Abstract
Monomeric IgA has been proposed as an alternative antibody format for cancer therapy. Here, we present our studies on the production, purification and functional evaluation of anti-HER2 IgA antibodies as anti-cancer agents in comparison to the anti-HER2 IgG1 trastuzumab. MALDI-TOF MS analysis showed profound differences in glycosylation traits across the IgA isotypes and cell lines used for production, including sialylation and linkage thereof, fucosylation (both core and antennary) and the abundance of high-mannose type species. Increases in sialylation proved to positively correlate with in vivo plasma half-lives. The polymerization propensity of anti-HER2 IgA2m2 could be suppressed by an 18-aa deletion of the heavy chain tailpiece - coinciding with the loss of high-mannose type N-glycan species - as well as by 2 cysteine to serine mutations at positions 320 and 480. The HER2 F(ab')2-mediated anti-proliferative effect of the IgA2m1 and IgA2m2 subtypes was similar to IgG1, whereas the IgA1 isotype displayed considerably lower potency and efficacy. The Fc-mediated induction of antibody-dependent cell-mediated cytotoxicity (ADCC) using human whole blood ADCC assays did not demonstrate such clear differences between the IgA isotypes. However, the potency of the anti-HER2 IgA antibodies in these ADCC assays was found to be significantly lower than that of trastuzumab. In vivo anti-tumor activity of the anti-HER2 IgA antibodies was compared to that of trastuzumab in a BT-474 breast cancer xenograft model. Multiple dosing and sialylation of the IgA antibodies compensated for the short in vivo half-life of native IgA antibodies in mice compared to a single dose of IgG1. In the case of the IgA2m2 antibody, the resulting high plasma exposure levels were sufficient to cause clear tumor stasis comparable to that observed for trastuzumab at much lower plasma exposure levels.
Abbreviations
| ACN | = | acetonitrile |
| ADCC | = | antibody-dependent cell-mediated cytotoxicity |
| ADCP | = | antibody-dependent cellular phagocytosis |
| ASGPR | = | asialoglycoprotein receptor |
| AUC | = | area under the curve |
| CDC | = | complement-dependent cytotoxicity |
| CL | = | clearance rate |
| Cmax | = | peak plasma concentration |
| ER | = | endoplasmic reticulum |
| FcαRI | = | Fc-α receptor I |
| FcγR | = | Fcγ-receptor |
| HER2 | = | human epidermal growth factor receptor 2 |
| HMW | = | high molecular weight |
| MALDI-TOF MS | = | matrix-assisted laser desorption/ionization time-of-flight mass spectrometry |
| mAbs | = | monoclonal antibodies |
| monocytes | = | MNC |
| NK | = | natural killer |
| PK | = | pharmacokinetic |
| PMN | = | polymorphonuclear leukocytes |
| SEC | = | size exclusion chromatography |
| SPR | = | surface plasmon resonance |
| t1/2 | = | elimination half-life |
| UPLC | = | ultra performance liquid chromatography |
| Vss | = | apparent volume of distribution at steady state. |
Introduction
The cancer cell-killing capacity of monoclonal antibodies (mAbs) is determined by Fab- and Fc-mediated effects. Antigen-binding of a mAb to its cancer cell target may trigger a number of Fab-mediated responses, i.e., internalization, apoptosis and growth inhibition. The antibody isotype can influence these Fab-mediated responses as for instance in the case of dimeric IgA or pentameric IgM isotypes having 4 or 5 Fabs, respectively. Yet, therapeutic efficacy of the different isotypes is deemed to be predominantly determined by the Fc-mediated interactions with myeloid effector cells and complement, i.e., interactions that can induce cell killing via antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP) and complement-dependent cytotoxicity (CDC).Citation1,2 The most commonly used antibody format comprises an IgG1 backbone or engineered versions thereof designed to modulate interactions with activating and inhibitory Fcγ-receptors (FcγRs), and thereby increase cancer cell killing even further.Citation3
