 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Gating of mechanosensitive (MS) channels is driven by a hierarchical cascade of movements and deformations of transmembrane helices in response to bilayer tension. Determining the intrinsic mechanical properties of the individual transmembrane helices is therefore central to understanding the intricacies of the gating mechanism of MS channels. We used a constant-force steered molecular dynamics (SMD) approach to perform unidirectional pulling tests on all the helices of MscL in M. tuberculosis and E. coli homologs. Using this method, we could overcome the issues encountered with the commonly used constant-velocity SMD simulations, such as low mechanical stability of the helix during stretching and high dependency of the elastic properties on the pulling rate. We estimated Young's moduli of the α-helices of MscL to vary between 0.2 and 12.5 GPa with TM2 helix being the stiffest. We also studied the effect of water on the properties of the pore-lining TM1 helix. In the absence of water, this helix exhibited a much stiffer response. By monitoring the number of hydrogen bonds, it appears that water acts like a ‘lubricant’ (softener) during TM1 helix elongation. These data shed light on another physical aspect underlying hydrophobic gating of MS channels, in particular MscL.
Introduction
The mechanosensitive channel of large conductance, MscL acts as a safety valve in bacterial membranes allowing the bacteria to release the turgor pressure under hypoosmotic conditions.Citation1-3 For this to occur, tension is transmitted directly from the lipid bilayer to MscL resulting in a conformational change that leads to channel gating.Citation4,5 To date, the structure and function of MscL are well characterized using a plethora of experimental and computational approaches.Citation1,6-13 MscL is a homopentamer with each monomer consisting of 4 α-helices, 2 transmembrane (TM1 and TM2) and 2 cytoplasmic (N- and C-terminal) helices ().Citation14 The TM1 helix, which lines the pore, is coupled to the membrane via a juxtaposed horizontal amphipathic N-terminal helix residing at the lipid-solvent interface.Citation15,16 The TM2 helix faces the bilayer and spans the full bilayer thickness () and is connected to a coiled-coil C-terminal helical bundle of the channel pentamer.Citation14,17 Due to in-plane expansion and thinning of the bilayer, the pore-lining TM1 helix is dragged by the N-terminus and tilts and rotates, resulting in solvation of a hydrophobic gate ().Citation6,9,18-20 Therefore, both the TM1 helix and the N-terminus undergo a considerable axial force in the open state.Citation16,21 MscL activation leads to opening of a large non-selective pore with a diameter of ∼ 3 nm and a unitary conductance in the range of ∼ 3 nS.Citation6,11,20
Figure 1. Three dimensional structures of the closed (resting) state of (A) MtMscL (PDB code: 2OAR) on the left and a homology model of EcMscL obtained based on 2OAR and 4LKU on the right side.Citation14,16,17 (B) A subunit of EcMscL has been shown after equilibrated in POPE lipid bilayer. The residues that anchor the protein to the membrane (F7, F10) have been highlighted.Citation15 (C) TM1 helix becomes aligned with the N-terminus in the open state.Citation16
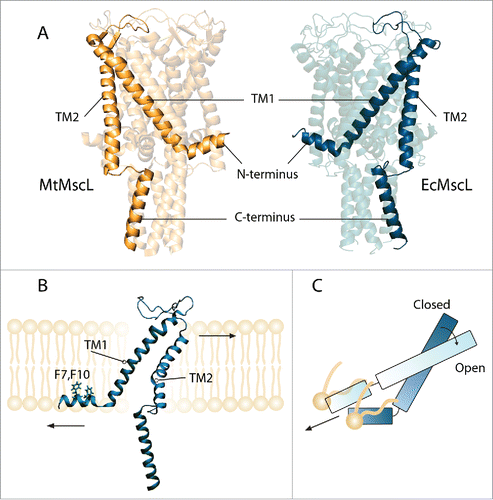
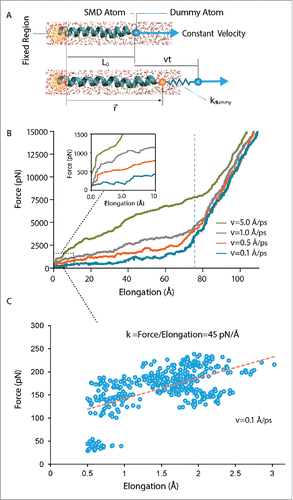
Figure 2. Steered molecular dynamics (SMD) simulation using constant-velocity (CV) method. In this method, the α carbons of the first 2 residues (ILE14 and VAL15) in TM1 helix of MtMscL have been fixed on the left hand side. Helical properties such as helix initial length, L0, pulling rate, v, time t, vector position of the helix end, , and the assigned spring constant of the dummy atom,
, have been indicated. (A) A schematic unidirectional pulling test on the TM1 helix of MtMscL solvated in water. (B) The force versus elongation (ΔL) curve during constant-velocity simulations. The typical spring constant here is 3 kcal/mol/Å2 (i.e. ∼210 pN/Å). Four different constant velocities have been assigned to the α carbon of the dummy atom, from 0.1 Å/ps, to 5 Å/ps. The helix elongation trend can be divided into 2 main quasi-linear regions. As shown in the inset multiple regions can be observed in the first regions which are indicative of sequential rupture of the hydrogen bonds (i.e., breakage of the hydrogen bonds during the pulling test). The length of TM1 increases up to a maximum length (>100 % of elongation), when the helix becomes almost unfolded due to the applied stretch. Before this point, helix response is rate dependent, i.e. the higher the pulling rate, the stiffer is the helix response. After this point the helix behavior becomes much stiffer and not rate dependent. It should be noted that the rationale for testing the helix behavior upon application of such high forces and large elongations was to find the reason for the rate dependency observed in the CV simulations. (C) A typical helical behavior under low forces. The spring constant here was 0.6 kcal/mol/Å2 (i.e., ∼42 pN/Å) and the pulling rate was 0.1 Å/ps. Similar to previous studies,Citation39 the slope of the best linear fit to the initial part of the diagram (dashed red line) indicates the elasticity modulus, which is ∼0.7 kcal/mol/Å2 (i.e., ∼45 pN/Å).
Gating in MscL occurs on a timescale in the order of milliseconds and is accompanied by series of movements resulting in large helical deformations that are dependent on the mechanical properties of each individual helix. In order to understand the intricacies of the gating mechanisms of these MS channels it is important that we fully determine the intrinsic mechanical properties of the individual TM helices. This information will also provide useful inputs and constraints for future computational analyses including coarse-grain molecular dynamics (MD) and continuum mechanics simulations that are routinely employed for investigating the global structural changes during MS channel activation.Citation22-29 Furthermore, given the growing interest in using MscL as nanosensor and/or nanovalve in biotechnology,Citation30-37 there are still unanswered questions regarding the intra-molecular mechanics of this family of MS channels. For example, how much force does each α-helix bear during the gating process? How do the helix properties differ in different environments (e.g., in water)? Also, is there any difference between mechanical properties of the helices in different MscL homologs?
The mechanical properties of different proteins have been studied using various theoreticalCitation38-40 and experimental techniques such as steered molecular dynamics (SMD), atomic force microscopy (AFM), and laser optical tweezers.Citation38,39,41-45 These methods, in combination with X-ray crystallography and cryo-EM structures, have greatly advanced our knowledge of protein structure, mechanical strength and function. Among these approaches, SMD provides atomistic descriptions of the mechanical behavior of different proteins.Citation46-48 This can be achieved using 2 different approaches, either by applying a constant-force (CF) or a constant-velocity (CV). The advantages and disadvantages of these approaches are however not entirely clear.
