Abstract
Monoclonal antibodies and antibody-like molecules represent a fast-growing class of bio-therapeutics that has rapidly transformed patient care in a variety of disease indications. The discovery of antibodies that bind to particular targets with high affinity is now a routine exercise and a variety of in vitro and in vivo techniques are available for this purpose. However, it is still challenging to identify antibodies that, in addition to having the desired biological effect, also express well, remain soluble at different pH levels, remain stable at high concentrations, can withstand high shear stress, and have minimal non-specific interactions. Many promising antibody programs have ultimately failed in development due to the problems associated with one of these factors. Here, we present a simple high-performance liquid chromatography (HPLC)-based screening method to assess these developability factors earlier in discovery process. This method is robust and requires only microgram quantities of proteins. Briefly, we show that for antibodies injected on a commercially available pre-packed Zenix HPLC column, the retention times are inversely related to their colloidal stability with antibodies prone to precipitation or aggregation retained longer on the column with broader peaks. By simply varying the salt content of running buffer, we were also able to estimate the nature of interactions between the antibodies and the column. We believe this approach should generally be applicable to assessment of the developability of other classes of bio-therapeutic molecules, and that the addition of this simple tool early in the discovery process will lead to selection of molecules with improved developability characteristics.
Abbreviations
| mAb | = | monoclonal antibody |
| scFv | = | single chain variable fragment |
| SMAC | = | standup monolayer chromatography |
| CIC | = | cross-interaction chromatography |
| SEC | = | size exclusion chromatography |
| AC-SINS | = | affinity capture self-interaction nanoparticle spectroscopy |
| HTP | = | high throughput |
| SEC | = | size exclusion chromatography |
| DSF | = | differential scanning fluorimetry |
| Tm | = | melting temperature |
Introduction
In the last 3 decades, substantial advances have been made in antibody research and development (R&D) technologies; as a result, therapeutic antibodies have become the fastest growing class of biopharmaceuticals.Citation1-5 Strong demand for antibodies with high affinity and specificity has fueled the growth of antibody discovery technologies, and arrays of in vitro and in vivo approaches are now available for rapid generation of antibodies against any target of interest.Citation6-12
To be a viable drug candidate, any new antibody from a discovery program needs to have high bioactivity and certain developability attributes, such as high expression, good solubility over wide pH and salt range, and a relatively long shelf life. In addition, for subcutaneous applications (due to the constraints of low dosage volume), it should be capable of being formulated at very high concentrations under conditions that maintain low viscosity, and homogeneity.Citation13-15 Thus, solubility and stability are 2 important biophysical properties for the success of any antibody development program. In the past, however, most discovery programs have been focused on finding high affinity antibodies against targets of interest, and little attention was paid early on to other developability attributes. There have been multiple failures of promising programs in development due to substantial problems with at least one of the developability factors, and these issues have led to considerable reevaluation of subsequent discovery approaches. Because of this, multiple techniques are now employed to assess these developability factors earlier in discovery programs.Citation16-18 Approaches include: (1) use of in silico tools, e.g., SAPCitation19, that predict aggregation propensity of antibodies via hydrophobic and electrostatic interactions; (2) use of thermalCitation20 or guanidine stress to assess the relative stability of various variants; (3) use of cross interaction chromatographyCitation21 where mAbs are passed through a column conjugated with a pool of polyclonal serum antibodies, with the mAbs that have a tendency to self-associate being retained longer on the column; and (4) affinity capture self-interaction nanoparticle spectroscopy (AC-SINS)Citation22,23 where gold nanoparticles are first coated with a polyclonal anti-human IgG, and then the mAb of interest is captured from dilute solutions. In the latter approach, antibodies that have a tendency to self-aggregate lead to a reduction in inter-particle separation distance, which can then be quantified by change in wavelength of maximum absorbance (plasmon wavelength).