The first indications that IgA might be an alternative antibody format for cancer therapy came from ADCC experiments that were either done with bispecific F(ab')2 antibodies, in which one Fab was directed at the Fc-α receptor I (FcαRI) and the other at the target antigen, or with recombinant monomeric IgA1.Citation4,5 One of the anticipated advantages of using IgA was its capacity to also act via the abundant polymorphonuclear leukocytes (PMN), whereas IgG1 is thought to mainly employ natural killer (NK) cells and macrophages as effector cells.Citation6,7 Moreover, IgA-mediated FcαRI activation appears to actively recruit PMN toward tumor cells.Citation8,9 Since tumor-killing by IgA depends on activation of the FcαRI and that of IgG1 is assumed to be mainly mediated by FcγRIIIa, it might be superior to the corresponding IgG1 when treating patients carrying the FcγRIIIa F158 allele in whom IgG1 efficacy is reduced compared to carriers of the V158 allele.Citation10,11,12 Very recently, it has been demonstrated not only that macrophages can mediate tumor cell killing by IgA2, but more importantly that this occurs in vivo.Citation13
IgA antibodies have attracted much less attention than IgG antibodies. One of the (practical) reasons might be the fact that this isotype utilizes the FcαRI, a receptor that is absent from mice, i.e., the standard animal for pre-clinical testing of cancer therapeutics. However, transgenic mice expressing the human FcαRI were developed more than a decade ago.Citation14,15 Additional discouragements might have been the significantly lower half-life of IgA compared to IgG1, the complexity of IgA N- (and O-) glycosylation profiles complicating control of product consistency and the general inexperience with commercial scale IgA production in mammalian cell culture and the subsequent purification.
In humans, secretory IgA is by far the most abundantly produced immunoglobulin, with rates up to about 6 gram per day in epithelial layers. Serum levels of mainly monomeric and some dimeric IgA are 15-20% of total immunoglobulin levels and consist of isotypes IgA1 and IgA2, with the latter occurring as one of 2 allotypes, IgA2m1 being predominant in Europe and IgA2m2 having the highest frequency in Africa.Citation16 The main differences between IgA1 and IgA2 are located in the heavy chain hinge region where the IgA1 isotype is 13-amino acid residues longer than IgA2, and where only this IgA isotype is decorated with up to 5 O-glycans.Citation17 In addition, IgA1 has 2 N-glycosylation sites, whereas the IgA2m1 and IgA2m2 isotypes comprise 4 or 5 N-glycosylation sites, respectively, containing N-glycans with higher complexity than what is usually found for IgG antibodies.Citation18 The IgA2m1 allotype is remarkable among human IgA for lacking a covalent disulfide bond between heavy and light chains.Citation19
A meaningful investigation into the potential value of (monomeric) IgA in oncology can only be performed by direct comparison with a standard IgG1 having identical variable domains. In this study, trastuzumab was compared to different human IgA isotypes containing the trastuzumab variable domains. To this end, each of the 3 known human IgA isotypes (IgA1, IgA2m1, IgA2m2) and several variants carrying the trastuzumab variable domains were produced. Production formats include stable PER.C6 pools co-expressing a human α2,3-sialyltransferase or transient expression in HEK293F cells. Pharmacokinetic (PK) studies demonstrated a clear correlation between the N-glycan sialylation and half-life in serum. Cysteine to serine mutations in the Cα2 domain and the tailpiece of an anti-HER2 IgA2m2 antibody were shown to reduce undesirable polymerization of (monomeric) IgA thus reducing a purification bottleneck. ADCC assays using human whole blood from different donors as effector cells showed that IgA potency tended to be notably lower than that of IgG1, though maximum cell killing by IgA could sometimes be higher than by IgG1. Isolated human PMN confirmed specific ADCC activity mediated by IgAs and lack of activity by IgG1. Finally, a subcutaneous BT-474 xenograft model was used in transgenic severe combined immunodeficient (SCID) mice expressing the human FcαRI to test in vivo efficacy of the various anti-HER2 IgA isotypes compared to trastuzumab. These studies indicate that, in this particular model, the tumor-inhibitory capacity of the IgG1 antibody was evidently more efficacious than the best IgA antibody, despite IgA serum levels that were equal or even higher than that of the IgG1 antibody.
