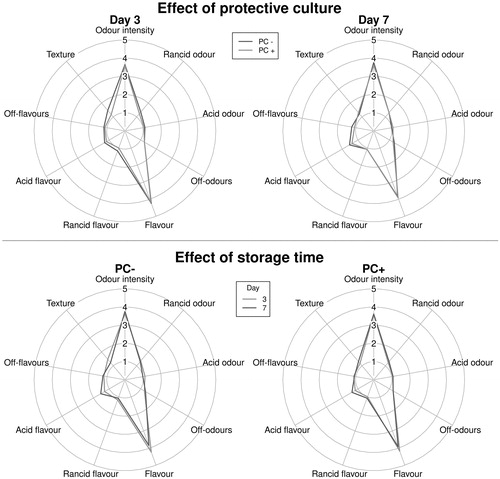
QDA results for raw and cooked samples are shown in Figures and , respectively. The sensory analysis on the raw samples confirmed the instrumental differences between PC + and PC– regarding colour and acidity and showed that, at t7, statistically significant differences were present, with PC + samples showing modifications of external appearance, colour of lean and fat portion, intensity of characteristic and acid odour, and texture. At t9, after the end of the set shelf life, PC + samples also had significantly higher off-odours; the effect of storage time was more pronounced in PC + samples, showing that PC was not useful to extend the sausage shelf life. On the contrary, cooked samples analysis did not highlight any significant difference in the sensory properties between types of sausage for the whole shelf life period.
Discussion
Listeria monocytogenes
The effect of PCs of L. sakei on L. monocytogenes growth was explored in several studies on sliced ham, fermented meat products and fresh meat (Zagorec and Champomier-Vergès Citation2017), but data about fresh sausages are limited (Liserre et al. Citation2002), even if other Lactobacillus species recently demonstrated inhibitory properties on the pathogen (De Castilho et al. Citation2020). In the technical sheet of the tested PC, it is stated that the included L. sakei strain is able to suppress the growth of spoilage and pathogenic microorganisms and enhance the sensory quality of fresh meat preparations and products. We tested its actual effect against L. monocytogenes in fresh salsiccia stored at different temperatures, to assess its safety potential in case of incorrect storage temperature, and, at the same time, to verify the limiting action on spoilage evolution in such a not uncommon scenario. We found a strong anti-Listeria potential. Indeed, the use of the PC successfully limited Listeria growth, leading to a difference between PC– and PC + samples of approximately 2 log CFU/g at t9, at both 7 °C and 12 °C. Moreover, the growth potential was below the limit of 0.50 log CFU/g in the period of assigned shelf life even at 7 °C, demonstrating an effective safety contribution. Indeed, on the basis of the results, PC + salsiccia did not support the pathogen growth during 7 days of shelf life, not only at the correct storage temperature (δ: 0.15 log CFU/g), but also in the case of an abusive temperature 5 °C higher (δ: 0.42 log CFU/g). On the contrary, PC– sausages supported the pathogen growth already at 2 °C (δ: 0.54 log CFU/g). It is true that fresh salsiccia is intended to be cooked before consumption, and that L. monocytogenes does not survive thorough cooking. Nonetheless, it is not uncommon to eat fresh sausages raw. Moreover, even when adequately cooked before consumption, they can be a source of cross-contamination during meal preparation. Therefore, the protective effect on L. monocytogenes is noteworthy considering that in Italy 64 out of 129 fresh sausages were found positive for L. monocytogenes by Bonardi et al. (Citation2002), while De Cesare et al. (Citation2007) reported 112 positive sausages out of 288, with L. monocytogenes loads higher than 100 MPN/g for 20.5% of positive fresh sausages.
Microbial evolution
As for the quality aspects, microbiological data at t0 indicated a good hygiene of the product with TMC in PC– sausages of 4.16 ± 0.39 log CFU/g and even lower TPC counts. These data are in agreement with those of Tremonte et al. (Citation2005), and lower than those reported by other authors (Kamdem et al. Citation2007; Chiavaro et al. Citation2008; Nuvoloni et al. Citation2012) for Italian fresh sausages. Regardless of the good initial hygiene, PC– sausages reached at the end of the shelf life (t7) a TMC of almost 7 log CFU/g at abusive temperature. Indeed, total viable aerobic counts higher than 6 log CFU/g are usually reported for Italian fresh sausage after 7 days of storage (Tremonte et al. Citation2005; Kamdem et al. Citation2007; Chiavaro et al. Citation2008; Nuvoloni et al. Citation2012).
Regarding the PC, the supplier indicates that the L. sakei strain grows even at 2 °C while S. xylosus requires a higher temperature (10 °C). LAB and staphylococci loads in PC + samples confirmed this information. Thus, in our trials, at 2 °C and 7 °C the contribution of S. xylosus was presumably insignificant.