All the approaches to assess the developability of mAbs offer some advantages, but most also include challenges that prevent their widespread use. One challenge common to most of the techniques is reproducibility, or batch-to-batch variation. Therefore, there is still need for improved screening assays that are compatible with large numbers of antibody variants, require only microgram quantities of protein, are easily reproducible, and do not require any special expertise, equipment or substantial commitment of resources and time. Here, we report a novel high throughput high-performance liquid chromatography (HPLC)-based screening method called standup monolayer adsorption chromatography (SMAC) that addresses some of these challenges and can be used to assess developability factors much earlier in the discovery process by using only microgram quantities of proteins. Briefly, we found retention times of antibodies injected on a Zenix HPLC column to be inversely related to their colloidal stability, with antibodies prone to aggregation or precipitation retained longer on the column. One advantage of this technique is that the column we used is commercially available and produced under controlled conditions. Another advantage is that the Zenix column is commonly used for size exclusion analysis; therefore, this technique has the potential to be used for simultaneous assessment of multiple developability factors (such as monomer percentage and solubility), which could be beneficial for the triage of molecules, especially at an early stage when the material availability is limited. We believe this approach should generally be applicable to assessing the developability of other classes of therapeutic molecules and can also help in the selection of molecules with improved biophysical characteristics, thus improving the efficiency of R&D.
Results
To evaluate the utility of the SMAC technique, 15 mAbs with varying developability characteristics were analyzed on Zenix and TSKgel columns, and their retention times determined. These mAbs exhibited a range of distinct retention times on the Zenix column (), suggesting some sort of interactions with it; the variation was low on TSKgel column ().
Figure 1. (A–B) SEC profiles of a representative antibody (mAb1) on TSKgel and Zenix column, respectively. This mAb had no interaction with either column. (C–D) SEC profiles of another representative antibody (mAb 2) on TSKgel and Zenix column, respectively. This mAb showed more retention on the Zenix column. The elution peak was not only delayed but also broader.
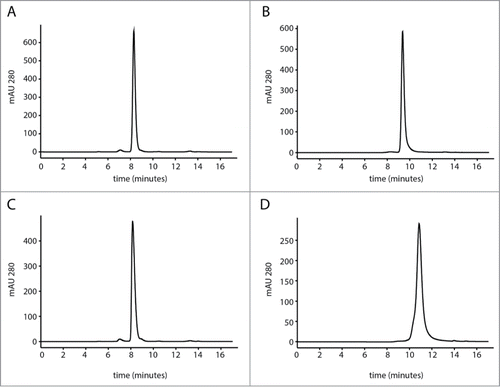
Table 1. Retention time for various mAbs on TSKgel, and Zenix columns. SEC was performed using either 50 mM sodium phosphate (+ 0.4 M NaClO4, pH 7.0, TSKgel), or 150 mM sodium phosphate (pH 7.0, Zenix) as running buffer. The retention time was very similar for all mAbs on TSKgel but distinct on Zenix column
The exact chemistry of both columns is proprietary. However, the TSKgel column, which is the current industry standard for size exclusion chromatography (SEC), is a silica-based column that has been modified with a bonded phase to minimize interactions with the protein and silica backbone. The Zenix column has a hydrophobic standup monolayer with terminal hydrophilic groups, reminiscent of the exterior of a protein, so we hypothesized that antibodies with colloidal instability may be more prone for non-specific interactions with the column leading to longer retention times.
In order to confirm this hypothesis, we measured the melting temperature (Tm) by differential scanning fluorimetry (DSF) and stability of mAbs at 37°C in 1X phosphate-buffered saline (PBS). All the mAbs had high Tm (>70°C by DSF), suggesting good conformational stability (). As shown in , however, they behaved differently after incubation at 37°C, suggesting differences in colloidal stability. All the mAbs that eluted normally on both the Zenix and TSKgel columns had a low rate of aggregation (). Antibodies that interacted with the Zenix column either had a high rate of aggregation (mAb5, mAb7 and mAb13), or showed significant precipitation over time (mAb4, mAb9, mAb10, mAb11 and mAb12); mAb2 was the only antibody that had delayed retention on the Zenix column but was found to be stable at 37°C. However, based on our prior scale-up experience with mAb2, the antibody had substantial developability challenges, including excessive clogging of virus clearance filters during the downstream processing, leading to a higher bioprocessing costs.
Table 2. Melting temperatures (Tm) of various mAbs, as measured by DSF
Though there are various mechanisms for antibody/protein aggregation, hydrophobic interactions have been shown to be the most predominant interaction in various extensive studies.Citation24,25 To test whether retention on the Zenix column is indicative of hydrophobicity, we evaluated the retention of antibodies in a low, medium, and high salt buffer (pH 7.4, 10 mM sodium phosphate + 75 mM/150 mM/300 mM NaCl, 0.5X/1X/2X PBS). As shown in , for some mAbs, relative to 1X PBS, there was a small increase in retention time under high salt conditions. However, the increase in retention time was much more substantial under low salt conditions. This result suggests that these mAbs have multiple modes of interactions with the column, with electrostatic interactions being the main interaction in this salt concentration range. Some mAbs (9 to 13) interacted irreversibly with the Zenix column, and did not elute under any of the tested salt/buffer conditions, suggesting strong hydrophobic or some other modes of interactions with the column.