In this study, we compared both CV and CF SMD for performing unidirectional pulling tests on the TM helices of MscL from M. tuberculosis and E. coli homologs. Using large number of simulations and the TM1 helix of MscL as our model α-helix, we demonstrated that the CF method is more reliable and accurate compared to the CV method in determining the mechanical properties of α-helices but more expensive in terms of the computational costs. We also examined the effect of water on the elastic behavior of the pore lining TM1 helix of MscL. We showed that in its hydrated state, the TM1 helix of MtMscL became more than 5 times more flexible, while the elasticity of the TM1 helix in EcMscL did not vary significantly. The framework and mechanical properties estimated here have important consequences for ‘hydrophobic lock-dependent gating’Citation49-54 of ion channels and may apply to many other prokaryotic and eukaryotic ion channels and proteins in general.
Materials and methods
All simulations were performed with the NAMD 2-10 package, where CHARM36 Force Field was employed. Visual Molecular Dynamics (VMD)Citation55 and Pymol were used for all visualizations. The crystal structure of the M. tuberculosis MscL (2OAR) was used from the Protein Data Bank (). The 3D structure of EcMscL () was generated based on the crystal structure of the MscL homolog of M. tuberculosis (PDB ID: 2OAR) and crystal structure of E. coli C-terminus (PDB ID: 4LKU)Citation17 using Phyre2Citation56 and Swiss-Model.Citation57
Steered molecular dynamics (SMD) simulation
In SMD, external forces/velocities are applied to biomolecules to manipulate them in order to probe and determine their different mechanical properties.Citation46,58,59 The application of external forces/velocities accelerates processes that are otherwise too slow to model by using non-steered molecular simulations.Citation6,28 We used 2 different types of steered molecular dynamics simulation, namely constant-velocity (CV) and constant-force (CF). The CV method is also called “Moving Constraints” method in some versions of NAMD and other studies.Citation60 In this method, we have restrained the 2 α carbons of the first 2 residues (e.g., ILE14 and VAL15 in the case of TM1 helix of MtMscL) on each helix using a strong harmonic restrain constant of 12 kcal/mol/Å2. Table S1 and S2 in the Supporting Material contain more details about all the helices studied here. The last α carbon at the end of the helix, which is called the SMD atom, is attached to another ‘dummy’ atom by a harmonic spring (e.g., ILE46 in the TM1). The dummy atom is then moved with a given constant-velocity vector (). We used a wide range of velocities for the TM1 helix (0.1 to 5 Å/ps) to fully investigate the effect of this parameter on estimation of the elastic moduli of our helices. In the CV method the force vector, , that has been applied on the SMD atoms can be calculated using Eq. Equation1
(1)
(1) ,Citation39
(1)
(1)
Where k is the stiffness of the system, is the pulling velocity, t is time,
is the position of the helix end (i.e.,
is the displacement) and
is the vector that indicates the pulling direction. It is also noteworthy, that the elongation (or strain) presented in the figures of this study, are the helical elongation which is the distance between the fixed atom and the SMD atom. Therefore, the resulting k from the slope of force versus elongation diagrams only indicates the elasticity constant of the helix. To estimate the Young's modulus, E, from the spring stiffness, k, we used Eq. Equation2
(2)
(2) as follows.
(2)
(2)
Where is the initial length of helix (Table S1 and S2), and A is the average cross-sectional area of helix. For calculating the helical cross-sectional area, we needed to estimate a radius of gyration for each helix as an average radius to be able to calculate their cross-sectional area using Eqs 3 and 4. The radius of gyration here,
, is the root mean square distance of the atoms from the helix central axis as following.
(3)
(3)
(4)
(4)
Where d and are the in-plane coordinates of each atom and central axis, respectively. N is the total number of atoms. x and y are the coordinates of each atom on the helix except hydrogens when the helix is aligned to the z axis.
and
are the in-plane coordination of the central axis of the helix when the helix is aligned to the z axis. For example, in the case of the TM1 helix, Eq. Equation3
(3)
(3) yields the helical radius of 2.98 Å and thus annular cross-sectional area of 27.9 Å2, which is similar to previously estimated values for the MscL helices.Citation11,61
In the CF method, a constant-force vector is applied onto selected atoms on one end of the helix, while the other end has been restrained. In this method, the α carbons of the first 2 residues (e.g., ILE14 and VAL15 in TM1 helix of MtMscL) were restrained, and the force was applied on the α carbon of the last residue (e.g., ILE46). See Table S1 and S2 for the information about the fixed residues and dummy atoms on each helix. In the case of TM1 for example, a constant-force in the range of 0.1-1.5 kcal/mol/Å (∼7 to 104 pN) was applied on the α carbon atoms of the last residue in the direction defined by a vector. This vector links center of the mass of the fixed atoms to the SMD atom, i.e., it is along the helix axis. For the other helices, the force range may alter given that the applied force was gradually increased to reach the helical strain of ∼6 %. We chose to measure the elastic response of each α-helix in this range as it is the maximum range that the MscL helices undergo during gating. This is a common range that has previously been used for other proteins as well.Citation39,41 After application of each force, the system was allowed to run for 10 ns to stabilize the equilibrium length (Fig. S1). The resultant strain of each helix was calculated by dividing the length of elongation, , by the initial length of the helix,
(i.e.,
). See Table S1 and S2 for the initial lengths of all the helices. The initial 0% strain corresponds to the non-stretched state. For each applied force, the system was permitted to equilibrate for 10 ns (Fig. S1). For calculating the stress resultant, we calculated force/cross - sectional area of the helix (
), where A is calculated using Eq. Equation3
(3)
(3) . The slope of the stress-strain diagram indicates the Young's modulus of the helix. It should be noted that each point on each stress-strain diagram (e.g., ) is the average of 3 separate simulations. Therefore, with the number of forces we used to create reliable stress-strain curves for each helix, we performed an average of 51 simulations for each individual helix.
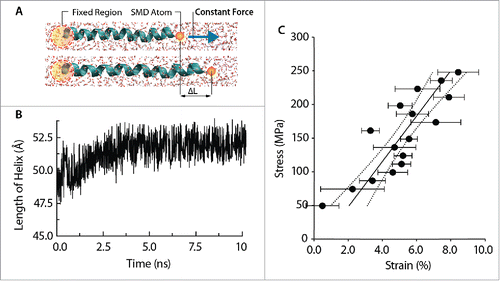
Figure 3. Mechanical behavior of the TM1 helix of MtMscL using constant-force (CF) method (A) A schematic unidirectional pulling of the TM1 helix of MtMscL solvated in water. (B) TM1 helix elongates over time as a result of constant pulling force applied on its end. The TM1 length increases to maximum length of ∼52 Å then fluctuates around this value. (C) Stress-strain curve of the unidirectional traction applied to the TM1 helix in water. The range of axial force applied in these simulations ranges from 0.1 to 1.5 kcal/mol/Å (i.e., from ∼7 to 70 pN). The Young's modulus of the TM1 helix can be calculated from the slope of this curve, which in this case is ∼3.2 ± 0.9 GPa (Mean ± SEM). To obtain each point on the stress-strain curve, 3 simulations were performed. The strain at each force has been averaged over 3 simulations. The Young's modulus has been estimated based on 95 % confidence of both stress and strain axis.
