Abstract
Macrophages are key players in controlling the immune response that can adapt to microenvironmental signals. This results in distinct polarization states (classical M1 or alternative M2), that play a differential role in immune regulation. In general, the M1 contribute to onset of inflammation, whereas the M2 orchestrate resolution and repair, whereby failure to switch from predominantly M1 to M2 reinforces a pro-inflammatory environment and chronic inflammation. Here, we show selective elimination of M1 macrophages in vitro by a range of CD64-targeted immunotoxins, including H22(scFv)-ETA'. After re-polarization of already polarized macrophages, still only M1 polarization showed sensitivity toward CD64-directed immunotoxins. The selectivity for M1 was found linked to reduced endosomal protease activity in M1 macrophages as demonstrated by inhibition of endosomal proteases. Using the H22(scFv)-ETA' in a transgenic mouse model for chronic cutaneous inflammation, the M1 specificity was confirmed in vivo and a beneficial effect on inflammation demonstrated. Also ex vivo on skin biopsies from atopic dermatitis and diabetes type II patients with chronically-inflamed skin, a clear M1 specific effect was found. This indicates the potential relevance for human application. Our data show that targeting M1 macrophages through CD64 can be instrumental in developing novel intervention strategies for chronic inflammatory conditions.
Abbreviations
| SLS | = | sodium lauryl sulfate |
| hCD64 | = | human CD64 |
| hCD64tg | = | human CD64 transgenic |
| scFv | = | single chain fragment variable |
| ETA | = | Pseudomonas exotoxin A |
Introduction
Macrophages are part of the innate immune system and play a principal role in the regulation of inflammationCitation1 as well as physiological processes such as tissue remodeling.Citation2 Differentiated macrophages and their precursors are versatile cells that adapt to microenvironmental signals by altering their phenotype and function.Citation3 Although they have been studied for many decades, polarization of macrophages, e.g., toward classical M1 or alternative M2, has in recent years received substantial attention and is a continuously developing field.
M1 macrophages, induced by interferon (IFN)-γ and lipopolysaccharide (LPS), are described as the pro-inflammatory subtype. They produce reactive oxygen species and pro-inflammatory cytokines (e.g., interleukin (IL)-12, tumor necrosis factor (TNF), and IL-6) and trigger Th1 polarized responses.Citation4 M2 macrophages, induced by IL-4 or IL-13, are anti-inflammatory and characterized by the expression of IL-10high (especially M2b+M2c), RELM-αhigh (mouse only), CD206high and IL-12low (M2a).Citation5
In the usual course of inflammation, macrophages undergo dynamic switching between these polarization states. While M1 macrophages predominate during the early stage, mediating clearance of pathogens and recruitment of other effector cells, M2 macrophages prevail toward the end of inflammation, and promote vascularization and new tissue formation.Citation6,7 A proper course of inflammation is strongly dependent on this correctly-balanced dynamic ratio of M1 and M2 macrophages. Failure to switch from a predominance of M1 to M2 causes to the perpetuation of chronic inflammation.Citation8
Imbalance in the M1/M2 ratio has been associated with cardiovascular diseases such as atherosclerosis,Citation9 metabolism-associated diseases such as diabetes and metabolic syndrome,Citation10 and autoimmune diseases such as multiple sclerosis,Citation11 systemic lupus erythematosus,Citation12 Crohn's diseaseCitation13 and rheumatoid arthritis. Persistence of M1 macrophages in the local inflammatory response can also prevent the resolution of inflammation in several chronic skin diseases, such as diabetes-associated skin ulcerations,Citation14 chronic venous ulcers,Citation15 and atopic dermatitis.Citation16
Targeting M1 macrophages during chronic inflammation could therefore be a promising intervention strategy to correct an imbalance between M1 and M2 macrophages, thus promoting the resolution of chronic inflammation. Although FcγRs are present in both human and mouse, there are several species-dependent differences.Citation17 The use of transgenic mice expressing human FcγRs allows direct evaluation of, for example, human CD64 (FcγRI) specific drugs in vivo and the development of appropriate therapeutic models.Citation18,19 More than a decade ago, Thepen and colleagues showed that using a human CD64 (hCD64)-targeting immunotoxin H22-ricin A completely resolved skin inflammation in a hCD64 transgenic mouse model. Resolution of inflammation was not only confirmed by the elimination of activated, hCD64-positive macrophages, but also by the decline of clinical parameters associated with inflammation (e.g., local skin temperature, vasodilation). In addition, the elimination of CD64+ macrophages was accompanied by the clearance of other inflammatory cells, including T cells and dendritic cells.Citation19 However, at that time no discrimination could be made between M1 and M2 macrophages. The goal of this work was to investigate whether targeting CD64 results in pan-macrophage elimination or discrimination between pro-inflammatory M1 and inflammation-resolving M2 macrophages. Therefore, we used the recombinant immunotoxin H22(scFv)-ETA', which is a hCD64-targeting human single chain fragment [H22(scFv)], genetically fused to a truncated version of the Pseudomonas aeruginosa exotoxin A (ETA').Citation20,21 ETA' is a bacterial enzyme with ADP-ribosyl-transferase activity that irreversibly inactivates the eukaryotic elongation factor-2, thus catalytically inhibiting protein synthesis and inducing cell death at very low concentrations. The latest generation of immunotoxins, containing a human enzyme as toxic moiety, are generally referred to as human cytolytic fusion proteins (hCFPs). Several hCFPs have demonstrated specific activity and efficiency in vitro and in preclinical studies.Citation22,23
We show here for the first time that targeting CD64 on both murine (transgenic for hCD64) and human macrophages results in selective elimination of M1, but not M2 cells, although both subtypes express CD64. Compared to M2, M1 macrophages have reduced endosomal protease activity, which limits the proteolytic degradation of the immunotoxin after internalization through CD64. These in vitro findings could be confirmed in a hCD64-transgenic mouse model for chronic cutaneous inflammation in vivo, where local application of H22(scFv)-ETA' resulted in the specific elimination of M1 macrophages, leaving M2 macrophages unaffected. Moreover, ex vivo treatment of skin explants from patients (n = 3) with atopic dermatitis and diabetes type II patients (n = 2) with severe chronic wounds also reduced the number of M1 macrophages and changed the microenvironmental conditions in such a way as to promote the expansion of anti-inflammatory M2 macrophages, a prerequisite for resolution of chronic inflammation.