Table 3. Retention time for various mAbs on a Zenix column under 3 different salt conditions. These results suggest that most mAbs have multiple modes of interactions with the column with either electrostatic or hydrophobic being the dominant one
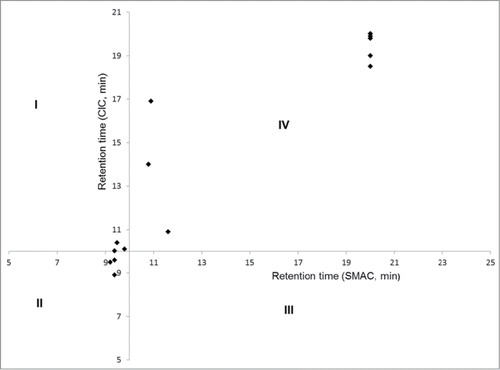
Cross-interaction chromatography (CIC) is gaining popularity for the triage of molecules at the discovery stage.Citation21,22,26 Therefore, we compared the SMAC results with the CIC results (). For this analysis, we used a threshold value of 10 min for both columns, which results in 4 quadrants. The cut-off/threshold value for CIC assay was taken from literature.Citation22 The cut-off value for SMAC was set at the average retention time (∼ +2σ) obtained from an in-house panel of well-behaved mAbs. Most of the mAbs fall into quadrant II or IV, suggesting good or poor developability, respectively. One mAb in quadrant I was identified as problematic by CIC, but not by SMAC. In summary, we found ∼90% correlation between the 2 assays in predicting colloidal stability, suggesting that SMAC can be a good surrogate for CIC. It is interesting to note that the correlation was 100% for the mAbs that interacted irreversibly with the Zenix column.
Figure 2. Comparison (CIC vs SMAC): Retention time for mAbs on CIC column (Y axis) and SMAC column (X axis). Quadrant II and IV represent mAbs which were found to have good or poor developability by both assays, respectively. There was approximately 90% correlation between both assays. All the IgGs which either bound irreversibly to the column, or didn't elute within the run time are shown as dots at 20 min.
In order to assess whether SMAC is also applicable to other classes of antibody-like molecules, we converted 6 mAbs into single chain fragments (scFvs) and determined their retention time. For this purpose, we chose 3 mAbs (mAb1/3/8) that were well behaved and stable as an IgG and 3 mAbs (mAb9/10/11) that had poor solubility. Consistent with their counterpart IgGs behavior (), scFvs 1 and 8 eluted normally on both TSKgel and Zenix (retention time <12 min); scFvs 9, 10 and 11 eluted normally on TSKgel but were retained on the Zenix column. However, scFv 3 showed some retention on the Zenix, suggesting that it lost some intrinsic stability during conversion and this scFv may not be as stable as its counterpart IgG. To further validate these observations, we measured the stability of all scFvs at 37°C. As expected, scFvs 1 and 8 were the most stable (data not shown) and had relatively low rates of aggregation; scFvs 3, 9, 10 and 11 were least developable as they showed excessive precipitation both during purification and storage.
Table 4. Retention time for various scFvs on TSKgel and Zenix column. SEC was performed using either 50 mM sodium phosphate (+ 0.4 M NaClO4, pH 7.0, TSKgel), or 150 mM sodium phosphate (pH 7.0, Zenix) as running buffer. The retention time was very similar for all scFvs on TSKgel but distinct on Zenix column
Discussion
The surface of an antibody molecule is very heterogeneous due to the presence of various moieties, such as hydroxyl, carbonyl, amide, aromatic and hydrophobic groups, that are capable of providing hydrogen bonding, electrostatic and hydrophobic interactions. These diverse interactions confer it with binding affinity and specificity for other molecules that may have similar heterogeneous surfaces. This heterogeneity, however, can also create self-interaction forces that can lead to colloidal instability, which could manifest itself as antibody aggregation or precipitation. Aggregation or precipitation can also occur if the antibody has low conformational stability, which can lead to the formation of reversible or irreversible aggregates via various transitional conformations. Therefore, the primary goal of any successful formulation development campaign is to minimize these interactions forces.