In all of our simulations, a modified Nosé-Hoover Langevin piston pressure control provided in NAMD was utilized to control fluctuations in barostats (at 1 atm). This method is combined with a method of temperature control (at 300 K) (Langevin dynamics) to simulate the NPT ensemble. In order to study the role of water on the mechanical properties of the pore-lining α helix TM1, a series of SMD simulations were carried out in the NVT ensembles for 2 cases: (i) with water and (ii) without water (i.e. in vacuum). The TM1 helix was stretched along its axis in water and in vacuum using CF method. In the cases with water, the α helix was solvated in a 40 × 40 × 90–Å water box using the “SOLVATE” module, with the TIP3P water molecule, in visual molecular dynamics (VMD) software. The helix was held fixed for the first 1 ns by restraining the atomic positions and then, only its backbone was maintained fixed while the rest of the system was allowed to relax for the following 1 ns. Thereafter, the whole system was equilibrated in a NPT ensemble for 10 ns with no restraint. An example of RMSD values during equilibration for the TM1 helix of MtMscL is shown in Figure S1. Care was taken of the charge neutrality in the simulations with water. We used HeliQuest and VMD for our bioinformatics data presented in Table S1 and S2.
Results
The mechanical response of the pore lining TM1 helix
We first compared the 2 SMD methods, constant-velocity (CV) and constant-force (CF), in determining Young's elasticity modulus of the TM1 helix of MtMscL solvated in water. In the CV method, the helix elongates as a result of assigning a velocity to the dummy atom attached to the end of the helix by a virtual spring, while the other end is fixed (, Movie S1). Due to the helix elongation, force is generated in the helix which can be calculated from Eq. Equation1(1)
(1) . The force vs. elongation was monitored over the simulation time for 4 different pulling velocities in the range 0.1 to 5 Å/ps (). Two main semi-linear regions are evident (). In the first, the slope of force versus elongation, representing the stiffness of the helix, is shallower and rate dependent. This region relates to hydrogen bonds and non-bonded interactions in general, which determine the secondary structure of the helix. In the second region the slope of force vs. elongation is steeper, corresponding to greater stiffness than the first region, and is independent of the pulling velocity. This region corresponds to the scenario after all the hydrogen bonds have been broken, so represents the elasticity or stiffness of the helical backbone, which is mainly determined by bonded interactions. Importantly, the rationale for examining the helix behavior upon application of such high forces and large elongations was merely to understand the rate dependency observed in the CV simulations, which also occurs even at very low forces (e.g. <10 pN). It should be noted that the elasticity measurements (elastic constants and Young's moduli) and comparison between the CV and CF methods were done at forces <100 pN (∼6% strain for TM1 helix) as described in the methods.
We have to note that the first region is not perfectly linear. This can be more clearly seen from the inset in . As shown, first there was a steep jump in the force value with little to no change in the helix length, but then the helix length increased in multiple steps as the force increased. Each of these semi-linear domains is shown in the force versus elongation graph (), which indicates the strain dependent behavior of TM1 helix in response to uniaxial force when the CV method was used. Since there was a slight nonlinearity in the helical response and to be able to compare our results with the results obtained from the CF method, we measured the helical properties corresponding to strain < 6 % region (). This is done similar to what has been done in previous studies,Citation39 by measuring the slope of the best linear fit to the portion of force-elongation diagram which is in the strain range of <6% (e.g., for TM1 helix, this strain value corresponds to 2.9 Å elongation). This is the range we chose to measure the elastic response of each α-helix throughout this study unless otherwise specified.
The rationale for doing it is that this is within the range of the strain that the α-helices of EcMscL experience during gating.Citation6,11,16 Also, this range has previously been used for other proteins in previous studies.Citation39,41 As described in the methods, for finding the apparent Young's modulus from the spring constant, we had to define the cross-section for the helix. For example, in the case of the TM1 helix, Eq. Equation3(3)
(3) yields the radius of 2.98 Å and thus annular cross-sectional area of 27.9 Å2, which is similar to previously estimated values for the MscL helices, ∼2.5 Å.Citation11,61 There is however, uncertainty in estimating the Young's elastic modulus of the α-helices from their spring constant (k), since they are not perfectly isotropic structures. One way to overcome this problem is to divide each helix into multiple domains with different geometries. But this would require many more computations, a more complex theory and a detailed geometric model of the helix. Hence, we consider an α-helix to behave as a homogenous material so that the apparent Young's modulus, E, can be expressed as a simple scalar from Eq. Equation2
(2)
(2) . Although there are limitations in applying such a simplified model, it provides insight and describes well the overall mechanical behavior of different α-helices.Citation61 For future work, the helix cross-sectional area could be modeled as its van der Waals (vdW) shape, allowing stress values within the helix to be more precisely calculated based on force over local cross-section, instead of force over a uniform cross-section. This issue may be important if the helix dynamics are associated with bending (flexural) or torsional stiffness—where calculation of the actual cross-sectional area is more important. These more realistic surfaces will also better reflect the “physicochemical” intricacies of MscL in future continuum models, such as when helical charge density has to be taken into account.Citation62
In the constant-force (CF) method, an external constant-force has been applied on one end of the TM1 helix, while the other end was restrained (See Table S1 for the fixed and stressed residues for each individual helix). As a result of axial pulling force, TM1 helix elongates to a stable length very quickly (e.g., in 0.5 ns of simulation). However, we continued the simulation for at least another 10 ns to equilibrate helical length at each force value ( and Fig. S1). This procedure is repeated for the forces up to ∼100 pN which is enough to stretch the helix beyond the length observed in the open state structure of MscL.Citation28 The stress-strain curve describing the helix response to a range of pulling forces is semi–linear (). The slope of this curve indicates the Young's modulus of TM1 solvated in water, which is estimated to be 3.2 ± 0.9 GPa.
Comparison between constant-velocity (CV) and constant-force (CF) method
Given the differences observed in TM1 helix behavior in response to the axial force applied using the CV and CF methods () we further investigated the role of the pulling rate and spring constant of the dummy atoms in our CV simulations in low strain regions (<6%). We performed more than 200 SMD simulations using the CV method with pulling rates between 0.1 Å/ps to 5 Å/ps, as well as a wide range of dummy atom spring constant values from 0.1 kcal/mol/Å2 to 2 kcal/mol/Å2. shows that the helix Young's modulus is dependent on both the pulling rate as well as the spring constant assigned to the dummy atoms. In general, for faster pulling rates, the Young's modulus was larger, i.e., the helix was stiffer. Specifically, as the rate of pulling decreased from 5.0 Å/ps to 0.1 Å/ps, the average of E values decreased from ∼42 GPa to ∼11 GPa. For some pulling rates (e.g., 5.0 Å/ps), higher spring constants of the dummy atoms resulted in a stiffer helix response. Also at higher pulling rates (i.e., 5 Å/ps), there was a considerable fluctuation in the Young's modulus of the helix which became larger as the spring constant of the dummy atoms increased. When we decreased the rate of pulling to 5 × 10−4 Å/ps the resulting spring stiffness of the TM1 reduced to ∼25 pN/Å which corresponds to the Young's modulus ∼4.3 GPa (Eq. Equation2(2)
(2) ; Fig. S2). This rate is closer to but still faster than the actual extension rate of this helix during the MscL gating, which is <5 × 10−7 Å/ps, given that MscL gating occurs in several μs and TM1 helix elongates ∼5 Å during gating.Citation16
Figure 4. Comparing the constant-velocity (CV) method with the constant-force (CF) method for testing the mechanical behavior of α-helices using TM1 helix of MtMscL in water as an example. (A) The effect of pulling rate and stiffness of spring constant of the dummy atoms using constant-velocity method on the mechanical behavior of the TM1 helix of MtMscL. For higher pulling rates (> 1 Å/ps), there is a considerable fluctuation in the Young's modulus depending on the spring constant assigned to the dummy atoms. This fluctuation becomes larger as the spring constant becomes higher. As the rate of pulling decreases from 5.0 Å/ps to 0.1 Å/ps, the average of E values decreases from 42 to 11 GPa. (B) Change in the number of hydrogen bonds of TM1 α helix of MtMscL during the simulation time. The black trace shows the number of hydrogen bonds in the TM1 helix in the CV method, compared to those when the CF method was used. For this typical CV example, the spring constant is 0.6 kcal/mol/Å2 (i.e. ∼42 pN/Å) and the pulling velocity is 0.1 Å/ps. The force used in the CF method was 27 pN. The values of Young's moduli are Mean ± SEM. One-way ANOVA was used for statistical analysis with p-value < 0.05 and it was confirmed in groups with low n number using non-parametric Kruskal-Wallis test.
