Abstract
The aim of this study was to confirm the role of AMP-activated protein kinase (AMPK) in the regulation of post-mortem breast muscle glycolysis in the broiler, we evaluated the effects of intraperitoneal injection of the specific AMPK activator (5-aminoimidazole-4-carboxamide ribonucleoside, AICAR) and inhibitor (6-[4-(2-piperidin-1-yl-ethoxy)-phenyl]-3-pyridin-4-yl-pyrazolo[1,5-a] pyrimidine, Compound C) on AMPK phosphorylation (p-AMPK) level, meat quality, glycolytic metabolites and glycolytic enzymes activities in post-mortem breast muscle of broilers. Compared to the CON group, intraperitoneal injection of AIRCR increased p-AMPK level, glycolytic enzyme activities and lactate content in post-mortem breast muscle, increased lightness (L*) and drip loss, but decreased pH45min and pH24h. However, intraperitoneal injection of Compound C, decreased p-AMPK level, glycolytic enzyme activities, glucose-6-phosphate and lactate content in post-mortem breast muscle, and increased pH24h. Based on these results, we conclude that AMPK played a vital role in regulating post-mortem glycolysis in the breast muscle of broilers.
Injection of AIRCR promoting muscle glycolysis.
Injection of Compound C alleviating muscle glycolysis.
AMPK played a vital role in regulating post-mortem muscle glycolysis.
HIGHLIGHTS
Introduction
Pale, soft, and exudative (PSE)-like meat was characterised by pale-colored, high drip loss, soft appearance, and tough texture after cooking (Van Laack et al. Citation2000). Due to its inferior meat quality, PSE-like meat resulted in huge economic loss in poultry industry. According to an investigation in 2012, PSE-like meat induced annual economic losses of over $30 million in Brazil. In 2014, the economic loss increased to $50 million in Brazil and over $200 million in the USA (Owens et al. Citation2009; Droval et al. Citation2012). Nowadays, with the rapid development of poultry meat production and consumption, PSE-like meat in poultry was an important issue due to its high incidences. The average occurrence of PSE-like meat in China was more than 20% and even reached 40% in some regions during summer (Zhu et al. Citation2012; Xing et al. Citation2016). PSE-like meat was caused by fast and excessive glycolysis in post-mortem muscle, which induced lactate accumulation and rapid pH decline. Previous studies indicated that there was a strong correlation between AMP-activated protein kinase (AMPK) activation and pH decline in post-mortem muscle. The activation of AMPK was responsible for higher lactic acid content and lower pH in PSE meat (Shen and Du Citation2005; Shen, Means, Thompson, et al. Citation2006; Wang et al. Citation2017; Sun et al. Citation2019). Intraperitoneal injection of 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR), a specific AMPK activator, increased AMPK activity and resulted in lower pH in post-mortem mouse longissimus dorsi (LD) muscle (Du et al. Citation2005). Intraperitoneal injection with 6-[4-(2-piperidin-1-yl-ethoxy)-phenyl]-3-pyridin-4-yl-pyrazolo[1,5-a] pyrimidine) (Compound C), a specific inhibitor of AMPK decreased AMPK activity and resulted in higher pH, and lower lactate content in post-mortem mouse LD muscle (Shen et al. Citation2008). The AMPK knockout mice had lower AMPK activity and lactate content, but higher pH in post-mortem LD muscle (Liang et al. Citation2013). These studies indicated that there was a strong correlation between pH and AMPK activity in post-mortem muscle, suggesting that AMPK played a critical role in regulating the post-mortem glycolysis rate. However, former studies focussed on the role of AMPK in the regulation of post-mortem muscle glycolysis in mouse and pig (Shen and Du Citation2005; Shen et al. Citation2008; Liang et al. Citation2013), relatively few studies were conducted in broilers (Xing et al. Citation2016; Zhang et al. Citation2017; Sun et al. Citation2019). To clarify the role of AMPK in post-mortem muscle glycolysis in broilers, we evaluated the effects of intraperitoneal injection of AICAR or Compound C on AMPK activation and glycolysis in post-mortem breast muscle. Therefore, the aim of this study was to confirm the role of AMPK in post-mortem muscle glycolysis rate in broilers.