Prior to being formulated, the antibody must also go through several purification/chromatography steps, which are generally done over a range of pH and salt conditions. For an antibody to be considered developable, it thus needs to have good conformational and colloidal stability over a wide range of buffer conditions. Needless to say, high throughput screening tools that can help identify troublesome antibodies early in the discovery process are very helpful.
At the discovery stage, the conformation stability of an antibody can easily be assessed in a high-throughput manner using techniques like differential scanning fluorescence (DSF);Citation27 however, screening for colloidal instability is much more challenging. One measure that is commonly used as a predictor of colloidal instability or antibody-antibody self-interaction is an osmotic second virial coefficient (B22).Citation28,29 A positive B22 value means that antibody-antibody interaction is repulsive and the solution is stable, while a negative value suggests attractive forces that can lead to crystallization or precipitation. There are several ways of measuring B22, including neutron scattering, light scattering, and osmometry,Citation29,30 but these techniques require special expertise, equipment and large quantities of proteins, which prevents their widespread use especially at an early discovery stage. These drawbacks have led to the popularity of alternate techniques like SIC, CIC and AC-SINS, which, although do not provide a direct measure of B22, seem to give information consistent with techniques that do directly measure the B22 value.
Here, we present a novel screening technique, SMAC, that can be used to assess colloidal stability of antibodies using only microgram quantities of protein. For this study, we chose mAbs that had known developability challenges as well as well-behaving clinical-stage mAbs. We showed that by simply measuring the retention time of antibodies on a commercially available Zenix column, we could separate problematic antibodies from promising antibodies. Though rare, there are some literature reports (and also our experience) of even highly stable and soluble mAbs also interacting non-specifically with SEC columns such as TSKgel. For such antibodies, if there is no other scalability issue, the analytical method development mobile phase is altered with various co-solvents to improve resolution, peak symmetry and recovery. However, in our experience, only the Zenix SEC column yielded data indicating a clear correlation between retention and developability. Though the exact column chemistry is proprietary, we believe this could be due to the fact that the Zenix column surface, in comparison to other SEC columns, is unusually heterogeneous, probably because of the presence of hydrophobic standup monolayer and terminal hydrophilic groups. Therefore, antibodies with colloidal instability may be more prone to have non-specific interactions with it. The exact nature of these interactions is still not clear because, for some mAbs, electrostatics seems to be the dominant mode. However, several other mAbs (mAb9/10/11/12/13) bound irreversibly to the column and did not elute under any of the tested salt ranges. A careful sequence analysis of these problematic mAbs suggested hydrophobic interactions as the main mode of interaction because of the overrepresentation of hydrophobic/aromatic residues (e.g., tyrosines) in their complementarity-determining regions, which could provide an interaction surface for aggregates to nucleate. As shown in , there was also a very strong correlation between this technique and CIC, suggesting that the Zenix exterior surface adequately mimics an IgG exterior; and as a result, the interaction forces that cause a delay on the Zenix column may be similar to the one in CIC. The results of both chromatography methods have not correlated with DSF results, confirming the previous observationCitation31,32 that conformational stability and colloidal stability may be determined by different characteristics of the antibody surface.
For this study, we focused exclusively on the manufacturer's recommended buffer (150 mM sodium phosphate), but this technique can easily be extended to other buffer conditions and one can envision an experiment where multiple buffer, pH and salt conditions can be interrogated. While this technique has been validated only on a limited number of mAbs, we believe this approach can be used as a screening tool to enrich for the molecules with promising developability much earlier in a discovery program, with the caveat that no retention on Zenix column is not a guarantee that the antibody is developable, as there are multiple other factors that can make a molecule non-scalable. Similarly, it is also possible an antibody that shows retention on Zenix column can be purified or formulated by altering the solution conditions (e.g., pH) and adding appropriate excipients.
For this technique to gain popularity, the useful life of the column is also a very important parameter, although this is very hard to predict because it will depend upon the nature of mAbs in the analysis. For example, sticky or highly impure proteins will accelerate the decay. For this technique to be predictive, the users will periodically need to check the column resolution by injecting a control antibody. In our experience with well-behaved and pure mAbs, up to 150 injections could be made without any loss of peak resolution.