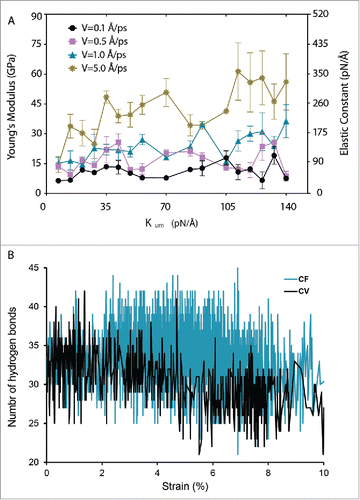
We next compared the number of hydrogen bonds in the TM helix after 10% strain in CV and CF simulations (). As indicated in this figure, the number of hydrogen bonds in the TM1 helix only marginally decreased for the strain <10% compared to the CF method.
Comparing the mechanical properties of the α-helices in the MtMscL and EcMscL
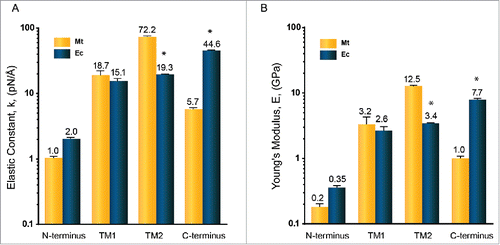
A comparison of the mechanical properties of transmembrane helices between homologues of MscL from M. tuberculosis and E. coli is shown in . The TM1 helix and the N-terminus of MtMscL have similar Young's moduli to those in EcMscL, while the TM2 helix of MtMscL is about 4 times stiffer than in EcMscL. Conversely, the C-terminal helix of EcMscL is about 8 times stiffer than the C-terminal helix of MtMscL. There is no apparent correlation between the length and stiffness of the helix in either homolog (Fig. S3). There is also no correlation between the Young's modulus and hydrophobic moment or number of charges (Fig. S4 and S5 and Table S1 and S2). We also showed that the Young's moduli of the TM2 helix in MtMscL and EcMscL are vastly different, although they have very similar number of hydrogen bonds (Fig. S6).
Figure 5. Mechanical properties of MtMscL and EcMscL α-helices in the presence of water measured by all-atom steered molecular dynamic (SMD) simulation. We used constant-force (CF) method here to estimate (A) the elasticity constant and (B) the Young's modulus. The elasticity constant is between 1.0 to 72.2 pN/Å (Young's moduli are between 0.2 to 12.5 GPa). Overall, the mechanical properties of MscL α-helices of both species are similar to each other except for their TM2 and C-terminal helices. TM2 helix in MtMscL is almost 4 times stiffer than the TM2 helix of EcMscL. But the C-terminal helix of EcMscL is about 8 times stiffer than the C-terminal helix of MtMscL. The values of Young's moduli are Mean ± SEM for n = 3. Each Young's modulus is obtained from the stress-strain graphs exemplified in . Student's t-test was used for statistical analysis between each α-helix in MtMscL with its corresponding α-helix in EcMscL. The differences were considered significant for *p-value < 0.05.
Applying steered forces on the specific parts of the pentameric MscL to gate the channel has been studied before to explore the gating mechanism of MscL.Citation6,63 Applying force on the MscL does not give us clear information about the material properties of each component because the result of such SMD simulation depends on: 1) the position on the protein that these forces are applied to, 2) the effect of surrounding lipidCitation64 and 3) the level of difference between the material properties of different helices and/or loops. Therefore, full atomistic calculations are required to derive physical parameters also useful for mesoscopic and continuum modeling of MscL in “membrane protein lattices” models. This “bottom-up” approach enables modeling multiple proteins (channels) in an implicit membrane to capture larger time and length scale phenomena.
Effect of water on the elasticity of TM1 helix
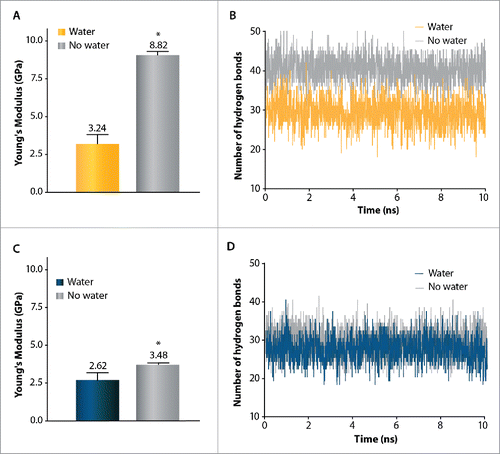
shows the influence of solvation in water on the properties of the TM1 helix obtained using constant-force method SMD simulation. We specifically chose TM1 helix as it forms the MscL pore and thus we were interested to see how its properties altered as it became hydrated. The intention here was to determine a range for the stiffness variation between these 2 conditions (hydrated and dehydrated). Solvation in water decreased the stiffness of the TM1 helix in both MtMscL and EcMscL (E = 3.2 ± 0.9 GPa in water and 8.8 ± 0.2 in vacuo for MtMscL and E = 2.6 ± 0.5 GPa in water and 3.5 ± 0.1 GPa in vacuo for EcMscL) ( and C). To examine why solvation resulted in a larger change in stiffness for MtMscL than EcMscL we determined the evolution of hydrogen bonds on the TM1 helix during solvation in each case. For MtMscL the number of hydrogen bonds was significantly reduced from ∼41 to ∼30 as the helix becomes solvated (). However, the number of hydrogen bonds did not vary as much when the TM1 helix of EcMscL was solvated in water ().
Figure 6. Effect of water on mechanical properties of the TM1 helix in MtMscL (A,B) and in EcMscL (C,D) using constant-force SMD simulation. (A) The Young's modulus of TM1 helix in MtMscL is E = 3.2 ± 0.9 GPa when it is solvated in water, and E = 8.8 ± 0.2 GPa in the absence of water (vacuum). Thus, in the absence of water (vacuum), TM1 helix is more than 3 times stiffer in MtMscL. (B) Change in number of the hydrogen bonds of TM1 α helix of MtMscL during 10 ns of SMD simulation. About 41 hydrogen bonds stabilize the secondary structure of TM1 helix in vacuum, while when it is solvated in water, the number of hydrogen bonds becomes significantly reduced to ∼30 bonds. This indicates that water acts as a ‘lubricant’ (softener) during TM1 helix elongation in water. (C) The Young's modulus of the TM1 helix of EcMscL solvated in water is E = 2.6 ± 0.5 GPa whereas it is E = 3.5 ± 0.1 GPa in the absence of water (vacuum). (D) The number of hydrogen bonds does not change considerably when the TM1 helix of EcMscL is solvated in water, thus its elasticity modulus only increases by ∼30 %. For all the hydrogen bond calculations, we used a donor-acceptor distance of 3.5 Å and angle cut-off of 40°. The values presented in (A) and (C) are Mean ± SEM for n = 3. Student's t-test was used for statistical analysis and differences were considered significant for *p-value < 0.05.
Discussion
Alpha helices play central roles in various biological processes such as molecular and cellular mechanotransduction, cell mechanics and tissue mechanics and are one of the most important elementary building components of proteins.Citation65 Due to a lack of understanding of how these secondary structural elements respond to mechanical force and how they interact with each other, the stiffness and unfolding of proteins remain unclear at atomistic resolution. In this study we have used steered molecular dynamics approaches to investigate the mechanical properties of transmembrane α helices in MscL.