Results
Production of monomeric IgA from stably transformed PER.C6 or transiently transfected HEK293F
The three anti-HER2 IgA antibodies representing the 3 known human IgA isotypes were generated using stable pools of PER.C6 cells co-transfected with 2 vectors, one encoding the human IgA heavy chain and a kappa light chain gene with the corresponding trastuzumab variable domains and the other encoding the human α2,3-sialyltransferase ST3GAL4 (). In addition, a stable pool expressing the human anti-HER2 IgA2m1 isotype in the absence of ectopic ST3GAL4 was also used for antibody production. The actual production runs were performed in 10 liter cultures using 20 liter wave bags and yielded IgA levels between 110–190 mg/L as detected by AlphaLISA. Size exclusion chromatography (SEC) analysis of KappaSelect-purified IgA revealed the presence of substantial amounts of higher molecular weight (HMW) species in addition to monomeric IgA and free light chain (). The molecular and biological traits of IgA were determined following a finishing round of purification by SEC. Using UPLC-SEC, the distribution of monomeric, dimeric and multimeric species was quantified. Thus, it was shown that all preparations contained more than 90% of the monomeric species (Table S1). The two preparations containing wild-type IgA2m2 from PER.C6 and HEK293F contained the highest levels of multimers at 3.4 and 3.6%, respectively, whereas in contrast none could be detected in the IgA2m2 preparation lacking the tailpiece. The final yields of purified monomeric IgA ranged between 10 and 15.7 mg/L. Besides IgA produced with PER.C6 pools, a number of antibodies were also produced by transient expression in HEK293F. All of these antibodies were based on the anti-HER2 IgA2m2 sequence used above, but contained one or more mutations, or deletion of the tailpiece. The aim of producing the mutants was to investigate the nature of the HMW species as well as the putative gel artifacts that were observed.
Figure 1. Preparative SEC profiles of protein samples from PER.C6 stable pools expressing IgA1-1g5 (A), IgA21-2g4 (B), IgA21-2g5 (C) and IgA22-4g5 (D) and from HEK293F cultures IgA22-4g2 (E) and IgA22Δ18-5g2 (F) after clarification and KappaSelect purification. HMW = high-molecular weight fraction; LC = light chain.
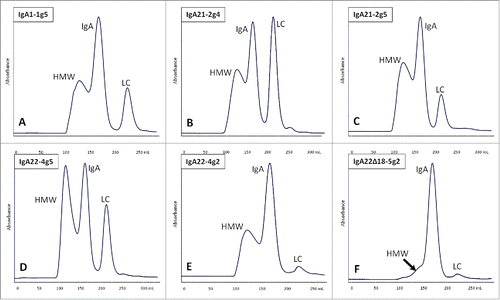
Table 1. Overview of anti-HER2 IgA antibodies used in this study.