One major advantage of this method over other high throughput techniques is that the Zenix column is commercially available where it is produced under controlled conditions, so there should be very little batch-to-batch or user-to-user variation. Moreover, the Zenix column is commonly used for SEC analysis, so this technique can also be used for simultaneously determining the aggregate content of purified antibodies. This could be very beneficial information for the triage of clones at the discovery stage, especially when the material availability is limited. We focused mainly on mAbs and the scFvs derived from them in this study. However, we believe that this technique can easily be extended to other classes of therapeutic molecules, provided the prior method validation work is carried out.
Materials and Methods
Materials
pCEP4 vector, 293-F cells, L-glutamine, and pluronic F-68 were purchased from Life Technologies. Linear polyethyleneimine (PEI, MW: 25 kDa) was purchased from Polysciences. 1X PBS was ordered from Lonza. TSKgel SuperSW3000 column (4.6 mm i.d. × 30 cm) was purchased from Tosoh Bioscience, and Zenix column (4.6 mm i.d. × 30 cm) was purchased from Sepax Technologies. All solutions were prepared in 18 MΩ DI water.
Expression and purification of antibodies
All the antibodies were expressed using transient transfection in 293-F cells. All the genes were synthesized at DNA 2.0 (Menlo Park, CA) and subcloned into pCEP4 for transient transfection using standard molecular biology techniques. The ratio of heavy chain and light chain DNA was kept 1:1 in all transfections.
Transient transfection
Cell culture media was prepared by adding 20 mL of 200 mM L-glutamine and 10 mL of 10% pluronic F-68 to 1 L of F17 media. For transient transfections, 450 mL of 293-F cells were grown in cell culture media (37°C, 5% CO2) to a density of 1.5–2.0 million/mL in a baffled shake flask. On the day of transfection, 500 ug of total DNA and 1 mL of PEI solution (1 mg/mL) were premixed in 50 mL of cell culture media, briefly vortexed, incubated for 15 min, and added to cell culture. After a week, the cells were harvested by centrifugation (4000 g, 20 min), and the supernatant filtered using a 0.22 μm filter.
Protein A chromatography
The filtered supernatant was loaded on a protein A column pre-equilibrated with PBS, and washed with high conductivity buffer (PBS+ 500 mM NaCl) to reduce non-specific interactions. The bound protein was eluted using 0.1 M acetic acid (pH 2.9), neutralized with 1 M tris base, and dialyzed against 1X PBS overnight.
Size exclusion chromatography
Unless otherwise stated, 50 μg of sample was injected on a TSKgel SuperSW3000 column using 50 mM sodium phosphate + 0.4 M NaClO4, pH 7.0, or a Zenix column using 150 mM sodium phosphate (pH 7.0) as running buffer. Both of these buffers are the standard manufacturer recommended buffers for SEC analysis. The run time was 20 min. All measurements were performed on Agilent 1100 HPLC which was equipped with an auto sampler, binary pump and diode array detector. Data was analyzed using Chemstation software.
Cross-interaction chromatography
A CIC column was prepared by coupling ∼30 mg of human polyclonal IgGs (Sigma I 4506) onto a HiTrap NHS-activated resin (GE Healthcare #17-0716-01) followed by passivation with ethanolamine according to published procedures. A “blank” column was prepared similarly except ethanolamine passivation was performed with no prior IgG coupling. The columns were then connected to an HPLC and run at 0.1 mL/min using 1X PBS as the mobile phase until a flat baseline was reached. 10 μL of mAbs at 1 mg/mL in PBS were then injected. Peak retention times on the column were monitored at 280 nm and compared with reference IgGs run on same column to determine level of sample IgG cross-interaction.
Differential scanning fluorescence (DSF)
A DSF assay was performed using IQ5 Real Time Detection System (Bio-Rad). Briefly, 19 μL of antibody solution (5 μM) was mixed with 1 μl of 20X SYPRO Orange solution and added to a 96 well plate. The plate was heated from 20°C to 90°C at a rate of 1°C/min and the resulting fluorescence data collected. The data was transferred to GraphPad prism for analysis and Tm was calculated by taking the maximal value of the first derivative of the resulting fluorescence data with respect to temperature.
Disclosure of Potential Conflicts of Interest
All authors were employees of Merrimack Pharmaceuticals with ownership interests in the company at the time this work was undertaken.