Constant-force SMD is a more reliable measure of helix stiffness than constant-velocity
We compared 2 widely used methods of steered molecular dynamics (SMD) namely, constant-velocity (CV) and constant-force (CF) in a more detailed and systematic way compared to previous studies using a large number of SMD simulations.Citation66 We monitored the number of hydrogen bonds during pulling the TM1 helix using both methods. We showed that the number of hydrogen bonds in CV method, which determine/stabilize the secondary structure of the helix, quickly and dramatically decreased compared to the CF method during the stretching (). Hence, we postulate that the most important advantage of CF method over CV method is that the secondary structure of the helix remains better conserved during the pulling test in the former method compared to the latter one. Although, this is the case for common pulling rates (1-5 pÅ/s), we should note that the CV and CF methods will not be much different, in terms of breaking the H-bonds, if both performed at the same force level (). The hydrogen bonding state of the helix was also reflected in the rate dependent unfolding during CV SMD which has also been shown in previous studies for different proteins.Citation39,67 Helix behavior was rate dependent only for the strains <100 % because the helical strain in this region is mainly due to the breakage of hydrogen bonds ( and Movie S2). Whereas in higher strains, the helix behavior is not rate dependent as it manifests the helical backbone elasticity.Citation40,67 Therefore, unlike in the CF method, the Young's moduli obtained from CV method are dependent on the pulling rate and the assigned spring constant to the dummy atoms. For example, the Young's modulus of TM1 helix measured by CV method can vary from ∼11 GPa to ∼42 GPa depending on the puling rate ().
Consequently, the absolute Young's modulus obtained from CF method is generally more reliable and reproducible compared to those estimated by using CV method. This information is essential given the great need for employing precise tools for determining the mechanical properties of α-helices of membrane proteins, cytoskeletal elements and collagen-like microfibrils. For instance, it is essential to accurately determine the elasticity of tip links in hair cells given its importance in the force transduction in auditory physiology.Citation46 Previous studies have rigorously used the CV method combined with experiments for measuring the elasticity of tip links which is formed by protocadherin 15 and cadherin 23.Citation46,47 Instead, we suggest more accurate computational elasticity measurements by employing the CF method, which may be used to ensure the accuracy of elasticity constants. Also, provided the essential role of tip links in deafness-related structural defects, this may further uncover the molecular elasticity and physical principles underlying the function of tip links.Citation68,69 However, the down side of this approach is that the number of simulations required to be done to generate a stress-strain curve using the CF method is much larger than using the CV method (51 CF simulations vs. 3 CV simulations for each helix). Thus, the CF method requires a much larger computational cost. Moreover, it has recently been shown that SMD-like techniques can be vastly improved for relatively fast pulling rates, if one combines Minh-Adib's bidirectional estimator with nonlinear WHAM equations to reconstruct and assess PMFs from trajectories.Citation70
Comparison of mechanical properties of T helices of MtMscL and EcMscL
Using the CF method, we determined the Young's moduli of all the helices of MtMscL and EcMscL (). The lowest Young's modulus was found for the N-terminus of MtMscL which is ∼0.2 GPa and the highest determined is for the TM2 helix of MtMscL, which is ∼12.5 GPa. This is well in the range of the elasticity moduli previously measured using various experimental and computational approaches for different proteins ranging from 1 GPa to 30 GPa.Citation71-75 TM1 helix and N-terminal helices of MtMscL have similar mechanical properties to those in EcMscL, which could be due to their higher level of amino acid sequence conservation and identity in these regions. Among all the helices, the Young's modulus of the TM2 helix and the C-terminal helix appear to be vastly different between M. tuberculosis and E. coli. The TM2 helix in MtMscL is much stiffer than TM2 helix in EcMscL. Hence, overall in the transmembrane region, MtMscL is stiffer than EcMscL. Given that TM2 helix is embedded in the lipid bilayer and determines the bilayer hydrophobic length around the channel,Citation20,76 we suggest that the stiffer TM2 helix may also correspond to less sensitivity of MtMscL to bilayer thinning during the gating compared to EcMscL, as previously shown by patch-clamp experiments.Citation77 Conversely, the C-terminal helix in EcMscL is about 8 times stiffer than in MtMscL. However, removal of up to 20 amino acids from the end of EcMscL has been shown to have no significant effect on gating of MscL, but rather may be involved in pH sensitivity.Citation78 At this stage, we are not sure what would be the physiological meaning of having stiffer C-terminal bundle. This will remain to be addressed in the future studies to understand the physiological role of the C-terminus in MscL channels.
Previous reports have shown a dependence of the elasticity and strength of some helix types on their length.Citation67 For example it has been shown that the stiffness of the stack of α-helical ankyrin repeats increases with a decreasing number of repeats.Citation79 In our study, the elasticity modulus did not correlate with the helix length or hydrophobic moment or number of charged residues in this study. In addition, we demonstrated here that the charge and other factors such as hydrophobic moment did not correlate with the helix stiffness of MtMscL and EcMscL (Figs S3, S4, S5 and S6).
Effect of hydration on mechanical properties of TM helices of MscL
MD simulations have previously shown that TM1 helix, which forms the pore, is only partially hydrated in the closed state and it becomes fully hydrated in the open state.Citation6,16 Therefore, we were interested to see how the TM1 helix elasticity changes when it is hydrated. By pulling the TM1 helix of both MtMscL and EcMscL in vacuum and water, we have found that exposure of the TM1 helix to water greatly affects its stiffness. Based on our results (), we suggest that water by interfering with the hydrogen bonds and electrostatic interactions within the helix, acts as a “lubricant” (softener) during the helix elongation. Thus, the Young's modulus of a helix like TM1 of MtMscL decreases significantly in the presence of water. This is not surprising given the previous reports on similar effects on different proteins such as the effect of water on collagen-like micro-fibrils, immunoglobulin domains and spectrin-like proteins.Citation60,80,81 In fact, the physiological function of this class of proteins is regulated by water since they should become softer and thus unfold easier as they become hydrated.Citation80 However, there are other studies which suggest that the elasticity of those α-helices that are primarily determined by the backbone hydrogen bonds do not change significantly when the helix is solvated in water.Citation82 An interesting point here is that water did not change the stiffness of TM1 helix of EcMscL to the same extent as it did in MtMscL. In fact, the effect of water on the elasticity of the TM1 helix in MtMscL was much larger than on the elasticity of the TM1 helix of EcMscL (). We showed that this is analogous to the change in the number of the hydrogen bonds in vacuum and in water (, D). The exact physiological meaning of this difference is unclear at this point. Given the recent computational MscL study, which showed that MscL gates in the presence of water easier than in vacuum, our results further support the importance of hydrophobic interactions for the gating of MS channels and nanopores.Citation49,52,83-85
Conclusion
In summary, we calculated Young's moduli and elastic constants for all the helices in MtMscL and EcMscL, which are 2 important members of the bacterial MS channel family. Given a considerable number of all-atom MD simulations, we demonstrated in this study that the widely used constant-velocity approach is less reliable compared to the constant-force method, which is more accurate for determining Young's modulus of α-helices, although it is computationally much more expensive. Interestingly, we showed not only the helical properties among the helices of MscL were quite different, but also the properties of some of the MscL helices (e.g. TM2 helix) turned out to be different between E. coli and M. tuberculosis homologs. Therefore, in these cases we checked if there was any correlation between the elastic properties and helical length, charge or amino acid sequence. We also demonstrated that water could act as a “lubricant” (softener) during TM1 helix elongation, which lines the channel pore in MscL. Consequently, the data and methods resulting from this study have wide implications for understanding the force-enduring properties of the α-helices of MscL and their mechanistic role in MscL gating. In addition, atomistic calculations to derive parameters are also useful for a mesoscopic bead-spring and “hybrid continuum-atomistic” models of channels using energy and force matching. This “bottom-up” approach will allow us to assemble multiple protein structures by springs (or elastic beam/rods) for capturing larger time and length scale phenomena, such as modeling of a cluster of proteins (channels) in lipid patches or attached to other intra- /extra-cellular elements.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
N.B., O.B., B.M. and Y.J. designed the research; N.B. and O.B. performed the simulations. M.V., R.N., A.P.H., B.M. and Y.J. contributed the computational/analytic tools; N.B., O.B., B.M. and Y.J. analyzed the data; and N.B., O.B., A.P.H., Y.J. and B.M. wrote the manuscript. All authors read and approved the final manuscript.