Characterization of purified IgA
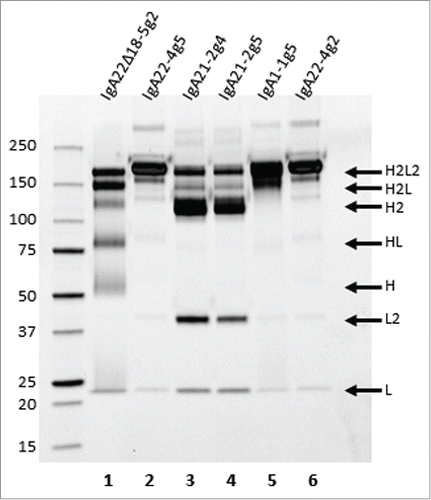
Purified, mainly monomeric IgA variants () were also characterized by non-reducing SDS-PAGE. IgA22-4g5 and IgA1-1g5 showed one predominant band at a molecular weight around 160 kDa (), which likely represents the fully assembled molecule with 2 heavy chains and 2 light chains (H2L2). The same 160 kDa band was also visible in IgA21-2g4 and −2g5, albeit at a relatively low level, but instead these samples displayed major amounts of 115 kDa and 45 kDa proteins, which likely represented heavy (H2) and light chain dimers (L2), respectively. Several more, but less conspicuous, bands could be detected, especially in the IgA21-2g4 and −2g5 samples that most likely corresponded to species consisting of folded and assembled heavy-heavy-light chains (H2L) and light chain (L). The HMW species above 160 kDa probably consisted of di- and polymeric forms of IgA that seemed to be coupled through their tailpieces since, when the 18-aa C-terminal tailpiece (containing the penultimate C-terminal cysteine) was removed from the anti-HER2 IgA2m2, hardly any HMW species were formed upon transient expression in HEK293F cells (; IgA22Δ18-5g2). However, the removal of the tailpiece seemingly caused a deterioration of antibody stability during electrophoresis as a number of additional bands emerged under non-reducing conditions compared to intact IgA22-4g2 also produced in HEK293F (see below). HER2 binding of the IgA antibodies using either surface plasmon resonance (SPR) or cellular binding showed that binding strengths of the antigen to the monomeric IgA preparations were very similar to trastuzumab (Table S2 and Fig. S2). Using SPR to compare binding to the FcαRI receptor, approximate 2-fold differences were found between the monomeric IgA preparations in observed KD (KDobs)(Table S3)
Figure 2. Non-reducing SDS-PAGE of IgA22Δ18-5g2 (lane 1), IgA22-4g5 (lane 2), IgA21-2g4 (lane 3), IgA21-2g5 (lane 4), IgA1-1g5 (lane 5), and IgA22-4g2 (lane 6) obtained by consecutive KappaSelect and SEC purification steps, and visualized using Criterion TGX Stain-free, precast gels and Krypton staining. Putative composition of the various bands has been indicated on the right side of the gel.
Mutation and deletion analysis of anti-HER2 IgA2m2
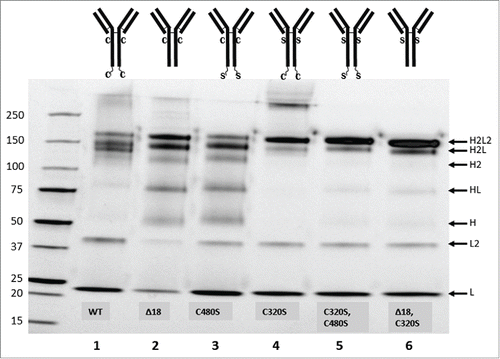
The ostensible instability of IgA22Δ18-5g2 on non-reducing SDS-PAGE prompted a further investigation into the role of free thiols in the appearance of the additional bands. To this end, free thiols were chemically blocked with N-ethylmaleimide (NEM). Non-reducing SDS-PAGE of NEM-treated IgA22Δ18-5g2 greatly reduced the intensity of these bands, which suggested that the penultimate cysteine in its tailpiece-containing IgA22-4g2 counterpart was involved in stabilizing this antibody during gel electrophoresis (Fig. S3). A series of mutants were designed in which either of the 2 cysteines in the IgA heavy chain at positions 320 and 480, which are potentially engaged in SC- and J-chain interactions when present in a secretory IgA, were mutated to serine, or in which the tailpiece was deleted. These mutant antibodies and the wild-type sequences were transiently expressed in HEK293F and affinity purified using KappaSelect resin only, which yielded intact IgA and light chain monomers and dimers. Tailpiece deletion () greatly affected the banding pattern compared to the wild-type () and yielded a profile that was very similar to antibody with the C480S mutation (). C320S mutation generated a very neat profile lacking some of the lower molecular weight by-products, but it still showed a significant level of HMW product (). Strikingly, combining C320S and C480S into one antibody, or combining C320S with tailpiece deletion, provided virtually identical profiles consisting mainly of (monomeric) IgA and hardly any gel artifacts or HMW species ().