The microorganisms with the greatest impact on spoilage of meat products packaged in aerobic conditions are usually Pseudomonadaceae, Enterobacteriaceae, LAB and B. thermosphacta. L. sakei has been reported to successfully inhibit spoilage bacteria in ground meat and sausages (Chaillou et al. Citation2014; Zagorec and Champomier-Vergès Citation2017).
In our study, the presence of the PC successfully contained Enterobacteriaceae and Pseudomonadaceae. Particularly, at t7 and t9, PC + samples showed for both microbial groups average counts at least 1.80 and 2.40 log CFU/g lower than those of the corresponding PC– samples.
PC effect was less successful on B. thermosphacta counts. This data is in agreement with Chaillou et al. (Citation2014) who reported a very limited effect of a L. sakei PC against B. thermosphacta inoculated in ground beef, although other authors (Comi et al. Citation2015) observed in the same food matrix an inhibiting effect. Considering that there was no or minimal growth of the PC S. xylosus strain at 7 °C, our data suggest that, while the added culture can have a strong limiting effect on Gram negative bacteria at this temperature, higher temperatures, that allow for the growth of S. xylosus, might be necessary for a higher limiting effect on B. thermosphacta, as we verified in some PC + samples stored at 12 °C (data not shown).
The activity of L. sakei within a PC against L. monocytogenes and spoilage microflora may be ascribed to different actions: competitive mechanisms, production of bacteriocins, organic acids, or other substances with antimicrobial activity, such as hydrogen peroxide. Although an analysis of the bacterial mechanisms responsible for the protective effect of the culture was not the object of this study, it seems probable that the acidifying activity of the PC could have played a role in our experiment.
Physico-chemical parameters
Recorded pH values, as well as sensory data on raw sausages, confirmed that the presence of the PC, and particularly of L. sakei, led to an acidification of the samples, probably boosted by the added dextrose.
As for TBARS, after a minor increase (lower than 0.1 mg/kg), observed after 3 days of storage, no significant subsequent increase of values in the analysed samples was recorded; this can be related with the short shelf life and with the presence of antioxidant additives and spices in the recipe, both preventing lipid oxidation (Munekata et al. Citation2020).
Regarding colour evolution, and particularly a* values evolution, these were significantly lower in PC + samples starting at t7 on the external surface and at t9 for internal surface. Colour changes, and particularly red discolouration, are physiological at the end of the shelf-life of sausages (Chiavaro et al. Citation2008; Nuvoloni et al. Citation2012). The more pronounced discolouration of PC + samples, particularly on the external surface, could be related to the simultaneous production of organic acids and hydrogen peroxide by L. sakei. Indeed, we verified that the PC L. sakei strain was able to produce hydrogen peroxide when screened by the method of Eschenbach et al. (Citation1989) (data not shown). Thus, in the presence of a sufficient amount of oxygen in the package, peroxides may have reached a significant amount, due to the lack of an adequate catalase production by S. xylosus at temperatures lower than its minimum. Peroxides can react with iron in the myoglobin and generate grey tones in the sausage (Centeno and Carballo Citation2014). Moreover, it is known that hydrogen peroxide produced by lactobacilli can inhibit microorganisms, including L. monocytogenes, often in synergy with lactic acid production (Tharrington and Sorrells Citation1992).
Overall, the greater colour changes in PC + sausages represent a drawback, even if this effect appeared only at the end of the shelf life of products stored at abusive temperature, whereas the producer did not experience the issue in correctly stored sausages.
Sensory analysis
Sensory data confirmed the instrumental data on colour changes and evidenced overall modifications of appearance, odour and texture determined by PC. Differences were found both by discriminative analysis and QDA on raw samples at t7. Considering that sensory characteristics of cooked sausages were not affected by PC during the shelf life period, discolouration of raw sausages seems to be the most important drawback. Indeed, colour is one of the main criteria of consumers’ choice when buying a meat product (Pérez-Alvarez and Fernández-Lopez Citation2010); in any case, in the tested sausages this drawback should have a quite limited impact on consumers’ choice given that it is not present in the first days of storage, not even when the indicated storage temperature is not respected.
Conclusions
Biopreservation is an ecological strategy that can be adopted in both new and traditional foods. Commercial PCs for fresh sausages have been available on the market for years, but the benefits, for both producers and consumers, are often not fully defined. Moreover, the actual effect of the microbial components of each culture is determined by the unique characteristics of each product, in terms of main factors, such as recipe, temperatures, additives, autochthonous microflora; thus, a culture may work differently even in products of the same category. The tested culture, generically referred as ‘for fresh sausages, hamburgers and other fresh cured meat products’, demonstrated a not negligible activity in the tested conditions.