Supplemental_material.docx
Download MS Word (14.1 KB)Funding
This research was funded by Merrimack Pharmaceuticals.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website
References
- Buss NAPS, Henderson SJ, McFarlane M, Shenton JM, De Haan L. Monoclonal antibody therapeutics: history and future. Curr Opin Pharmacol 2012; 12:615-22
- Maggon K. Monoclonal antibody “gold rush”. Curr Med Chem 2007; 14:1978-87; PMID:17691940; http://dx.doi.org/10.2174/092986707781368504
- Ludwig DL, Pereira DS, Zhu Z, Hicklin DJ, Bohlen P. Monoclonal antibody therapeutics and apoptosis. Oncogene 2003; 22:9097-106; PMID:14663488; http://dx.doi.org/10.1038/sj.onc.1207104
- Von Mehren M, Adams GP, Weiner LM. Monoclonal antibody therapy for cancer. Annu Rev Med 2003; 54:343-69; PMID:12525678; http://dx.doi.org/10.1146/annurev.med.54.101601.152442
- Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science [Internet] 2013; 341:1192-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24031011; http://dx.doi.org/10.1126/science.1241145
- Chao G, Lau WL, Hackel BJ, Sazinsky SL, Lippow SM, Wittrup KD. Isolating and engineering human antibodies using yeast surface display. Nat Protoc 2006; 1:755-68; PMID:17406305; http://dx.doi.org/10.1038/nprot.2006.94
- Boder ET, Wittrup KD. Yeast surface display for screening combinatorial polypeptide libraries. Nat Biotechnol 1997; 15:553-7; PMID:9181578; http://dx.doi.org/10.1038/nbt0697-553
- Hoogenboom HR. Selecting and screening recombinant antibody libraries. Nat Biotechnol 2005; 23:1105-16; PMID:16151404; http://dx.doi.org/10.1038/nbt1126
- Tiller T. Single B cell antibody technologies. N Biotechnol 2011; 28:453-7; PMID:21473940; http://dx.doi.org/10.1016/j.nbt.2011.03.014
- Filpula D. Antibody engineering and modification technologies. Biomol Eng 2007; 24:201-15; PMID:17466589; http://dx.doi.org/10.1016/j.bioeng.2007.03.004
- Lee EC, Owen M. The application of transgenic mice for therapeutic antibody discovery. Methods Mol Biol 2012; 901:137-48; PMID:22723098; http://dx.doi.org/10.1007/978-1-61779-931-0_8
- Xu L, Kohli N, Rennard R, Jiao Y, Razlog M, Zhang K, Baum J, Johnson B, Tang J, Schoeberl B, et al. Rapid optimization and prototyping for therapeutic antibody-like molecules. MAbs 2013; 5:237-54; PMID:23392215; http://dx.doi.org/10.4161/mabs.23363
- Dani B, Platz R, Tzannis ST. High concentration formulation feasibility of human immunoglubulin G for subcutaneous administration. J Pharm Sci 2007; 96:1504-17; PMID:17387698; http://dx.doi.org/10.1002/jps.20508
- Yadav S, Liu J, Shire SJ, Kalonia DS. Specific interactions in high concentration antibody solutions resulting in high viscosity. J Pharm Sci 2010; 99:1152-68; PMID:19705420; http://dx.doi.org/10.1002/jps.21898
- Connolly BD, Petry C, Yadav S, Demeule B, Ciaccio N, Moore JMR, Shire SJ, Gokarn YR. Weak interactions govern the viscosity of concentrated antibody solutions: high-throughput analysis using the diffusion interaction parameter. Biophys J 2012; 103:69-78; PMID:22828333; http://dx.doi.org/10.1016/j.bpj.2012.04.047
- Ponsel D, Neugebauer J, Ladetzki-Baehs K, Tissot K. High affinity, developability and functional size: the holy grail of combinatorial antibody library generation. Molecules 2011; 16:3675-700; PMID:21540796; http://dx.doi.org/10.3390/molecules16053675
- Saxena V, Panicucci R, Joshi Y, Garad S. Developability assessment in pharmaceutical industry: an integrated group approach for selecting developable candidates. J Pharm Sci 2009; 98:1962-79; PMID:18855914; http://dx.doi.org/10.1002/jps.21592