Results
Phenotypic characterization of polarized macrophages
In order to discriminate between M1 and M2 phenotypes in our in vitro system, we first established a panel of surface and soluble markers. Therefore, we analyzed the relative expression levels of a number of surface proteins on transgenic murine macrophages expressing human CD64 (hCD64tg), polarized in vitro by stimulation with IFN-γ + LPS (M1) or IL-4 (M2). Eight receptors were found to be upregulated in M1 macrophages, including the human FcγRI (hCD64), the co-receptor for LPS (CD14), and MHC class I and II (Fig. S1A). In contrast, M2 macrophages expressed lower levels of both hCD64 and CD14, but expressed 9 other receptors at higher levels than M1 macrophages, including the mannose receptor (CD206) and the macrophage galactose-type C-type lectin (CD301) (Fig. S1A). We also investigated the expression profiles of surface markers on human macrophages, again selecting and validating appropriate markers from literature. The upregulation of CD64 and CD14 in human M1 macrophages was similar to murine cells, and human M2 macrophages also showed elevated levels of CD206 and CD301, but lower levels of CD64 and CD14 (Fig. S1B). Some of these markers have already been assigned to M1 or M2 macrophages, but we here identified a set of cell surface antigens that allowed us to discriminate between the M1 and M2 subpopulations in vitro and in vivo (Fig. S1A and S1B).
Polarization also induced changes in the expression of soluble cytokines. Whereas both murine and human M1 macrophages secreted the pro-inflammatory cytokine IL-12, this molecule was not produced by M2 macrophages. IL-6 and TNF were also strongly upregulated in M1 macrophages, whereas the murine M2 marker RELM-α was over 2-fold more abundant in M2 compared to M1 macrophages. The anti-inflammatory cytokine IL-10, which is known to mediate resolution,Citation24 was detected at comparable levels in both macrophage populations (Fig. S1C).
Elimination of M1 macrophages in vitro
After defining the M1 and M2 expression profiles in our system and confirming the preferential expression of hCD64 by M1 macrophages, we investigated the manipulation of these cells by targeting hCD64. It has thus far proven impossible to target M1 macrophages without adversely affecting the anti-inflammatory M2 cell population. Here, we show that H22(scFv)-ETA' selectively kills hCD64tg murine M1 macrophages, without affecting the M2 population in vitro. The targeting unit H22(scFv) alone in an equimolar range had no effect ().
Figure 1. M1-specific elimination of polarized macrophages in vitro. (A) H22(scFv)-based immunotoxins selectively confer cytotoxicity toward M1 macrophages. H22(scFv) alone was used as control (means ± SD, n = 3). (B) Apoptosis assay of polarized macrophages after incubation with 100 nM of H22(scFv)-ETA' for 72 h. (C) Quantitative analysis of dot blots shown in B. Zeocin was used as a control (means ± SD, n = 3); *P ≤ 0.05, ***P ≤ 0.001 (one-way ANOVA). (D) Design of silica beads for the assessment of endosomal protease activity. (E) Relative protease activity in polarized macrophages (means ± SD, n = 3); ***P ≤ 0.001 (unpaired 2-tailed Student's ttest). (F) Calpain inhibitor lowers endosomal protease activity in M2 macrophages (means ± SD, n = 2); ***P ≤ 0.001 (unpaired 2-tailed Student's ttest). (G) Calpain Inhibitor overrides the resistance of M2 macrophages to H22(scFv)-ETA' (means ± SD, n = 3). Ang, angiogenin; Gb, granzyme B.
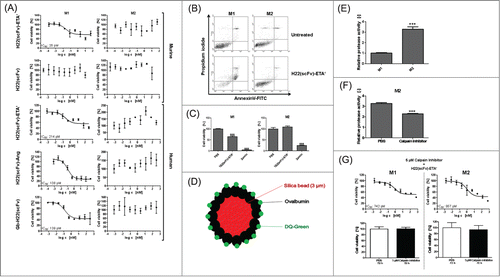
We subsequently also tested H22(scFv)-ETA' on human macrophage subpopulations with similar results. Furthermore, 2 human H22(scFv)-based CFPs, H22(scFv)-AngiogeninCitation25 and Granzyme B-H22(scFv),Citation26 were tested and again selectively killed the human M1 macrophages. This shows that the cytotoxic specificity is independent of the effector protein (). Of note, not all M1 macrophages could be killed even at increasing concentrations of the immunotoxin. This was potentially due to the fact that not all cells could acquire the same susceptible M1 phenotype. This variability could also be associated with the use of primary cells rather than cell lines. Figure S2 illustrates that the M1 population bears high heterogeneity in terms of surface marker expression and the corresponding susceptibility of these sub-population toward H22(scFv)-ETA' treatment. This result confirms that it is realistic to assume that, among M1 macrophages, there are also M2-like cells that resist the treatment with the immunotoxin H22(scFv)-ETA'. This is especially due to the non-synchronized life cycle of primary cells compared to cell lines; however, this is inherent to the nature and function of the original isolated monocytic cell populations and might better represent the real situation. Staining with AnnexinV-FITC after incubation with H22(scFv)-ETA' confirmed the mode of action as the selective induction of cell death in M1 macrophages (). Although hCD64 was expressed by both M1 and M2 macrophages (Fig. S1D), the relatively few hCD64 molecules on M2 should nevertheless be sufficient to internalize an adequate number of toxin molecules to induce apoptosis. As there is no correlation between expression of CD64 and sensitivity toward CD64-based immunotoxins (data not shown), we hypothesized that the selective sensitivity of M1 macrophages therefore must be determined by other factors, for instance differential routing and processing after internalization.
Endosomal protease activity in polarized macrophages
As both M1 and M2 express CD64, albeit at different levels, the selectivity for M1 must be determined by post-internalization processes. To gain insight into this, we constructed silica beads coated with DQ-Green-ovalbumin for the assessment of endosomal protease activity. Following the hydrolysis of ovalbumin, DQ-Green dye molecules dissociate and display fluorescence (). This experiment revealed that both human (data not shown) and murine M2 macrophages have a significantly increased endosomal protease activity compared to M1 macrophages (). We were able to counteract the resistance of M2 macrophages toward H22(scFv)-ETA' by inhibiting endosomal proteases such as cathepsins B and L using Calpain Inhibitor (), confirming the basis of the observed differences in sensitivity. Inhibition of endosomal proteases in M1 macrophages did not change their susceptibility toward the immunotoxin. Proteins internalized in M1 cells therefore have more time to escape from the endosomes before being degraded by proteases, which accounts for their sensitivity toward immunotoxins.