Indeed, the PC examined in our study successfully limited L. monocytogenes growth already at 2 °C and more evidently and significantly at abusive temperature, with a clear food safety improvement. Moreover, it proved to have an effective action on Gram negative bacteria at 7 °C, with a more limited effect on B. thermosphacta. The undesired side effects on the organoleptic properties of the raw product could represent a partial obstacle for consumer acceptance, limited by the fact that they only appeared at the end of the shelf life in the tested conditions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Beaufort A, Bergis H, Lardeux A-L, Lombard B. 2014. EURL (European Union Reference Laboratory for Listeria monocytogenes) Lm technical guidance document for conducting shelf-life studies on Listeria monocytogenes in ready-to-eat foods. Version 3, 6 June. [accessed 2020 Jan 7]. https://ec.europa.eu/food/sites/food/files/safety/docs/biosafety_fh_mc_technical_guidance_document_listeria_in_rte_foods.pdf.
- Bonardi S, Brindani F, Pizzin G, Bacci C, Cenci A, D’Incau M, Liebana E. 2002. Microbiological hazard of fresh pork sausages. Results of a one-year study. Ind Aliment. 41:782–788.
- Centeno JA, Carballo J. 2014. Starter and adjunct microbial cultures used in the manufacture of fermented and/or cured or ripened meat and dairy products. In: Rai VR and Bai JA, editors. Beneficial microbes in fermented and functional foods. New York: CRC Press; p. 35–54.
- Chaillou S, Champomier-Vergès MC, Cornet M, Crutz-Le Coq AM, Dudez AM, Martin V, Beaufils S, Darbon-Rongère E, Bossy R, Loux V, et al. 2005. The complete genome sequence of the meat-borne lactic acid bacterium Lactobacillus sakei 23K. Nat Biotechnol. 23(12):1527–1533.
- Chaillou S, Christieans S, Rivollier M, Lucquin I, Champomier-Vergès MC, Zagorec M. 2014. Quantification and efficiency of Lactobacillus sakei strain mixtures used as protective cultures in ground beef. Meat Sci. 97(3):332–338.
- Chiavaro E, Zanardi E, Bottari B, Ianieri A. 2008. Efficacy of different storage practices in maintaining the physicochemical and microbiological properties of fresh pork sausage. J Muscle Foods. 19(2):157–174.
- CIE (Commission Internationale de l’Eclairage). 1976. Official recommendations on uniform colour spaces, colour differences equations and metric colour terms, Suppl. 2, Publication 15 Colourimetry, Paris.
- Comi G, Tirloni E, Andyanto D, Manzano M, Iacumin L. 2015. Use of bio-protective cultures to improve the shelf-life and the sensorial characteristics of commercial hamburgers. LWT-Food Sci Technol. 62(2):1198–1202.
- Consigliere R, Meloni D, Mazzette R. 2018. Key hurdles in the Mediterranean-style dry fermented sausage “Salsiccia Sarda” as influenced by different ingredients related to product safety. J Food Process Preserv. 42(1):e13321.
- Dal Bosco A, Mugnai C, Mourvaki E, Cardinali R, Moscati L, Paci G, Castellini C. 2009. Effect of genotype and rearing system on the native immunity and oxidative status of growing rabbits. Ital J Anim Sci. 8(sup2):781–783.
- De Castilho NPA, Todorov SD, Licursi Oliveira L, Dos Santos Bersot L, Nero LA. 2020. Inhibition of Listeria monocytogenes in fresh sausage by bacteriocinogenic Lactobacillus curvatus UFV-NPAC1 and its semi-purified bacteriocin. LWT-Food Sci Technol. 118:108757.
- De Cesare A, Mioni R, Manfreda G. 2007. Prevalence of Listeria monocytogenes in fresh and fermented Italian sausages and ribotyping of contaminating strains. Int J Food Microbiol. 120(1–2):124–130.
- Desai AN, Anyoha A, Madoff LC, Lassmann B. 2019. Changing epidemiology of Listeria monocytogenes outbreaks, sporadic cases, and recalls globally: a review of ProMED reports from 1996 to 2018. Int J Infect Dis. 84:48–53.
- EFSA, European Food Safety Authority. 2019. The European Union One Health 2018 Zoonoses Report. EFSA J. 17(12):5926.
- Eschenbach DA, Davick PR, Williams BL, Klebanoff SJ, Young-Smith K, Critchlow CM, Holmes KK. 1989. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol. 27(2):251–256.