- Yang X, Xu W, Dukleska S, Benchaar S, Mengisen S, Antochshuk V, Cheung J, Mann L, Babadjanova Z, Rowand J, et al. Developability studies before initiation of process development: improving manufacturability of monoclonal antibodies. MAbs 2013; 5:787-94; PMID:23883920; http://dx.doi.org/10.4161/mabs.25269
- Chennamsetty N, Voynov V, Kayser V, Helk B, Trout BL. Prediction of aggregation prone regions of therapeutic proteins. J Phys Chem B 2010; 114:6614-24; PMID:20411962; http://dx.doi.org/10.1021/jp911706q
- Hawe A, Kasper JC, Friess W, Jiskoot W. Structural properties of monoclonal antibody aggregates induced by freeze-thawing and thermal stress. Eur J Pharm Sci 2009; 38:79-87; PMID:19540340; http://dx.doi.org/10.1016/j.ejps.2009.06.001
- Jacobs SA, Wu SJ, Feng Y, Bethea D, O'Neil KT. Cross-interaction chromatography: a rapid method to identify highly soluble monoclonal antibody candidates. Pharm Res 2010; 27:65-71; PMID:19911257; http://dx.doi.org/10.1007/s11095-009-0007-z
- Liu Y, Caffry I, Wu J, Geng SB, Jain T, Sun T, Reid F, Cao Y, Estep P, Yu Y, et al. High-throughput screening for developability during early-stage antibody discovery using self-interaction nanoparticle spectroscopy. MAbs 2014; 6:483-92; PMID:24492294; http://dx.doi.org/10.4161/mabs.27431
- Sule SV, Dickinson CD, Lu J, Chow CK, Tessier PM. Rapid analysis of antibody self-association in complex mixtures using immunogold conjugates. Mol Pharm 2013; 10:1322-31; PMID:23383873; http://dx.doi.org/10.1021/mp300524x
- Lowe D, Dudgeon K, Rouet R, Schofield P, Jermutus L, Christ D. Aggregation, stability, and formulation of human antibody therapeutics. Adv Protein Chem Struct Biol 2011; 84:41-61
- Nieba L, Honegger A, Krebber C, Plückthun A. Disrupting the hydrophobic patches at the antibody variable/constant domain interface: improved in vivo folding and physical characterization of an engineered scFv fragment. Protein Eng 1997; 10:435-44; PMID:9194169; http://dx.doi.org/10.1093/protein/10.4.435
- Spencer S, Bethea D, Raju TS, Giles-Komar J, Feng Y. Solubility evaluation of murine hybridoma antibodies. MAbs 2012; 4:319-25; PMID:22531448; http://dx.doi.org/10.4161/mabs.19869
- Lavinder JJ, Hari SB, Sullivan BJ, Magliery TJ. High-throughput thermal scanning: a general, rapid dye-binding thermal shift screen for protein engineering. J Am Chem Soc 2009; 131:3794-5; PMID:19292479; http://dx.doi.org/10.1021/ja8049063
- Saito S, Hasegawa J, Kobayashi N, Kishi N, Uchiyama S, Fukui K. Behavior of monoclonal antibodies: relation between the second virial coefficient (B 2) at low concentrations and aggregation propensity and viscosity at high concentrations. Pharm Res 2012; 29:397-410; PMID:21853361; http://dx.doi.org/10.1007/s11095-011-0563-x
- Tessier PM, Lenhoff AM, Sandler SI. Rapid measurement of protein osmotic second virial coefficients by self-interaction chromatography. Biophys J 2002; 82:1620-31; PMID:11867474; http://dx.doi.org/10.1016/S0006-3495(02)75513-6
- Einaga Y, Abe F, Yamakawa H. Light-scattering method of determining the 2nd virial-coefficient for simple molecules and oligomers. J Phys Chem 1992; 96:3948-53; http://dx.doi.org/10.1021/j100189a008
- Kheddo P, Tracka M, Armer J, Dearman RJ, Uddin S, Van Der Walle CF, Golovanov AP. The effect of arginine glutamate on the stability of monoclonal antibodies in solution. Int J Pharm 2014; 473:126-33; PMID:24992318; http://dx.doi.org/10.1016/j.ijpharm.2014.06.053
- Goldberg DS, Bishop SM, Shah AU, Sathish HA. Formulation development of therapeutic monoclonal antibodies using high-throughput fluorescence and static light scattering techniques: role of conformational and colloidal stability. J Pharm Sci [Internet] 2010; 100:1306-15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20960568; http://dx.doi.org/10.1002/jps.22371