Elimination of M1 macrophages in a murine model of chronic cutaneous inflammation
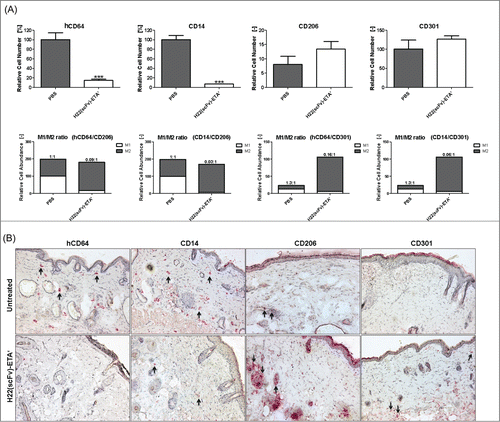
Sodium lauryl sulfate (SLS), which is a detergent present in the majority of ‘rub on / rub off’ skin care products like shampoos and soaps, was used to induce chronic cutaneous inflammation. Topical application of SLS resulted in local skin inflammation with a predominance of M1 macrophages (). Local intradermal injection with H22(scFv)-ETA' resulted in the reduction of hCD64+ CD14+ (M1) macrophages, whereas CD206+ CD301+ (M2) macrophages remained unaffected (). Removal of M1 macrophages from the site of inflammation led to an M2-biased microenvironment, shifting the M1/M2 ratio toward an overall anti-inflammatory status (). This was confirmed for all combinations (hCD64/CD206, hCD64/CD301, CD14/CD206 and CD14/CD301) of M1- and M2-specific surface markers.
Figure 2. Selective elimination of M1 macrophages in transgenic mice in vivo. (A) Upper panel: Treatment with H22(scFv)-ETA' reduced the number of hCD64+CD14+ M1 macrophages while leaving CD206+CD301+ M2 macrophages unaffected as analyzed by immunohistochemistry (means ± SEM, n = 5); ***P ≤ 0.001 (unpaired 2-tailed Student's t-test). Lower panel: Selective elimination of M1 macrophages shifts the M1/M2 ratio to an anti-inflammatory state. (B) Representative images of murine skin sections stained for M1-specific and M2-specific markers. Examples of stained cells are indicated by arrows. Objective: 10x.
Potential clinical relevance of M1-selective elimination of macrophages
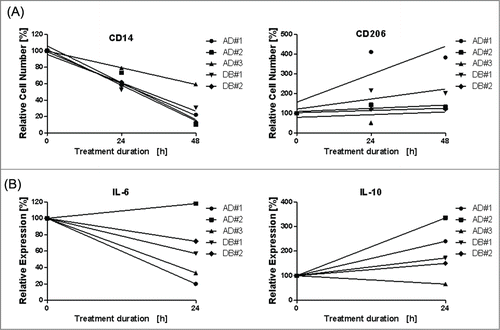
To provide first evidence that indicates the clinical relevance of our findings, we treated skin biopsies taken from lesional skin of human atopic dermatitis (n = 3) and diabetes type II patients (n = 2) with chronic wounds ex vivo using H22(scFv)-ETA'. Histological staining (Fig. S3) for CD14 (M1) and CD206 (M2) confirmed that treatment with H22(scFv)-ETA' reduces the number of M1 macrophages without negatively affecting the M2 population (). In addition to surface receptors, we also analyzed the effect of H22(scFv)-ETA' on the production of soluble cytokines. Supernatants from the human skin biopsies were analyzed for the presence of relevant cytokines before and after treatment. Although the concentrations of IL-12, IFN-γ, TNF and IL-4 were below the detection limit, we were able to measure the levels of the pro-inflammatory cytokine IL-6 and the anti-inflammatory cytokine IL-10. In agreement with the distribution of surface receptors, IL-6 levels had a clearly declining tendency 24 h after treatment with H22(scFv)-ETA', whereas IL-10 levels were increased strongly ().
Figure 3. Ex vivo elimination of M1 macrophages in chronically-inflamed skin of atopic dermatitis and diabetes type II patients. Skin biopsies were taken from atopic dermatitis patients (n = 3) and diabetes type II patients (n = 2). (A) Treatment with H22(scFv)-ETA' reduced the number of CD14+ M1 cells compared with the control incubated in medium only. In contrast, the number of CD206+ M2 macrophages was unaffected. Each point is the mean value of n = 3. (B) Elimination of M1 macrophages reduces the level of the pro-inflammatory IL-6 and induces the production of the anti-inflammatory IL10 after 24 h. Each point is the mean value of n = 3. Linear regression was performed using Graph Pad Prism 5.0.
Phenotypic plasticity of polarized macrophages
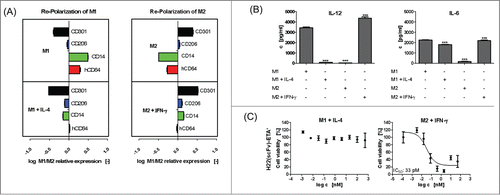
Phenotypic and functional plasticity is a key feature of polarized macrophages,Citation2,5,27 and the pathological inability of macrophages to switch phenotype from M1 to M2 can lead to chronic inflammation.Citation28 Both in our mouse model and in human skin explants, there was a tendency toward an increase in M2 after selective elimination of M1 macrophages. In particular with regard to the skin explants, which represent an isolated system without infiltration of new macrophages from the circulation, this observation could be explained by proliferation of M2 cells or re-polarization of remaining differently polarized macrophages in the tissue. To confirm this, we re-polarized murine peritoneal macrophages by reversion of the initial stimuli (IL-4 for M1 and IFN-γ for M2). Successful re-polarization was demonstrated by both surface () and soluble () markers. However, in addition to the already known phenotypic plasticity of polarized macrophages, we were also able to demonstrate their functional plasticity by reversing their sensitivity toward the immunotoxin H22(scFv)-ETA'. The stimulation of previously sensitive M1 macrophages with murine IL-4 resulted in the development of resistance to H22(scFv)-ETA', whereas the stimulation of previously resistant M2 macrophages with murine IFN-γ resulted in the development of sensitivity toward H22(scFv)-ETA' (). These results again underline that, despite the expression of CD64 on both (M1 and M2) macrophage subsets, ultimately only M1 macrophages are susceptible to the CD64-directed immunotoxin H22(scFv)-ETA'.