Figure 3. Non-reducing SDS-PAGE of IgA from transiently producing HEK293F following KappaSelect purification. Lanes: (1) IgA22-4g2, (2) IgA22Δ18-5g2, (3) IgA22-7, (4) IgA22-8, (5) IgA22-9, (6) IgA22-10. Diagrams depict cysteines at heavy chain positions 320 and 480 (C), serines at heavy chain positions 320 and 480 (S) and the C-terminal 18 aa tailpiece. Gels were visualized using Criterion TGX Stain-free, precast gels and Krypton staining. Putative composition of the various bands has been indicated on the right side of the gel.
N-glycosylation of the IgA preparations
MALDI-TOF MS was used to study the N-glycans released from the various antibodies, with derivatization of the sialic acids to enhance their stability and allow the discrimination of α2,3- and α2,6-linked variants. Profound N-glycosylation differences were revealed between the IgA isotypes produced in PER.C6 stable pools and between antibodies of the same IgA isotype but from different expression systems. Structural characterization was performed by MALDI-TOF/TOF MS/MS, proving informative in determining antennary compositions and fucose locations (Table S3).
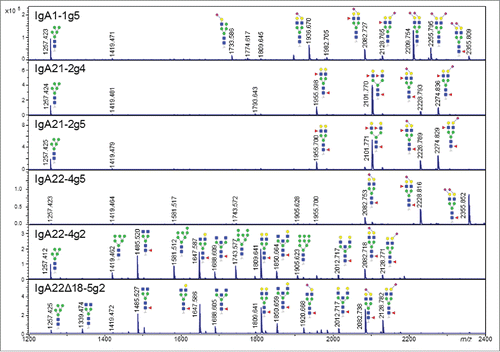
N-glycans from the antibodies IgA1-1g5 and IgA22-4g5 produced in PER.C6 cells with ectopic α2,3-sialyltransferase turned out to be highly sialylated with those from IgA1-1g5 being remarkable in having very low levels of core fucose (). The bulk of the sialic acid residues in these N-glycans were α2,3-linked and a minority of N-glycans was mono-substituted with α2,6-linked residues. In contrast, N-glycans from antibodies IgA21-2g4 and −2g5 mainly consisted of bi-antennary oligosaccharides that were poorly substituted with single α2,3- or α2,6-linked sialic acid residues. At first glance, the 2 antibodies IgA21-2g4 and −2g5 produced without and with ectopic α2,3-sialyltransferase activity, respectively, displayed very similar N-glycan profiles dominated by a small number of peaks representing bi-antennary N-glycans with both antennae either carrying a fucose (Lewis A or X epitopes) or with one antenna instead carrying a sialic acid (visible at m/z 2101.771, 2228.793 and 2274.836). Yet, average sialylation of IgA21-2g5 (41.3%) produced in the presence of ectopic α2,3-sialyltransferase was considerably higher than that of IgA22-2g4 (23.6%) (Table S4). In both IgA1-1g5 and IgA22-4g5, average sialylation - mostly by α2,3-linked sialic acid residues - is 2-4 fold higher and amounts to 85.3% and 94.0%, respectively. In addition, IgA22-4g5, and especially IgA1-1g5, contained very few LewisA/X structures, while these glyco-epitopes were found to be abundant on IgA21-2g4 and −2g5. Three of the 4 IgA antibodies contained a small, but significant level of the high-mannose species Man5GlcNAc2 (m/z = 1257.423). N-glycan profiles of the HEK293F-produced antibodies IgA22-4g2 and IgA22Δ18-5g2 were found to be quite different from those of PER.C6-produced IgA. The striking dissimilarity between N-glycan profiles from HEK293F- and PER.C6-produced antibodies was due to those from HEK293F-produced antibodies being relatively poorly galactosylated and sialylated, lacking LewisA/X epitopes and displaying significant levels of bisected N-glycans. Remarkably, the loss of the tailpiece in IgA22Δ18-5g2 was associated with a decrease of high-mannose N-glycans beyond Man5GlcNAc2, as seen by the percentage of high-mannose type compositions dropping from 21.0% in IgA22-4g2 to 2.1% in IgA22Δ18-5g2, and the average high mannose size changing from 7.08 to 5.91 mannose residues (Table S5). As expected, sialic acid residues on N-glycans of antibodies coming from the human cell line HEK293F were found to be either α2,3- or α2,6-linked and the sialylation level of these IgA antibodies was as low as that of IgA21-2g4 and −2g5.