Materials and methods
Animals, experimental design, diets and sample collection
A total of 18 female 84-day-old Chinese indigenous chicken (Luhua chicken) was randomly allocated to 3 treatments with 6 replication and 1 broiler per replicate: control group (CON), AICAR group, and Compound C group. The broilers were fed separately, one broiler in one cage, in a temperature- and humidity-controlled environment. The temperature was maintained at 24 ± 2 °C, and the humidity was maintained at 55–65%. Artificial light was provided 23 h/day by fluorescent lights. In the AICAR and Compound C group, broilers were intraperitoneally injected with AIRCR (5 mg/kg body weight) and 1 mg/kg of Compound C (1 mg/kg body weight), respectively. The CON group was intraperitoneally injected with the same volume of vehicle solution phosphate buffer saline (PBS). After injection, broilers were returned to their cages and free access to water, two-hour later, the broilers were intravenously injected with pentobarbital sodium (25 mg/kg) and slaughtered via exsanguination. The entire right breast muscle was collected and stored at 4 °C for 24 h to measure meat quality. The left breast muscle was also collected and stored at 4 °C, and about 1 g of the samples at post-mortem 45-min and 24-h were collected, quick freezing in liquid nitrogen and stored at −80 °C for later determination of the muscle glycogen, glucose, glucose-6-phosphate (G-6-P), pyruvate acid, lactate and glycolytic enzymes activity. The experimental protocol used in this study followed the Animal Care and Use Committee of Guangdong Ocean University.
AMPK phosphorylation
The AMPK phosphorylation (p-AMPK) level in the breast muscle was detected using a chicken p-AMPK enzyme-linked immunosorbent assay kit (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China). One-gram frozen muscle sample was homogenised in 9 ml of 0.9% ice-cold saline, then centrifuged at 3000 g for 10 min at 4 °C and the supernatant was used for the following measurement according to the manufacture’s instruction. Each sample was assayed in triplicate and the values were averaged. The p-AMPK level was quantified using commercial ELISA kits at 450 nm. The sensitivity of the p-AMPK assay ranged from 10–320 ng/l and its intra- and inter-assay coefficients of variation were 7.6% and 8.5%, respectively.
Meat quality
Muscle pH value was measured on breast muscle at a depth of 2.5 cm below the surface using a pH metre for 45-min (pH45min) and 24-h (pH24h) post-mortem (Fisher Scientific, Pittsburgh, PA). Meat colour of the lightness (L*), redness (a*), and yellowness (b*) were measured using a Model CR-410 Chroma metre (Konica Minolta Sensing Inc., Osaka, Japan) with a CIE D65 illuminant, 8 mm aperture diameter. Before measurement, the colorimeter was standardised with a white tile (L* = 98.99, a* = −0.12, and b* = 0.98). For drip loss determination, approximately 4 g sample was weighed, the samples packaged in a transparent polythene bag and stored at 4 °C for 24 h, after which the excess moisture was wiped out and the breast samples were weighed, drip loss was calculated based on the following equation, Drip loss (%) = (Raw weight − Stored weight)/Raw weight × 100.
Muscle glycogen, glucose, glucose-6-phosphate, pyruvate acid and lactate measurement
The muscle glycogen (NO. A043-1-1), glucose (NO. F154-1-1), glucose-6-phosphate (NO. H580-1), pyruvate acid (NO. A081-1-1) and lactate (NO. A019-2-1) measurement were estimated spectrophotometrically using commercial kits (Nanjing Jiancheng Biochemical Institute, Nanjing, China).
Muscle glycolytic key enzymes activity analysis
Protein concentration was determined using a BCA protein assay kit (NO. A045-4-2). Hexokinase (HK, NO. A077-3-1), phosphofructokinase-1 (PFK-1, NO. A129-1-1), pyruvate kinase (PK, NO. A076-1-1), and the lactate dehydrogenase (LDH, NO. A020-1-2) activities were determined spectrophotometrically using commercial kits (Nanjing Jiancheng Biochemical Institute, Nanjing, China).
Statistical analysis
Data were analysed with the use of SAS version 9.1 (SAS Institute, Inc., Cary, NC, USA, 2004). All data were analysed using a one-way analysis of variance followed by Duncan’s multiple range test to analyse differences among treatments. The figures were developed by using GraphPad software. Significant differences were considered at p < 0.05.