Figure 4. Plasticity of polarized macrophages in vitro. Polarized murine macrophages were repolarized by inverse stimuli. Representative M1 and M2 markers were analyzed by flow cytometry and FlowCytomix. Both populations could be repolarized causing changes in the profile of (A) surface and (B) soluble markers. M1/M2 ratio > 0 = upregulation in M1 cells; M1/M2 ratio < 0 = upregulation in M2 cells. Values are means ± SD, n = 3; ***P ≤ 0.001 (one-way ANOVA). (C) Functional plasticity of polarized macrophages was demonstrated by their reversed susceptibility to H22(scFv)-ETA'. Non-linear regression and calculation of relative IC50 values were performed by GraphPad Prism 5 software (means ±SD, n = 3).
Discussion
Liposome-based technology to deplete macrophages was described in the literature as early as 1985.Citation29 This technology depends on the ingestion of liposome-encapsulated dichloromethylene diphosphonate by phagocytic cells, which are mainly macrophages. This method proved a breakthrough in the study of macrophages, but also showed that, depending on the condition, the effect of the macrophage elimination could be beneficial as well as detrimental.Citation30-34
The current perception that macrophages are composed of functionally distinct sub-populations, termed M1 and M2, now explains these differential effects and emphasizes the importance of selectively targeting these different sub-populations. During chronic inflammation, there is strong evidence for an inappropriate persistence of M1 macrophages, which are directly associated with the development and perpetuation of many chronic inflammatory and autoimmune diseases.Citation8,35 In rheumatoid arthritis, crosstalk between M1 and Th17 cells is implicated in severity and chronicity of the disease and targeting either population is proposed as a potent therapeutic strategy for autoimmune disease.Citation36
Human CD64 is the high-affinity FcγR in humans, and it displays several unique properties such as the ability to bind and rapidly internalize monomeric IgGCitation37 and constitutive expression restricted to macrophages, monocytes and their progenitors.Citation38 These properties make hCD64 a good therapeutic target on M1 polarized macrophages. Increased expression of hCD64 has indeed been reported in several chronic diseases, including atopic dermatitis during the chronic phaseCitation39 underlining the relevance of hCD64 in chronic inflammation and therapy.
In 2000, Thepen and colleagues reported the resolution of chronic skin inflammation by local elimination of CD64+ macrophages. Our study now adds substantial understanding to this outcome, showing for the first time that H22(scFv)-based immunotoxins (independent of the toxin molecule) can be suitable tools to selectively eliminate M1 polarized macrophages without affecting their counterpart, the M2 macrophages. This effect is clearly not dependent on the bacterial ETA' toxin, as also the human cytolytic effector proteins exert the same effect. Despite several alterations to the bacterial ETA' toxin to reduce immunogenicity, the major disadvantage of this toxin, there is still the potential that unwanted immune responses limit the use of ETA' based therapeutics in humans. Replacing the bacterial protein with human cytolytic effector proteins, thus creating a human toxin, circumvents this disadvantage and can make human application possible.
One potential mechanism for this selectivity could be a difference in endosomal protease activity, which was indeed – accordance with the literature – significantly lower in M1 compared to M2 cells.Citation40,41 The lower protease activity in the endosomes of M1 cells may allow internalized toxin molecules to escape proteolysis and subsequently leak into the cytosol to exert their pro-apoptotic effect. This mechanism for M1-selectivity is also in line with the distinct functions M1 and M2 macrophages fulfill during various phases of the immune response. Upon activation, for example by infection, the primary function of M1 cells is to fight the pathogen and to initiate an immune response.Citation42 Phagocytosed material is therefore not completely degraded, but rather processed in a size that is appropriate for presentation of antigenic epitopes. This is accompanied by increased expression of MHC molecules on M1 macrophages supporting this role in antigen presentation during inflammation.Citation43 In contrast, M2 macrophages require a higher level of protease activity in accordance with their role in tissue remodeling and repair.Citation40,44 It is however noteworthy that the observed effect of polarization on endosomal protease activity could be influenced by the nature of the activation, e.g., viral vs. autoimmune-based. The latter one could be similar to the scenario postulated in this manuscript.
Our in vivo and ex vivo data indicate that elimination of M1 cells changes the microenvironment in a way that favors M2 polarization. This could affect newly infiltrating monocytes, but also push remaining macrophages to develop into a more like M2 phenotype. This increased presence of M2 cells could then give rise to resolution by enhanced phagocytosis of debris and apoptotic neutrophils, and by secretion of anti-inflammatory mediators including cytokines (e.g., IL-10) and lipid mediators (e.g., lipoxins, resolvins, protectins).Citation45-47 In particular these soluble mediators are responsible for cross-talking to, and skewing of, other inflammatory effectors, such as T cells or dendritic cells.
With regard to phenotypical plasticity, a well-established phenomenon of polarized macrophages, re-polarization also reversed the sensitivity of the macrophages toward the hCD64 immunotoxin. It should also be noted that there are indications that the subdivision in M1 and M2 is not absolute. There are potentially intermediate stages regarding marker expression, cytokine production, functionality,Citation45,48-50 and potentially sensitivity for hCD64 immunotoxins. This is clearly reflected in our in vitro experiments, in which we induce the different subpopulations. There is always some degree of scatter in marker expression and cytokine profile. These are never exclusive for either subpopulation, nor completely uniform. Also in the cytotoxicity assays, a hundred percent killing is never achieved in the M1-polarized macrophages, neither of murine nor of human origin. Typically, the application of immunotoxins, using the same toxin moieties as here in the hCD64 targeted immunotoxins, to, for example, cancer cell lines induces much higher kill rates because these cell lines are more uniform. It can be expected that M1 and M2 skewing factors under pathological conditions in vivo will vary strongly, both spatial and in time. This would render intermediate polarized cells responsive to the changed, more M2-prone environment created by the elimination of the M1 cells, and polarize them in the M2 direction. How long this effect lasts will largely be dependent on the nature of the chronic condition and whether it is remitting, like in rheumatoid arthritis or atopic dermatitis, or not, like in the case of chronic diabetic wounds. For different conditions, a specific schedule and formulation could be developed for optimal treatment.
In conclusion, our results provide new insights into the physiology of polarized macrophages during chronic inflammation, pointing to a pivotal role of M1 cells in determining the local cytokine milieu and perpetuation of inflammation. The hCD64 targeted immunotoxins can efficiently eliminate murine as well as human M1 macrophages with high specificity both in vitro and in vivo. This elimination leads in vivo to an altered micro-environment, which favors polarization toward the M2 phenotype that supports resolution of inflammation. A hCD64-targeted therapeutic can therefore be a powerful instrument both to identify M1 macrophages in vivo and to reverse chronicity into resolution for M1-associated chronic diseases.