KCHL_S_1249077.zip
Download Zip (9.7 MB)Acknowledgments
We would like to thank Dr Charles D Cox for insightful discussions and for feedback on the manuscript. Dr Yoshitaka Nakayama and Dr Massimo Vassalli are kindly acknowledged for proofreading of the manuscript. We also acknowledge the Institute for Research in Fundamental Sciences (IPM), the Institute for Nanoscience and Nanotechnology (INST), Sharif University of Technology of Iran and the supercomputing facility at CSIRO, Australia for providing access to the supercomputer equipped with the standard software.
Funding
N.B. has been supported by a University International Postgraduate Award (UIPA) from the University of New South Wales. This project was supported by a grant (APP1044628) and a Principal Research Fellowship to B.M. from the National Health and Medical Research Council of Australia. No potential conflicts of interest were disclosed.
References
- Berrier C, Besnard M, Ajouz B, Coulombe A, Ghazi A. Multiple mechanosensitive ion channels from Escherichia coli, activated at different thresholds of applied pressure. J Membrane Biol 1996; 151:175-87; https://doi.org/10.1007/s002329900068
- Meyer GR, Gullingsrud J, Schulten K, Martinac B. Molecular dynamics study of MscL interactions with a curved lipid bilayer. Biophys J 2006; 91:1630-7; PMID:16751236; https://doi.org/10.1529/biophysj.106.080721
- Sukharev SI, Blount P, Martinac B, Blattner FR, Kung C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature 1994; 368:265-8; PMID:7511799; https://doi.org/10.1038/368265a0
- Phillips R, Ursell T, Wiggins P, Sens P. Emerging roles for lipids in shaping membrane-protein function. Nature 2009; 459:379-85; PMID:19458714; https://doi.org/10.1038/nature08147
- Kung C. A possible unifying principle for mechanosensation. Nature 2005; 436:647-54; PMID:16079835; https://doi.org/10.1038/nature03896
- Corry B, Hurst AC, Pal P, Nomura T, Rigby P, Martinac B. An improved open-channel structure of MscL determined from FRET confocal microscopy and simulation. J Gen Physiol 2010; 136:483-94; PMID:20876362; https://doi.org/10.1085/jgp.200910376
- Nomura T, Cranfield CG, Deplazes E, Owen DM, Macmillan A, Battle AR, Constantine M, Sokabe M, Martinac B. Differential effects of lipids and lyso-lipids on the mechanosensitivity of the mechanosensitive channels MscL and MscS. Proc Natl Acad Sci 2012; 109:8770-5; PMID:22586095; https://doi.org/10.1073/pnas.1200051109
- Perozo E, Cuello LG, Cortes DM, Liu YS, Sompornpisut P. EPR approaches to ion channel structure and function. Novartis Found Symp 2002; 245:146-58; discussion 58-64, 65-8; PMID:12027005; https://doi.org/10.1002/0470868759.ch10
- Sukharev S, Betanzos M, Chiang CS, Guy HR. The gating mechanism of the large mechanosensitive channel MscL. Nature 2001; 409:720-4; PMID:11217861; https://doi.org/10.1038/35055559
- Battle A, Ridone P, Bavi N, Nakayama Y, Nikolaev Y, Martinac B. Lipid–protein interactions: Lessons learned from stress. Biochim Biophys Acta 2015; 1848 (9):1744-56; PMID:25922225; https://doi.org/10.1016/j.bbamem.2015.04.012
- Wang Y, Liu Y, Deberg HA, Nomura T, Hoffman MT, Rohde PR, Schulten K, Martinac B, Selvin P. Single molecule FRET reveals pore size and opening mechanism of a mechano-sensitive ion channel. Elife 2014; 3:e01834; PMID:24550255
- Gullingsrud J, Kosztin D, Schulten K. Structural determinants of MscL gating studied by molecular dynamics simulations. Biophys J 2001; 80:2074-81; PMID:11325711; https://doi.org/10.1016/S0006-3495(01)76181-4
- Iscla I, Wray R, Eaton C, Blount P. Scanning MscL channels with targeted post-translational modifications for functional alterations. PLoS One 2015; 10:e0137994; PMID:26368283; https://doi.org/10.1371/journal.pone.0137994
- Steinbacher S, Bass R, Strop P, Rees DC. Structures of the prokaryotic mechanosensitive channels MscL and MscS. In: Hamill OP, ed. Mechanosensitive Ion Channels, Part A. San Diego: Elsevier Academic Press, Inc., 2007:1-24.
- Iscla I, Wray R, Blount P. On the structure of the N-terminal domain of the MscL channel: helical bundle or membrane interface. Biophys J 2008; 95:2283-91; PMID:18515388; https://doi.org/10.1529/biophysj.107.127423
- Bavi N, Cortes DM, Cox CD, Rohde PR, Liu W, Deitmer JW, Bavi O, Strop P, Hill AP, Rees D, et al. The role of MscL amphipathic N terminus indicates a blueprint for bilayer-mediated gating of mechanosensitive channels. Nat Commun 2016; 7:11984; PMID:27329693; https://doi.org/10.1038/ncomms11984
- Walton TA, Rees DC. Structure and stability of the C-terminal helical bundle of the E. coli mechanosensitive channel of large conductance. Protein Sci 2013; 22:1592-601; PMID:24038743; https://doi.org/10.1002/pro.2360
- Iscla I, Blount P. Sensing and responding to membrane tension: the bacterial MscL channel as a model system. Biophys J 2012; 103:169-74; PMID:22853893; https://doi.org/10.1016/j.bpj.2012.06.021
- Blount P, Moe PC. Bacterial mechanosensitive channels: integrating physiology, structure and function. Trends Microbiol 1999; 7:420-4; PMID:10498951; https://doi.org/10.1016/S0966-842X(99)01594-2
- Perozo E, Cortes DM, Sompornpisut P, Kloda A, Martinac B. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature 2002; 418:942-8; PMID:12198539; https://doi.org/10.1038/nature00992
- Bavi N, Cox CD, Perozo E, Martinac B. Towards a structural blueprint for bilayer-mediated channel mechanosensitivity. Channels 2016; 7:00-.