Results
AMPK phosphorylation
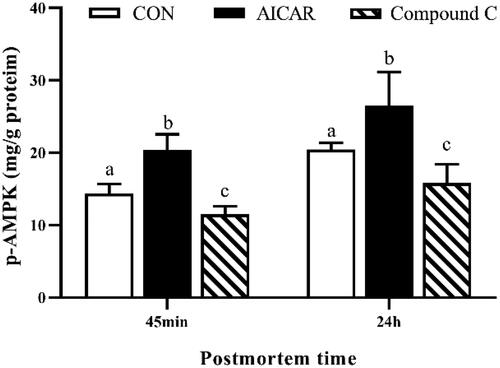
Compared to the CON group, inhibition of AMPK by AICAR significantly reduced (p < 0.05) p-AMPK level in post-mortem breast muscle of broiler, while activation of AMPK by AICAR significantly increased (p < 0.05) p-AMPK level at both 45 min and 24 h post-mortem breast muscle (Figure ).
Figure 1. Effects of activation of AMPK by AICAR or Compound C on AMPK phosphorylation (p-AMPK) level in post-mortem breast muscle of broiler. Results are presented as mean ± standard error (n = 6). a,b,cValues in the same row with different letters are significant different (p < 0.05). CON, control group, broilers injected with same volume of vehicle solution phosphate buffer saline; AICAR group, broilers were intraperitoneally injected with AICAR (5 mg/kg body weight); Compound C group, broilers were intraperitoneally injected with Compound C (1 mg/kg body weight).
AMPK: AMP-activated protein kinase; AICAR: 5-aminoimidazole-4-carboxamide ribonucleoside; CON: Control.
Meat quality
Compared to the CON group, Compound C group had higher (p < 0.05) pH45min, lower (p < 0.05) L*, while the AICAR group had lower (p < 0.05) pH45min and pH24h, higher (p < 0.05) L* and drip loss in breast muscle of broilers (Table ).
Table 1. Effects of activation of AMPK by AICAR or Compound C on meat quality in broiler breast muscle.
Glycolytic metabolites
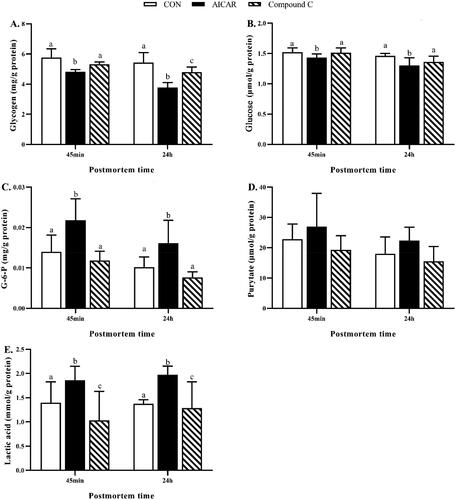
Compared to the CON group, activation of AMPK by AICAR decreased (p < 0.05) the content of glycogen (Figure ) and glucose (Figure ), increased (p < 0.05) the content of G-6-P (Figure ) and lactate (Figure ) in breast muscle 45 min and 24 h post-mortem. Inhibition of AMPK by AICAR decreased the content of glycogen (Figure ) in breast muscle 24 h post-mortem, and decreased (p < 0.05) the content of lactate (Figure ) in breast muscle 45 min and 24 h post-mortem when compared to the CON group.
Figure 2. (A–E) Effects of activation of AMPK by AICAR or Compound C on glycolytic metabolites in post-mortem breast muscle of broiler. Results are presented as mean ± standard error (n = 6). a,b,cValues in the same row with different letters are significant different (p < 0.05). CON, control group, broilers injected with same volume of vehicle solution phosphate buffer saline; AICAR group, broilers were intraperitoneally injected with AICAR (5 mg/kg body weight); Compound C group, broilers were intraperitoneally injected with Compound C (1 mg/kg body weight); G-6-P, Glucose-6-Phosphate.
AMPK: AMP-activated protein kinase; AICAR: 5-aminoimidazole-4-carboxamide ribonucleoside; CON: Control.