Materials and Methods
Ethics statement
The human skin biopsy was taken at the Sankt Franziskus Hospital in Aachen, Germany, in accordance with the guidelines of the ethical committee of University Hospital Aachen, Germany. The committee specifically approved this study. Written informed consent was obtained from the patient.
Human patient material
We analyzed lesional skin from 3 patients with atopic dermatitis. The specimen (4 mm in diameter) was taken from an inflamed region and divided into 4 pieces. Chronically-inflamed skin biopsies from 2 diabetes type II patients were taken from the edge area of the wound. All specimens were incubated in complete RPMI1640 medium supplemented with 10% fetal calf serum, 50 µg/ml penicillin and 100 µg/ml streptomycin (Invitrogen, Germany) to which was added 1 µM of the hCD64-targeted bacterial immunotoxin H22(scFv)-ETA' for 24 or 48 h. Control specimens were incubated for 24 h in complete medium only in order to determine the H22(scFv)-ETA'-independent death or extravasation of macrophages. One piece of the tissue was immediately frozen in liquid nitrogen and stored at −80°C prior to analysis by immunohistochemistry (here described as “untreated”).
Animals and induced cutaneous inflammation model
The animal experiments were approved by the local animal care and use review committee. All animals received humane treatment in accordance with the requirements of the German Tierschutzgesetz, §8 Abs. 1 and with the guide for the care and use of laboratory animals published by the National Institutes of Health in 2011. We used > 6-week-old nude hairless transgenic male C57/Bl6/SKH1-E mice expressing hCD64 in all experiments. This mouse model has been shown to closely parallel the hCD64 expression in humans.Citation18 Chronic cutaneous inflammation was induced as described elsewhere.Citation19 Briefly, 5% (w/v) SLS in phosphate-buffered saline (PBS) was applied to a 1.5 × 1.5 cm skin surface area on both flanks of each mouse for 11 consecutive days. For immunotoxin administration, animals were anaesthetized with isofluran. Three intradermal injections were administered in a triangular pattern with 20 µl of 1 µM immunotoxin, on the left flank, and with 20 μl of PBS as control on the right flank. The animals were sacrificed after 24 h and 4 mm skin punches were taken from the area of the injection sites, snap frozen in liquid nitrogen, and stored at −80°C prior to analysis.
Isolation and in vitro polarization of macrophages
Peritoneal macrophages were induced in hCD64 transgenic mice by intraperitoneal injection of 1 ml 2% (w/v) BioGel P–100 (BioRad, Germany). After 3 days, mice were sacrificed and macrophages were isolated by peritoneal lavage using 5 ml of cold PBS (pH 7.4). After the lysis of red blood cells, macrophages were cultured at a concentration of 0.5–1.0 × 106 cells/ml in complete medium in T75 tissue culture flasks for 2–4 h. Non-adherent cells were removed by washing with cold PBS and macrophages were detached by incubation with cold 0.5 mM EDTA in PBS (pH 7.4) for 10 min on ice. Appropriate numbers of cells were then seeded into assay plates and incubated overnight. The cells were then stimulated for 24 h with 100 U/ml murine IFN-γ (Peprotech, Germany) and 1 µg/ml LPS (Sigma-Aldrich, Germany) to generate M1 macrophages, or with 20 ng/ml murine IL-4 (Peprotech, Germany) for 24 h to generate M2 macrophages. Human monocytes were isolated from buffy coats by gradient centrifugation with Ficoll (VWR, Germany) and cultured overnight as described above. Polarization was carried out for 72 h using 100 U/ml human IFN-γ (Peprotech, Germany) and 1 µg/ml LPS (Sigma-Aldrich, Germany) for M1 and 20 ng/ml human IL-4 (Peprotech, Germany) for M2 macrophages. After 72 h, the polarization was boosted with 50 U/ml human IFN-γ (Peprotech, Germany) and 0.5 µg/ml LPS (Sigma-Aldrich, Germany) for M1 and 10 ng/ml human IL-4 (Peprotech, Germany) for M2 macrophage for additional 24 h and macrophages were used in functional assays.
In vitro plasticity of polarized macrophages
To study the plasticity of polarized macrophages, cells were exposed to the reverse stimulus for 24 h. Before repolarization, medium containing the initial stimulus was changed and cells were washed once with fresh medium. Surface expression and cytokine profiles were analyzed by flow cytometry and FlowCytomix, respectively.
Flow cytometry
The expression of cell-surface receptors on macrophages was analyzed by flow cytometry. Cells were incubated with a fluorophore-labeled antibody in PBS (pH 7.4) containing 2 mM EDTA and 0.5% (w/v) BSA for 30 min on ice followed by 2 washes with PBS. The fluorescence was then analyzed on a FACSCalibur flow cytometer (Becton Dickinson, Germany). The following antibodies were used: mouse-anti-human CD64 [10.1]:FITC; rat-anti-mouse CD301:AlexaFluor488; rat-anti-mouse CD206:FITC; hamster-anti-mouse CD11c:RPE; rat-anti-mouse CD204:FITC (all from Serotec, Germany); mouse-anti-mouse CD284:PE; mouse-anti-human CD284:PE; rat-anti-mouse CD273:PE; mouse-anti-human CD273:PE; rat-anti-mouse CD14:FITC; rat-anti-mouse CD205:AlexaFluor488; mouse-anti-human CD200R:PE; mouse-anti-mouse MHCI:FITC; rat-anti-mouse MHCII:FITC; mouse-anti-human CD206:PE; rat-anti-mouse CD25:AlexaFluor488; rat-anti-mouse CD36:PE; rat-anti-mouse CD39:PE (all from eBioscience, Germany); mouse-anti-human CLEC10A/CD301:AlexaFluor488 (R&D Systems, Germany). Mouse-anti-rat IgG:FITC (eBioscience, Germany) was used as a secondary antibody for MOMA-1, MOMA-2 and ERTR-9 (kindly provided by Dr. Kraal, Amsterdam, Netherlands). The rat IgG2a K isotype control was labeled with PE or FITC (both eBioscience, Germany). Results are presented as relative fluorescence intensity calculated as follows: relative surface expression = (MFIantibody – MFIisotype control)/MFIisotype control. Comparisons between M1 and M2 polarized macrophages are presented as log(M1/M2) or log(M2/M1). All experiments were carried out in triplicate.