- Bavi O, Vossoughi M, Naghdabadi R, Jamali Y. The Combined effect of hydrophobic mismatch and bilayer local bending on the regulation of mechanosensitive ion channels. PloS one 2016; 11:e0150578; PMID:26958847; https://doi.org/10.1371/journal.pone.0150578
- Bavi O, Cox CD, Vossoughi M, Naghdabadi R, Jamali Y, Martinac B. Influence of global and local membrane curvature on mechanosensitive ion channels: A finite element approach. Membranes (Basel) 2016; 6(1):14 PMID:26861405
- Bavi O, Vossoughi M, Naghdabadi R, Jamali Y. The effect of local bending on gating of MscL using a representative volume element and finite element simulation. Channels (Austin) 2014; 8:344-9; PMID:25478623; https://doi.org/10.4161/chan.29572
- Wiggins P, Phillips R. Analytic models for mechanotransduction: gating a mechanosensitive channel. Proc Natl Acad Sci U S A 2004; 101:4071-6; PMID:15024097; https://doi.org/10.1073/pnas.0307804101
- Chen X, Cui Q, Tang Y, Yoo J, Yethiraj A. Gating mechanisms of mechanosensitive channels of large conductance, I: a continuum mechanics-based hierarchical framework. Biophys J 2008; 95:563-80; PMID:18390626; https://doi.org/10.1529/biophysj.107.128488
- Bavi N, Nakayama Y, Bavi O, Cox CD, Qin QH, Martinac B. Biophysical implications of lipid bilayer rheometry for mechanosensitive channels. Proc Natl Acad Sci U S A 2014; 111:13864-9; PMID:25201991; https://doi.org/10.1073/pnas.1409011111
- Deplazes E, Louhivuori M, Jayatilaka D, Marrink SJ, Corry B. Structural investigation of MscL gating using experimental data and coarse grained MD simulations. PLoS Comput Biol 2012; 8:e1002683; PMID:23028281; https://doi.org/10.1371/journal.pcbi.1002683
- Sansom MS, Scott KA, Bond PJ. Coarse-grained simulation: a high-throughput computational approach to membrane proteins. Biochem Soc Trans 2008; 36:27-32; PMID:18208379; https://doi.org/10.1042/BST0360027
- Cox CD, Bae C, Ziegler L, Hartley S, Nikolova-Krstevski V, Rohde PR, Ng CA, Sachs F, Gottlieb PA, Martinac B. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat Commun 2016; 7:10366; PMID:26785635; https://doi.org/10.1038/ncomms10366
- Nakayama Y, Mustapić M, Ebrahimian H, Wagner P, Kim JH, Al Hossain MS, Horvat J, Martinac B. Magnetic nanoparticles for “smart liposomes”. Eur Biophy J 2015; 44:647-54; PMID:26184724; https://doi.org/10.1007/s00249-015-1059-0
- Iscla I, Eaton C, Parker J, Wray R, Kovacs Z, Blount P. Improving the design of a MscL-based triggered nanovalve. Biosensors (Basel) 2013; 3:171-84; PMID:23678232; https://doi.org/10.3390/bios3010171
- Yang LM, Wray R, Parker J, Wilson D, Duran RS, Blount P. Three routes to modulate the pore size of the MscL channel/nanovalve. ACS Nano 2012; 6:1134-41; PMID:22206349; https://doi.org/10.1021/nn203703j
- Yang LM, Blount P. Manipulating the permeation of charged compounds through the MscL nanovalve. FASEB J 2011; 25:428-34; PMID:20930114; https://doi.org/10.1096/fj.10-170076
- Kocer A, Walko M, Meijberg W, Feringa BL. A light-actuated nanovalve derived from a channel protein. Science 2005; 309:755-8; PMID:16051792; https://doi.org/10.1126/science.1114760
- Kocer A, Walko M, Feringa BL. Synthesis and utilization of reversible and irreversible light-activated nanovalves derived from the channel protein MscL. Nat Protoc 2007; 2:1426-37; PMID:17545979; https://doi.org/10.1038/nprot.2007.196
- Pacheco-Torres J, Mukherjee N, Walko M, Lopez-Larrubia P, Ballesteros P, Cerdan S, Kocer A. Image guided drug release from pH-sensitive Ion channel-functionalized stealth liposomes into an in vivo glioblastoma model. Nanomedicine 2015; 11:1345-54; PMID:25888277
- Buehler MJ. Atomistic and continuum modeling of mechanical properties of collagen: elasticity, fracture, and self-assembly. J Mat Res 2006; 21:1947-61; https://doi.org/10.1557/jmr.2006.0236
- Lorenzo AC, Caffarena ER. Elastic properties, Young's modulus determination and structural stability of the tropocollagen molecule: a computational study by steered molecular dynamics. J Biomechan 2005; 38:1527-33; https://doi.org/10.1016/j.jbiomech.2004.07.011
- Sikora M, Sułkowska JI, Cieplak M. Mechanical strength of 17 134 model proteins and cysteine slipknots. PLoS Comput Biol 2009; 5:e1000547; PMID:19876372; https://doi.org/10.1371/journal.pcbi.1000547
- Buehler MJ, Keten S, Ackbarow T. Theoretical and computational hierarchical nanomechanics of protein materials: Deformation and fracture. Prog Mat Sci 2008; 53:1101-241; https://doi.org/10.1016/j.pmatsci.2008.06.002
- Tskhovrebova L, Trinick J, Sleep J, Simmons R. Elasticity and unfolding of single molecules of the giant muscle protein titin. Nature 1997; 387:308-12; PMID:9153398; https://doi.org/10.1038/387308a0
- Gautieri AV, Vesentini S, Redaelli A, Buehler MJ. Nanomechanics of collagen microfibrils from the atomistic scale up. Nano Lett 2011; 11:757-66; PMID:21207932; https://doi.org/10.1021/nl103943u
- Wolny M, Batchelor M, Knight PJ, Paci E, Dougan L, Peckham M. Stable single α-helices are constant force springs in proteins. J Biol Chem 2014; 289:27825-35; PMID:25122759; https://doi.org/10.1074/jbc.M114.585679
- Shayegan M, Forde NR. Microrheological characterization of collagen systems: from molecular solutions to fibrillar gels. PloS One 2013; 8:e70590; PMID:23936454; https://doi.org/10.1371/journal.pone.0070590
- Sotomayor M, Weihofen WA, Gaudet R, Corey DP. Structure of a force-conveying cadherin bond essential for inner-ear mechanotransduction. Nature 2012; 492:128-32; PMID:23135401; https://doi.org/10.1038/nature11590
- Sotomayor M, Weihofen WA, Gaudet R, Corey DP. Structural determinants of cadherin-23 function in hearing and deafness. Neuron 2010; 66:85-100; PMID:20399731; https://doi.org/10.1016/j.neuron.2010.03.028
- Marszalek PE, Lu H, Li H, Carrion-Vazquez M, Oberhauser AF, Schulten K, Fernandez JM. Mechanical unfolding intermediates in titin modules. Nature 1999; 402:100-3; PMID:10573426; https://doi.org/10.1038/47083
- Aryal P, Sansom MS, Tucker SJ. Hydrophobic gating in ion channels. J Mol Biol 2015; 427:121-30; PMID:25106689; https://doi.org/10.1016/j.jmb.2014.07.030
- Anishkin A, Sukharev S. Water dynamics and dewetting transitions in the small mechanosensitive channel MscS. Biophys J 2004; 86:2883-95; PMID:15111405; https://doi.org/10.1016/S0006-3495(04)74340-4
- Yoshimura K, Batiza A, Schroeder M, Blount P, Kung C. Hydrophilicity of a single residue within MscL correlates with increased channel mechanosensitivity. Biophys J 1999; 77:1960-72; PMID:10512816; https://doi.org/10.1016/S0006-3495(99)77037-2
- Beckstein O, Sansom MS. Liquid-vapor oscillations of water in hydrophobic nanopores. Proc Natl Acad Sci U S A 2003; 100:7063-8; PMID:12740433; https://doi.org/10.1073/pnas.1136844100
- Sotomayor M, Schulten K. Molecular dynamics study of gating in the mechanosensitive channel of small conductance MscS. Biophys J 2004; 87:3050-65; PMID:15339798; https://doi.org/10.1529/biophysj.104.046045