Glycolytic enzymes activity
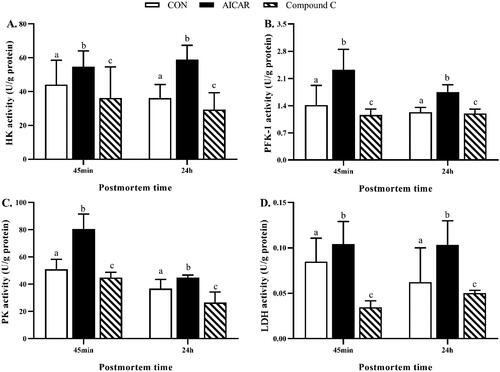
Compared to the CON group, activation of AMPK by AICAR increased (p < 0.05) the activity of HK (Figure ), PFK-1 (Figure ), PK (Figure ), and LDH (Figure ), while inhibition of AMPK by AICAR decreased (p < 0.05) the activity of HK (Figure ), PFK-1 (Figure ), PK (Figure ), and LDH (Figure ) in breast muscle 45 min and 24 h post-mortem.
Figure 3. (A–D) Effects of activation of AMPK by AICAR or Compound C on glycolytic enzymes activity in post-mortem breast muscle of broiler. Results are presented as mean ± standard error (n = 6). a,b,cValues in the same row with different letters are significant different (p < 0.05). CON, control group, broilers injected with same volume of vehicle solution phosphate buffer saline; AICAR group, broilers were intraperitoneally injected with AICAR (5 mg/kg body weight); Compound C group, broilers were intraperitoneally injected with Compound C (1 mg/kg body weight); HK, hexokinase; PFK-1, phosphofructokinase-1; PK, pyruvate kinase; LDH, lactate dehydrogenase.
AMPK: AMP-activated protein kinase; AICAR: 5-aminoimidazole-4-carboxamide ribonucleoside; CON: Control.
Discussion
AMPK was an obligate heterotrimer, containing three subunits: a catalytic α-subunit and two regulatory β- and γ-subunits. AMPK was a crucial cellular energy sensor and the master energy metabolic switch, which was activated by increasing in AMP/ATP or/and ADP/AMP ratio (Mihaylova and Shaw Citation2011). Once activated, AMPK regulated energy balance by promoting catabolic pathways to generate more ATP while inhibiting ATP depletion by down-regulating anabolic pathways (Hardie et al. Citation2012). Former works of literature indicated that activation of AMPK induced glycolysis in post-mortem muscle (Shen and Du Citation2005; Shen, Means, Thompson, et al. Citation2006; Shen, Means, Underwood, et al. Citation2006; Liang et al. Citation2013). The activation of AMPK by preslaughter transport stress or AICAR resulted in increasing AMPK activity, induced lower pH24min and higher lactate accumulation in post-mortem muscle (Shen et al. Citation2008; Xing et al. Citation2016; Li et al. Citation2017; Zhang et al. Citation2017). Conversely, AMPK knockout or inhibition of AMPK by Compound C led to decreased AMPK activity, enhanced pH value and lower lactate accumulation in post-mortem muscle (Shen and Du Citation2005; Shen et al. Citation2008; Liang et al. Citation2013). Consistent with these results, our results indicated that AICAR increased p-AMPK level, decreased pH value and enhanced lactate accumulation in post-mortem breast muscle. The lower pH45min and pH24h suggested increasing glycolysis in breast muscle with AICAR injection. In contrast, Compound C decreased p-AMPK level, increased pH value and lower lactate accumulation in post-mortem breast muscle, and lower lactate content suggested decreasing glycolysis in breast muscle with Compound C injection. These results supported former studies that AMPK played vital role in regulating glycolysis in post-mortem muscle (Shen and Du Citation2005; Li et al. Citation2017). Broiler meat is typically classified as ‘PSE-like meat’ based on its L* value and pH, where L* ≥ 53 and pH ≤ 5.80 (Soares et al. Citation2003). In this study, the L* value and pH were 55.44 and 5.24 in breast muscle 24 h post-mortem of the AICAR group, suggesting the involvement of AMPK activity and PSE-like broiler meat occurrence. Similar results were also reported by Shen, Means, Thompson, et al. (Citation2006), who indicated that preslaughter stress enhanced AMPK activity and PSE meat risk in pig. Fast and excessive glycolysis in post-mortem muscle, especially at the early post-mortem stage was the cause of PSE meat. The earlier and faster post-mortem AMPK activation was responsible for the lower pH and higher lactic acid accumulation in PSE-like meat.