Cytokine ELISA and FlowCytomix
The production of soluble cytokines such as IL-12, IL-6, TNF, IL-1α and IL-10 was measured by FlowCytomix according to the manufacturer's instructions (eBioscience, Germany). The concentration of murine RELM-α was determined by a sandwich enzyme-linked immunosorbent assay (ELISA). Therefore, high-binding 96-well flat-bottom microtiter plates (VWR, Germany) were coated with 1 µg/ml of rabbit-anti-mouse RELM-α capturing antibody (Peprotech, Germany) in PBS (pH 7.4) at 4°C overnight followed by blocking with 2% (w/v) BSA (Sigma, Germany) in PBS for 2 h at room temperature. All intermediate washing steps were carried out 5 times for 5 min using PBST (PBS + 0.05% Tween-20). Standard samples and supernatants were applied to the plate in a total volume of 100 µl and incubated at 4°C overnight. Captured cytokines were detected by incubating with 0.5 µg/ml of biotinylated rabbit-anti-mouse RELM-α (Peprotech, Germany) for 1 h at room temperature. Bound antibodies were detected using horseradish peroxidase-conjugated streptavidin (Jackson ImmunoResearch, USA) at a concentration of 1 µg/ml for 30 min at room temperature. Finally, wells were extensively washed with PBST and incubated with TMB substrate (eBioscience, Germany) until color development was observed. The staining reaction was stopped with 1 M H3PO4 and the signal detected at E450–570nm using an Epoch microplate spectrophotometer (Biotek, Germany). Data were analyzed using GraphPad Prism 5 software. All experiments were carried out in triplicate.
In vitro heterogeneity of H22(scFv)-ETA' treated macrophages
To investigate the heterogeneity of polarized macrophages exposed to H22(scFv)-ETA', differently treated cells were stained for M1 (CD14) and M2 (CD206) surface markers plotted against Annexin V. Polarized macrophages were incubated for 24 h with 100 nM H22(scFv)-ETA' at standard cell culture conditions. Thereafter, cells were detached and manually washed. The double staining was performed for 30 min on ice using mouse-anti-human CD14:FITC, mouse-anti-human CD206:PE and Annexin V:APC in Annexin V buffer. The fluorescence was then analyzed on a FACSCalibur flow cytometer (Becton Dickinson, Germany).
Immunohistochemistry
Biopsy specimens were cut into 8-µm sections on a Cryostat CM 3050 (Leica, Germany) and mounted on superfrost slides (Menzel, Germany). After drying for 48–72 h, sections were fixed for 10 min with dry acetone and air-dried. Incubation with the primary antibody was carried out at 4°C overnight using mouse-anti-human CD64 [10.1]:FITC (eBioscience, Germany, 1:40), mouse-anti-human CD14-FITC (eBioscience, Germany, 1:40), rat-anti-mouse CD14-FITC (eBioscience, Germany, 1:40) in 1% (v/v) normal mouse serum in PBS, or mouse-anti-human CD206 (eBioscience, Germany), mouse-anti-human CD301 (R&D Systems, Germany), and rat-anti-mouse CD206 (Abd Serotec) in 1% (v/v) normal goat serum in PBS. Slides were washed 3 times for 5 min with PBST and incubated with alkaline phosphatase (AP)-conjugated sheep anti-FITC (Southern Biotech, Germany, 1:400) in 1% (v/v) sheep serum in PBS (30 min) or AP-conjugated goat-anti-rat antibody (eBioscience, Germany, 1:400) in 1% (v/v) goat serum in PBS (30 min). After washing twice in PBST and once in Tris-HCl (0.1 M, pH 8.5), AP activity was detected using naphthol AS-BI phosphate (sodium salt, 50 mg/100 ml; Sigma, Germany) as the substrate and new fuchsin (10 mg/100 ml; Merck, USA) as the chromogen dissolved in 0.1 M Tris-HCl, pH 8.5, resulting in pink/red staining. Endogenous AP activity was inhibited by adding 0.35 mg/ml levamisole (Sigma, Germany) to the reaction mixture. Slides were lightly counterstained with hematoxylin.
Quantification of immunostained macrophages
Slides with stained specimens were analyzed using a Leica DMR-HC light microscope and digitalized using Leica QWin software. The grid was adapted to size of the sections so the complete section was covered. The individual images were stitched using Adobe Photoshop CS5.1. Thereby the entire stained section was recorded and used for analyses. Final quantification of immunostained sections was done using the HistoCad software developed at Fraunhofer Mevis. This intelligent software can be trained to either include or exclude elements in an image and can subsequently analyze in an identical fashion and with high accuracy, large numbers of sections.Citation51 After training the software, the results were concurred by 2 independent experienced evaluators in a blinded fashion. When the results were deviant from that of HistoCad, the software was trained anew. The software was kindly provided by Dr. Hans Meine (Fraunhofer MEVIS, Bremen, Germany).
Cell viability assay
The cytotoxic effect of H22(scFv)-based immunotoxins or human cytolytic fusion proteins was assessed by measuring the conversion of XTT to a water-soluble orange formazan dye as previously described.Citation52 The concentration required to achieve a relative 50% reduction of protein synthesis (IC50) relative to untreated control cells was calculated using GraphPad Prism 5 software. All experiments were carried out in triplicate. M1 and M2 macrophages showed diverse metabolic activity resulting in different scales of cell viability.
Assessment of endosomal protease activity
For the comparison of endosomal protease activity in different polarized macrophages, 3 µm (in diameter) amino-modified silica beads (Kisker Biotech, Germany) were coupled to DQ ovalbumin (Invitrogen, Germany), a pH-stable protease substrate that is fluorogenic following proteolysis. Therefore, 50 mg/ml silica beads (in PBS, pH 7.4) were activated using 10 mg/ml Sulfo-SMCC (in DMF; Sigma, Germany) for 1 h at room temperature with agitation. Beads were washed twice with PBS (pH 7.4) followed by incubation with 1 mg/ml DQ ovalbumin (in PBS, pH 7.4) for 2 h at room temperature and at 4°C overnight. Finally, beads were washed twice with PBS (pH 7.4), resuspended, and stored at 4°C. Inhibition of endosomal proteases by 5 µM calpain inhibitor (Sigma, Germany) was carried out for 2 h at 37°C prior to the addition of silica beads or H22(scFv)-ETA'. For the relative quantitation of endosomal protease activity, polarized macrophages were incubated with 0.25 mg/ml DQ ovalbumin-modified silica beads for 15 min at room temperature and then 15 min at 37°C. Cells were washed twice with warm PBS and 1 ml of fresh complete medium was added. Emitted fluorescence at 520 nm was measured every 15 min using a Tecan Genios Pro spectrophotometer (Tecan, Switzerland).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 software. Data were expressed as the mean ±standard deviation (SD) or standard error of the mean (SEM) as indicated. Statistical comparisons were made using an unpaired 2-tailed Student's t-test, and one- or 2-way ANOVA, where appropriate. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Disclosure of Potential Conflicts of Interest
R Fischer, T Thepen and S Barth are inventors on a European patent on recombinant anti-C64 immunotoxins assigned to Fraunhofer. S Barth is also co-inventor on further patent applications on human cytolytic fusion proteins based on different human enzymes/proteins assigned to Fraunhofer or Pharmedartis. The authors otherwise declare no conflict of interest.