- Blount P, Moe PC. Bacterial mechanosensitive channels: integrating physiology, structure and function. Trends Microbiol 1999; 7:420-4; PMID:10498951; https://doi.org/10.1016/S0966-842X(99)01594-2
- Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph 1996; 14:33-8, 27-8; PMID:8744570; https://doi.org/10.1016/0263-7855(96)00018-5
- Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 2015; 10:845-58; PMID:25950237; https://doi.org/10.1038/nprot.2015.053
- Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M, Bordoli L, Schwede, T. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acid Res 2014; p.gku340; PMID:24782522; https://doi.org/10.1093/nar/gku340
- Ackbarow T, Chen X, Keten S, Buehler MJ. Hierarchies, multiple energy barriers, and robustness govern the fracture mechanics of alpha-helical and beta-sheet protein domains. Proc Natl Acad Sci U S A 2007; 104:16410-5; PMID:17925444; https://doi.org/10.1073/pnas.0705759104
- Sotomayor M, Schulten K. The allosteric role of the Ca 2+ switch in adhesion and elasticity of C-cadherin. Biophys J 2008; 94:4621-33; PMID:18326636; https://doi.org/10.1529/biophysj.107.125591
- Zhang D, Chippada U, Jordan K. Effect of the structural water on the mechanical properties of collagen-like microfibrils: a molecular dynamics study. Annal Biomed Eng 2007; 35:1216-30; https://doi.org/10.1007/s10439-007-9296-8
- Tang Y, Cao G, Chen X, Yoo J, Yethiraj A, Cui Q. A finite element framework for studying the mechanical response of macromolecules: application to the gating of the mechanosensitive channel MscL. Biophys J 2006; 91:1248-63; PMID:16731564; https://doi.org/10.1529/biophysj.106.085985
- Zhu L, Wu J, Liu L, Liu Y, Yan Y, Cui Q, et al. Gating mechanism of mechanosensitive channel of large conductance: a coupled continuum mechanical-continuum solvation approach. Biomech Model Mechanobiol 2016:1-20; https://doi.org/10.1007/s10237-016-0783-4
- Gullingsrud J, Schulten K. Gating of MscL studied by steered molecular dynamics. Biophys J 2003; 85:2087-99; PMID:14507677; https://doi.org/10.1016/S0006-3495(03)74637-2
- Laganowsky A, Reading E, Allison TM, Ulmschneider MB, Degiacomi MT, Baldwin AJ, Robinson CV. Membrane proteins bind lipids selectively to modulate their structure and function. Nature 2014; 510:172-5; PMID:24899312; https://doi.org/10.1038/nature13419
- Kendrew J, Dickerson R, Strandberg B, Hart R, Davies D, Phillips D, et al. Structure of myoglobin: A three-dimensional Fourier synthesis at 2 Å. resolution. Nature 1960; 185(4711):422-7.
- Lu H, Schulten K. The key event in force-induced unfolding of titin's immunoglobulin domains. Biophys J 2000; 79:51-65; PMID:10866937; https://doi.org/10.1016/S0006-3495(00)76273-4
- Bertaud J, Hester J, Jimenez DD, Buehler MJ. Energy landscape, structure and rate effects on strength properties of alpha-helical proteins. J Phys Condens Matter 2009; 22:035102; PMID:21386278; https://doi.org/10.1088/0953-8984/22/3/035102
- Alagramam KN, Goodyear RJ, Geng R, Furness DN, van Aken AF, Marcotti W, Kros CJ, Richardson GP. Mutations in protocadherin 15 and cadherin 23 affect tip links and mechanotransduction in mammalian sensory hair cells. PLoS One 2011; 6:e19183; PMID:21532990; https://doi.org/10.1371/journal.pone.0019183
- Alagramam KN, Murcia CL, Kwon HY, Pawlowski KS, Wright CG, Woychik RP. The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat Genet 2001; 27:99-102; PMID:11138007
- Ngo VA, Kim I, Allen TW, Noskov SY. Estimation of potentials of mean force from nonequilibrium pulling simulations using both minh-adib estimator and weighted histogram analysis method. J Chem Theory Comput 2016; 12:1000-10; PMID:26799775; https://doi.org/10.1021/acs.jctc.5b01050
- Solar M, Buehler MJ. Comparative analysis of nanomechanics of protein filaments under lateral loading. Nanoscale 2012; 4:1177-83; PMID:22193831; https://doi.org/10.1039/C1NR11260K
- Hawkins RJ, McLeish TC. Dynamic allostery of protein alpha helical coiled-coils. J Royal Soc Interface 2006; 3:125-38; https://doi.org/10.1098/rsif.2005.0068
- Knowles TP, Fitzpatrick AW, Meehan S, Mott HR, Vendruscolo M, Dobson CM, Welland ME. Role of intermolecular forces in defining material properties of protein nanofibrils. Science 2007; 318:1900-3; PMID:18096801; https://doi.org/10.1126/science.1150057
- Kol N, Adler-Abramovich L, Barlam D, Shneck RZ, Gazit E, Rousso I. Self-assembled peptide nanotubes are uniquely rigid bioinspired supramolecular structures. Nano Lett 2005; 5:1343-6; PMID:16178235; https://doi.org/10.1021/nl0505896
- Heim AJ, Matthews WG, Koob TJ. Determination of the elastic modulus of native collagen fibrils via radial indentation. Applied Phys Lett 2006; 89:181902; https://doi.org/10.1063/1.2367660
- Perozo E, Kloda A, Cortes DM, Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat Struct Biol 2002; 9:696-703; PMID:12172537; https://doi.org/10.1038/nsb827
- Mukherjee N, Jose MD, Birkner JP, Walko M, Ingolfsson HI, Dimitrova A, Arnarez C, Marrink SJ, Koçer A. The activation mode of the mechanosensitive ion channel, MscL, by lysophosphatidylcholine differs from tension-induced gating. FASEB J 2014; 28:4292-302; PMID:24958207; https://doi.org/10.1096/fj.14-251579
- Kloda A, Ghazi A, Martinac B. C-terminal charged cluster of MscL, RKKEE, functions as a pH sensor. Biophys J 2006; 90:1992-8; PMID:16387769; https://doi.org/10.1529/biophysj.105.075481
- Lee G, Abdi K, Jiang Y, Michaely P, Bennett V, Marszalek PE. Nanospring behaviour of ankyrin repeats. Nature 2006; 440:246-9; PMID:16415852; https://doi.org/10.1038/nature04437
- Ortiz V, Nielsen SO, Klein ML, Discher DE. Unfolding a linker between helical repeats. J Mol Biol 2005; 349:638-47; PMID:15896349; https://doi.org/10.1016/j.jmb.2005.03.086
- Lu H, Schulten K. Steered molecular dynamics simulation of conformational changes of immunoglobulin domain I27 interprete atomic force microscopy observations. Chem Phys 1999; 247:141-53; https://doi.org/10.1016/S0301-0104(99)00164-0
- Choe S, Sun SX. The elasticity of α-helices. J Chem Phys 2005; 122:244912; PMID:16035821; https://doi.org/10.1063/1.1940048
- Sawada Y, Sokabe M. Molecular dynamics study on protein–water interplay in the mechanogating of the bacterial mechanosensitive channel MscL. Eur Biophys J 2015; 44:531-43; PMID:26233760; https://doi.org/10.1007/s00249-015-1065-2
- Kocer A. Mechanisms of mechanosensing - mechanosensitive channels, function and re-engineering. Curr Opin Chem Biol 2015; 29:120-7; PMID:26610201; https://doi.org/10.1016/j.cbpa.2015.10.006
- Anishkin A, Akitake B, Kamaraju K, Chiang CS, Sukharev S. Hydration properties of mechanosensitive channel pores define the energetics of gating. J Phys Condens Matter 2010; 22:454120; PMID:21339607; https://doi.org/10.1088/0953-8984/22/45/454120