- Gelinski JMLN, Baratto CM, Casagrande M, de Oliveira TP, Megiolaro F, de Martini-Soares FAS, Borges De Souza EM, Vicente VA, Fonseca GG. 2019. Control of pathogens in fresh pork sausage by inclusion of Lactobacillus sakei BAS0117. Can. J. Microbiol. 65(11):831–841.
- Hammami R, Fliss I, Corsetti A. 2019. Editorial: application of protective cultures and bacteriocins for food biopreservation. Front Microbiol. 10:1561.
- ISO (International Organization for Standardization). 2017a. Microbiology of the food chain – Horizontal method for the detection and enumeration of Listeria monocytogenes and of Listeria spp. – Part 1: Detection method. ISO 11290–1.
- ISO (International Organization for Standardization). 2017b. Microbiology of the food chain – Horizontal method for the detection and enumeration of Listeria monocytogenes and of Listeria spp. – Part 2: Enumeration method. ISO 11290–2.
- Kamdem S, Patrignani F, Guerzoni ME. 2007. Shelf-life and safety characteristics of Italian Toscana traditional fresh sausage (Salsiccia) combining two commercial ready-to-use additives and spices. Food Control. 18(5):421–429.
- Ke PJ, Ackman RG, Linke BA, Nash DM. 2007. Differential lipid oxidation in various parts of frozen mackerel. J Food Technol. 12(1):37–47.
- Laranjo M, Elias M, Fraqueza MJ. 2017. The use of starter cultures in traditional meat products. J Food Qual. 2017:1–18.
- Laukovà A, Simonovà M, Strompfovà V. 2010. Staphylococcus xylosus S03/1M/1/2, bacteriocin-producing meat starter culture or additive. Food Control. 21:970–973.
- Liserre AM, Landgraf M, Destro MT, Franco BDGM. 2002. Inhibition of Listeria monocytogenes by a bacteriocinogenic Lactobacillus sake strain in modified atmosphere-packaged Brazilian sausage. Meat Sci. 61(4):449–455.
- Lorenzo JM, Gómez M, Fonseca S. 2014. Effect of a commercial starter culture on physicochemical characteristics, microbial counts and free fatty acid composition of dry-cured foal sausage. Food Control. 46:382–389.
- Lücke F-K. 2000. Utilization of microbes to process and preserve meat. Meat Sci. 56(2):105–111.
- Macfie HJ, Bratchell N, Greenhoff K, Vallis LV. 1989. Designs to balance the effect of order of presentation and first-order carry-over effects in hall tests. J Sensory Studies. 4(2):129–148.
- Meloni D. 2015. Presence of Listeria monocytogenes in Mediterranean-style dry fermented sausages. Foods. 4(1):34–50.
- Munekata PES, Rocchetti G, Pateiro M, Lucini L, Domínguez R, Lorenzo JM. 2020. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: an overview. Curr Opin Food Sci. 31:81–87.
- Nuvoloni R, Pedonese F, Fratini F, Torracca B, Turchi B, Preziuso G, Serra A. 2012. Microbiological and physico-chemical profile of traditional Salsiccia toscana during storage. Ital J Anim Sci. 11(3):e59.
- Pérez-Alvarez JA, Fernández-Lopez J. 2010. Colour characteristics of meat and poultry processing. In: Nollet LML and Fidel Toldrá F, editors. Sensory analysis of foods of animal origin. Boca Raton: CRC Press; p. 101–120.
- Rodgers S. 2001. Preserving non-fermented refrigerated foods with microbial cultures. Trends Food Sci Technol. 12(8):276–284.
- Rossi LPR, Almeida RCC, Lopes LS, Figueiredo ACL, Ramos MPP, Almeida PF. 2011. Occurrence of Listeria spp. in Brazilian fresh sausages and control of Listeria monocytogenes using bacteriophage P100. Food Control. 22(6):954–958.
- Sperandii AF, Neri D, Romantini R, Santarelli GA, Prencipe V. 2015. Definition of a standard protocol to determine the growth potential of Listeria monocytogenes and Yersinia enterocolitica in pork sausage produced in Abruzzo region. Italy. Ital J Food Saf. 4:4575.
- Tharrington G, Sorrells KM. 1992. Inhibition of Listeria monocytogenes by milk culture filtrates from Lactobacillus delbrueckii subsp. lactis. J Food Prot. 55(7):542–544.
- Tremonte P, Sorrentino E, Succi M, Reale A, Maiorano G, Coppola R. 2005. Shelf life of fresh sausages stored under modified atmospheres. J Food Prot. 68(12):2686–2692.
- Zagorec M, Champomier-Vergès M-C. 2017. Lactobacillus sakei: a starter for sausage fermentation, a protective culture for meat products. Microorganisms. 5(3):56.