Supplemental_Material.zip
Download Zip (476.9 KB)Acknowledgments
We would like to thank Reinhard Rosinke for the production of H22(scFv)-ETA'. We also thank Dr. Richard M. Twyman for critically reading the manuscript.
Funding
This work was funded by the Fraunhofer project “Märkte von übermorgen – SkinHeal.” Radoslav Mladenov was supported by a scholarship from the Jürgen-Manchot-Foundation.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 2010; 32:593-604; PMID:20510870; http://dx.doi.org/10.1016/j.immuni.2010.05.007
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010; 11:889-96; PMID:20856220; http://dx.doi.org/10.1038/ni.1937
- Cassetta L, Cassol E, Poli G. Macrophage polarization in health and disease. ScientificWorldJournal 2011; 11:2391-402; PMID:22194670; http://dx.doi.org/10.1100/2011/213962
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004; 25:677-86; PMID:15530839; http://dx.doi.org/10.1016/j.it.2004.09.015
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8:958-69; PMID:19029990; http://dx.doi.org/10.1038/nri2448
- Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 2011; 22:317-26; PMID:21289217; http://dx.doi.org/10.1681/ASN.2009060615
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011; 11:723-37; PMID:21997792; http://dx.doi.org/10.1038/nri3073
- Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, Eming SA. Differential roles of macrophages in diverse phases of skin repair. J Immunol 2010; 184:3964-77; PMID:20176743; http://dx.doi.org/10.4049/jimmunol.0903356
- Cardilo-Reis L, Gruber S, Schreier SM, Drechsler M, Papac-Milicevic N, Weber C, Wagner O, Stangl H, Soehnlein O, Binder CJ. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol Med 2012; 4:1072-86; PMID:23027612; http://dx.doi.org/10.1002/emmm.201201374
- Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 2013; 339:172-7; PMID:23307735; http://dx.doi.org/10.1126/science.1230721
- Liu C, Li Y, Yu J, Feng L, Hou S, Liu Y, Guo M, Xie Y, Meng J, Zhang H, et al. Targeting the shift from m1 to m2 macrophages in experimental autoimmune encephalomyelitis mice treated with fasudil. PLoS One 2013; 8:13; PMID:23418431
- Orme J, Mohan C. Macrophage subpopulations in systemic lupus erythematosus. Discov Med 2012; 13:151-8; PMID:22369974
- Crielaard BJ, Lammers T, Morgan ME, Chaabane L, Carboni S, Greco B, Zaratin P, Kraneveld AD, Storm G. Macrophages and liposomes in inflammatory disease: friends or foes? Int J Pharmac 2011; 416:499-506; PMID:21238559; http://dx.doi.org/10.1016/j.ijpharm.2010.12.045
- Goren I, Muller E, Schiefelbein D, Christen U, Pfeilschifter J, Muhl H, Frank S. Systemic anti-TNFalpha treatment restores diabetes-impaired skin repair in ob/ob mice by inactivation of macrophages. J Invest Dermatol 2007; 127:2259-67; PMID:17460730; http://dx.doi.org/10.1038/sj.jid.5700842
- Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest 2011; 121:985-97; PMID:21317534; http://dx.doi.org/10.1172/JCI44490
- Thepen T, Langeveld-Wildschut EG, Bihari IC, van Wichen DF, van Reijsen FC, Mudde GC, Bruijnzeel-Koomen CA. Biphasic response against aeroallergen in atopic dermatitis showing a switch from an initial TH2 response to a TH1 response in situ: an immunocytochemical study. J Allergy Clin Immunol 1996; 97:828-37; PMID:8613640; http://dx.doi.org/10.1016/S0091-6749(96)80161-8
- Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 2012; 119:5640-9; PMID:22535666; http://dx.doi.org/10.1182/blood-2012-01-380121
- Heijnen IA, Van de Winkel JG. A human Fc gamma RI/CD64 transgenic model for in vivo analysis of (bispecific) antibody therapeutics. J Hematother 1995; 4:351-6; PMID:8581368; http://dx.doi.org/10.1089/scd.1.1995.4.351
- Thepen T, van Vuuren AJ, Kiekens RC, Damen CA, Vooijs WC, van De Winkel JG. Resolution of cutaneous inflammation after local elimination of macrophages. Nat Biotechnol 2000; 18:48-51; PMID:10625390; http://dx.doi.org/10.1038/71908
- Hristodorov D, Mladenov R, Huhn M, Barth S, Thepen T. Macrophage-targeted therapy: CD64-based immunotoxins for treatment of chronic inflammatory diseases. Toxins 2012; 4:676-94; PMID:23105975; http://dx.doi.org/10.3390/toxins4090676
- Ribbert T, Thepen T, Tur MK, Fischer R, Huhn M, Barth S. Recombinant, ETA'-based CD64 immunotoxins: improved efficacy by increased valency, both in vitro and in vivo in a chronic cutaneous inflammation model in human CD64 transgenic mice. Br J Dermatol 2010; 163:279-86; PMID:20426788; http://dx.doi.org/10.1111/j.1365-2133.2010.09824.x
- Schiffer S, Letzian S, Jost E, Mladenov R, Hristodorov D, Huhn M, Fischer R, Barth S, Thepen T. Granzyme M as a novel effector molecule for human cytolytic fusion proteins: CD64-specific cytotoxicity of Gm-H22(scFv) against leukemic cells. Cancer Lett 2013; 341:178-85; PMID:23973499; http://dx.doi.org/10.1016/j.canlet.2013.08.005
- Schiffer S, Rosinke R, Jost E, Hehmann-Titt G, Huhn M, Melmer G, Barth S, Thepen T. Targeted ex vivo reduction of CD64-positive monocytes in chronic myelomonocytic leukemia and acute myelomonocytic leukemia using human granzyme B-based cytolytic fusion proteins. Int J Cancer 2014; PMID:24523193
- Nathan C, Ding A. Nonresolving inflammation. Cell 2010; 140:871-82; PMID:20303877; http://dx.doi.org/10.1016/j.cell.2010.02.029
- Hetzel C, Bachran C, Fischer R, Fuchs H, Barth S, Stocker M. Small cleavable adapters enhance the specific cytotoxicity of a humanized immunotoxin directed against CD64-positive cells. J Immunother 2008; 31:370-6; PMID:18391759; http://dx.doi.org/10.1097/CJI.0b013e31816a2d23