The influence of AMPK activity on glycolysis was also evidenced by the changes in glycogen, glucose and G-6-P in post-mortem muscle. Lower glycogen and glucose, and increased G-6-P contents were observed in breast muscle post-mortem 45 min and 24 h with AIRCR injection, indicating that AICAR promoted glycolysis. Glycolysis in post-mortem muscle followed the same reactions in living animals and was medicated by some key enzymes, including HK, PFK-1, PK and LDH. HK was the first rate-limiting enzyme in glycolysis, which converted glucose to G-6-P, the other two rate-limiting enzymes were PFK-1 and HK, which converted fructose-6-phosphate to fructose-1,6- bisphosphate and phosphoenolpyruvic acid to pyruvate, respectively. LDH was involved in the last step of glycolysis, which converted pyruvate to lactate under anaerobic conditions (Scheffler and Gerrard Citation2007; Hardie et al. Citation2012). Our results showed that AIRCR injection increased HK, PFK-1, PK and LDH activities in post-mortem breast muscle compared to the CON group. While, Compound C injection decreased HK, PFK-1, PK and LDH activities. These results suggested that AMPK-regulated post-mortem glycolysis might be associated with the activity of key enzymes. Similar results were also reported by Holmes et al. (Citation1999), who indicated that AMPK was activated by AICAR, accompanied by increasing muscle HK activity.
The decline in pH in muscle post-mortem was due to the conversion of glycogen to lactic acid. The formation of lactic acid from stored glycogen including 12 enzyme reactions, the glycogen phosphorylase, HK, PFK, PK and LDH reactions were of main importance in the regulation of glycolysis (Strobel et al. Citation2021). AMPK increases glycolysis through two main pathways. Activated AMPK up-regulates glycolysis by phosphorylating and activating phosphorylase kinase, which then phosphorylated and activated glycogen phosphorylase, an enzyme that controlled glycogenolysis and catalysed the production of substrate for glycolysis (Rodríguez et al. Citation2021). In this study, we did not detect the glycogen phosphorylase activity in breast muscle, but the decreased glycogen content, increased glucose and lactic acid content were correlated well with AMPK activity. Another important mechanism by which AMPK regulated glycolysis was through phosphorylation and activation of PFK-2. PFK-2 catalyses the production of fructose-2,6-bisphosphate. Fructose-2,6-bisphosphate was an allosteric activator of PFK-1, which was the most important rate-controlling enzyme in glycolysis (Marsin et al. Citation2000). In our results, PFK-1 activity was in line with AMPK activity, supporting the vital role of AMPK in mediating post-mortem breast muscle.
Conclusions
In conclusion, the results indicated that intraperitoneal injection of AIRCR increased p-AMPK level and glycolytic enzyme activities, promoting glycolysis, decreased pH value and increased lactate content in post-mortem breast muscle compared to the control group, while injection of Compound C showed the contrast results. Based on these results, we conclude that AMPK played a vital role in regulating post-mortem breast muscle glycolysis and was a potential molecular target for the control of PSE-like meat incidence in broilers.
Ethical approval
The experimental protocol used in this study was approved by the Animal Care and Use Committee of Guangdong Ocean University (SYXK-2018-0147).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The original data of the paper are available upon request from the corresponding author.
Additional information
Funding
References
- Droval AA, Benassi V, Rossa A, Prudencio S, Paião F, Shimokomaki M. 2012. Consumer attitudes and preferences regarding pale, soft, and exudative broiler breast meat. J Appl Poult Res. 21(3):502–507.
- Du M, Shen QW, Zhu MJ. 2005. Role of β-adrenoceptor signaling and AMP-activated protein kinase in glycolysis of postmortem skeletal muscle. J Agric Food Chem. 53(8):3235–3239.
- Hardie DG, Ross FA, Hawley SA. 2012. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 13(4):251–262.
- Holmes BF, Kurth KE, Winder W. 1999. Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol. 87(5):1990–1995.
- Li Q, Li Z, Lou A, Wang Z, Zhang D, Shen QW. 2017. Histone acetyltransferase inhibitors antagonize AMP-activated protein kinase in postmortem glycolysis. Asian-Australas J Anim Sci. 30(6):857–864.
- Liang J, Yang Q, Zhu M, Jin Y, Du M. 2013. AMP-activated protein kinase (AMPK) α2 subunit mediates glycolysis in postmortem skeletal muscle. Meat Sci. 95(3):536–541.
- Marsin A, Bertrand L, Rider M, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L. 2000. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 10(20):1247–1255.
- Mihaylova MM, Shaw RJ. 2011. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 13(9):1016–1023.
- Owens CM, Alvarado CZ, Sams AR. 2009. Research developments in pale, soft, and exudative turkey meat in North America. Poult Sci. 88(7):1513–1517.
- Rodríguez C, Muñoz M, Contreras C, Prieto D. 2021. AMPK, metabolism, and vascular function. FEBS J. 288(12):3746–3771.
- Scheffler T, Gerrard D. 2007. Mechanisms controlling pork quality development: the biochemistry controlling postmortem energy metabolism. Meat Sci. 77(1):7–16.
- Shen Q, Means W, Thompson S, Underwood K, Zhu M, McCormick R, Ford S, Du M. 2006. Pre-slaughter transport, AMP-activated protein kinase, glycolysis, and quality of pork loin. Meat Sci. 74(2):388–395.
- Shen QW, Du M. 2005. Role of AMP-activated protein kinase in the glycolysis of postmortem muscle. J Sci Food Agric. 85(14):2401–2406.
- Shen QW, Gerrard DE, Du M. 2008. Compound C, an inhibitor of AMP-activated protein kinase, inhibits glycolysis in mouse longissimus dorsi postmortem. Meat Sci. 78(3):323–330.
- Shen QW, Means WJ, Underwood KR, Thompson SA, Zhu MJ, McCormick RJ, Ford SP, Ellis M, Du M. 2006. Early post-mortem AMP-activated protein kinase (AMPK) activation leads to phosphofructokinase-2 and-1 (PFK-2 and PFK-1) phosphorylation and the development of pale, soft, and exudative (PSE) conditions in porcine longissimus muscle. J Agric Food Chem. 54(15):5583–5589.
- Soares AL, Ida EI, Miyamoto S, HernÁndez-Blazquez FJ, Olivo R, Pinheiro JW, Shimokomaki M. 2003. Phospholipase A2 activity in poultry PSE, pale, soft, exudative, meat. J Food Biochemistry. 27(4):309–320.
- Strobel P, Galaz A, Villaroel-Espíndola F, Apaoblaza A, Slebe JC, Jerez-Timaure N, Gallo C, Ramírez-Reveco A. 2021. Temperature, but not excess of glycogen, regulates “in vitro” AMPK activity in muscle samples of steer carcasses. PLoS One. 16(1):e0229480.
- Sun XB, Huang JC, Li TT, Ang Y, Xu XL, Huang M. 2019. Effects of preslaughter shackling on postmortem glycolysis, meat quality, changes of water distribution, and protein structures of broiler breast meat. Poult Sci. 98(9):4212–4220.
- Van Laack R, Liu CH, Smith M, Loveday H. 2000. Characteristics of pale, soft, exudative broiler breast meat. Poult Sci. 79(7):1057–1061.
- Wang R, Liang R, Lin H, Zhu L, Zhang Y, Mao Y, Dong P, Niu L, Zhang M, Luo X. 2017. Effect of acute heat stress and slaughter processing on poultry meat quality and postmortem carbohydrate metabolism. Poult Sci. 96(3):738–746.
- Xing T, Xu X, Jiang N, Deng SL. 2016. Effect of transportation and pre-slaughter water shower spray with resting on AMP-activated protein kinase, glycolysis and meat quality of broilers during summer. Anim Sci J. 87(2):299–307.
- Zhang L, Wang X, Li J, Zhu X, Gao F, Zhou G. 2017. Creatine monohydrate enhances energy status and reduces glycolysis via inhibition of AMPK pathway in pectoralis major muscle of transport-stressed broilers. J Agric Food Chem. 65(32):6991–6999.
- Zhu XS, Xu XL, Min HH, Zhou GH. 2012. Occurrence and characterization of pale, soft, exudative-like broiler muscle commercially produced in China. J Integr Agr. 11(8):1384–1390.