- Stahnke B, Thepen T, Stocker M, Rosinke R, Jost E, Fischer R, Tur MK, Barth S. Granzyme B-H22(scFv), a human immunotoxin targeting CD64 in acute myeloid leukemia of monocytic subtypes. Mol Cancer Ther 2008; 7:2924-32; PMID:18790773; http://dx.doi.org/10.1158/1535-7163.MCT-08-0554
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002; 23:549-55; PMID:12401408; http://dx.doi.org/10.1016/S1471-4906(02)02302-5
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 2009; 27:451-83; PMID:19105661; http://dx.doi.org/10.1146/annurev.immunol.021908.132532
- van Rooijen N, van Nieuwmegen R, Kamperdijk EW. Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. Ultrastructural aspects of elimination of marginal zone macrophages. Virchows Archiv B Cell Pathol Includ Mol Pathol 1985; 49:375-83; PMID:2867636; http://dx.doi.org/10.1007/BF02912114
- Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med 1989; 170:499-509; PMID:2526847; http://dx.doi.org/10.1084/jem.170.2.499
- Nieuwenhuijzen GA, Haskel Y, Lu Q, Berg RD, van Rooijen N, Goris RJ, Deitch EA. Macrophage elimination increases bacterial translocation and gut-origin septicemia but attenuates symptoms and mortality rate in a model of systemic inflammation. Ann Surge 1993; 218:791-9; PMID:8257230; http://dx.doi.org/10.1097/00000658-199312000-00014
- Kotter MR, Setzu A, Sim FJ, Van Rooijen N, Franklin RJ. Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination. Glia 2001; 35:204-12; PMID:11494411; http://dx.doi.org/10.1002/glia.1085
- Borders AS, Getchell ML, Etscheidt JT, van Rooijen N, Cohen DA, Getchell TV. Macrophage depletion in the murine olfactory epithelium leads to increased neuronal death and decreased neurogenesis. J Compar Neurol 2007; 501:206-18; PMID:17226772; http://dx.doi.org/10.1002/cne.21252
- van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJ. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol 2007; 170:818-29; PMID:17322368; http://dx.doi.org/10.2353/ajpath.2007.060547
- Bannon P, Wood S, Restivo T, Campbell L, Hardman MJ, Mace KA. Diabetes induces stable intrinsic changes to myeloid cells that contribute to chronic inflammation during wound healing. Dis Models Mech 2013; PMID:24057002
- Li J, Hsu HC, Mountz JD. The dynamic duo-inflammatory M1 macrophages and Th17 cells in rheumatic DISEASES. J Orthop Rheumatol 2013; 1:4; PMID:25309946; http://dx.doi.org/10.13188/2334-2846.1000002
- Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol 1991; 9:457-92; PMID:1910686; http://dx.doi.org/10.1146/annurev.iy.09.040191.002325
- Daeron M. Fc receptor biology. Annu Rev Immunol 1997; 15:203-34; PMID:9143687; http://dx.doi.org/10.1146/annurev.immunol.15.1.203
- Kiekens RC, Thepen T, Bihari IC, Knol EF, Van De Winkel JG, Bruijnzeel-Koomen CA. Expression of Fc receptors for IgG during acute and chronic cutaneous inflammation in atopic dermatitis. Br J Dermatol 2000; 142:1106-13; PMID:10848732; http://dx.doi.org/10.1046/j.1365-2133.2000.03534.x
- Balce DR, Li B, Allan ER, Rybicka JM, Krohn RM, Yates RM. Alternative activation of macrophages by IL-4 enhances the proteolytic capacity of their phagosomes through synergistic mechanisms. Blood 2011; 118:4199-208; PMID:21846901; http://dx.doi.org/10.1182/blood-2011-01-328906
- Yates RM, Hermetter A, Taylor GA, Russell DG. Macrophage activation downregulates the degradative capacity of the phagosome. Traffic 2007; 8:241-50; PMID:17319801; http://dx.doi.org/10.1111/j.1600-0854.2006.00528.x
- Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood 2008; 112:935-45; PMID:18684880; http://dx.doi.org/10.1182/blood-2007-12-077917
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005; 5:953-64; PMID:16322748; http://dx.doi.org/10.1038/nri1733
- Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 2013; 229:176-85; PMID:23096265; http://dx.doi.org/10.1002/path.4133
- Bystrom J, Evans I, Newson J, Stables M, Toor I, van Rooijen N, Crawford M, Colville-Nash P, Farrow S, Gilroy DW. Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood 2008; 112:4117-27; PMID:18779392; http://dx.doi.org/10.1182/blood-2007-12-129767
- Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Cell Metab 2012; 15:432-7; PMID:22482726; http://dx.doi.org/10.1016/j.cmet.2011.11.013
- Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med 2005; 201:1355-9; PMID:15867089; http://dx.doi.org/10.1084/jem.20050640
- Dalrymple H, Barna BP, Malur A, Malur AG, Kavuru MS, Thomassen MJ. Alveolar macrophages of GM-CSF knockout mice exhibit mixed M1 and M2 phenotypes. BMC Immunol 2013; 14:1471-2172; http://dx.doi.org/10.1186/1471-2172-14-41
- Reinartz S, Schumann T, Finkernagel F, Wortmann A, Jansen JM, Meissner W, Krause M, Schwörer AM, Wagner U, Müller-Brüsselbach S, et al. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: correlation of CD163 expression, cytokine levels and early relapse. Int J Cancer 2014; 134:32-42; PMID:23784932; http://dx.doi.org/10.1002/ijc.28335
- Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014; 40:274-88; PMID:24530056; http://dx.doi.org/10.1016/j.immuni.2014.01.006
- Homeyer A, Schenk A, Arlt J, Dahmen U, Dirsch O, Hahn HK. Practical quantification of necrosis in histological whole-slide images. Comput Med Imaging Graph 2013; 37:313-22; PMID:23796718; http://dx.doi.org/10.1016/j.compmedimag.2013.05.002
- Huhn M, Sasse S, Tur MK, Matthey B, Schinkothe T, Rybak SM, Barth S, Engert A. Human angiogenin fused to human CD30 ligand (Ang-CD30L) exhibits specific cytotoxicity against CD30-positive lymphoma. Cancer Res 2001; 61:8737-42; PMID:11751393