Figure 4. MALDI-TOF MS analysis of N-glycans from IgA1-1g5, IgA21-2g4, IgA21-2g5, IgA22-4g5, IgA22-4g2 and IgA22Δ18-5g2 purified as described in analyzed in positive mode. The diagrams indicate peaks with S/N of >10 in the range of m/z 1200-2400. Blue square indicates N-acetylglucosamine; red triangle, fucose; green circle, mannose; yellow circle, galactose; purple diamond, N-acetylneuraminic acid. Linkage positions of sialic acid residues are indicated by differing angles.Citation38
Cell-based assays
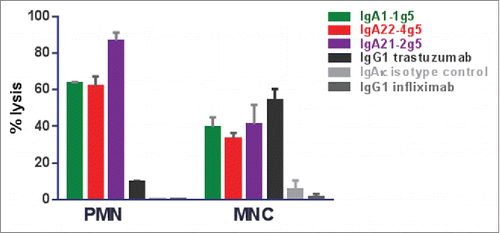
The ability to inhibit cell proliferation has been studied in HER2-expressing BT-474 breast carcinoma cells after 6 days of treatment with anti-HER2 IgA antibodies and compared to the IgG1 antibody trastuzumab. Cell growth was inhibited by IgA2 antibodies with a similar efficacy and IC50 value as IgG1, whereas IgA1-1g5 was 3-fold less potent and only able to partially inhibit cell growth (). In the ADCC assay using human whole blood as effector cells and the BT-474 human breast carcinoma as target cells, all IgA isotypes showed similar potency and efficacy after 4 h incubation (Fig. S4). Using three donors and the SK-BR-3 human breast carcinoma as target cells, the EC50 values for the IgA antibodies ranged between 30 and 340 ng/mL, and for IgG1 from 3-7 ng/mL (Fig. S5). The maximal antibody lytic activity was donor- and isotype-dependent, ranging from 50-80% lysis. Hence, whereas no clear difference in lysis efficacy was observed between IgA and the IgG1, the IgG1 variant was consistently at least 7-fold more potent using SK-BR-3 as target cells. When using whole blood in combination with BT-474 as target cells, IgG1 was 25-fold more potent than the most active IgA antibodies IgA1-1g5 and IgA22-4g5 (). Following separation of donor cells into different effector cell populations, the ADCC activity of IgA with PMN was found to be much higher than with IgG1, whereas IgA and IgG1 were equally effective in inducing ADCC activity exerted by monocytes (). Whole blood from FcαRI-transgenic mice that mainly contains PMN as effector cells was likewise observed to mediate ADCC activity in HER2-expressing SK-BR-3 tumor cells (Fig. S6)
Figure 5. Proliferation inhibition assay of IgA1-1g5, IgA21-2g4, IgA21-2g5, IgA22-4g5 purified as described in , IgG1 trastuzumab and isotype controls. BT-474 cells were incubated with the indicated antibodies for 6 days before cell viability was measured using ATP CellTiter-Glo assay. n= 3 replicates; ± SEM.
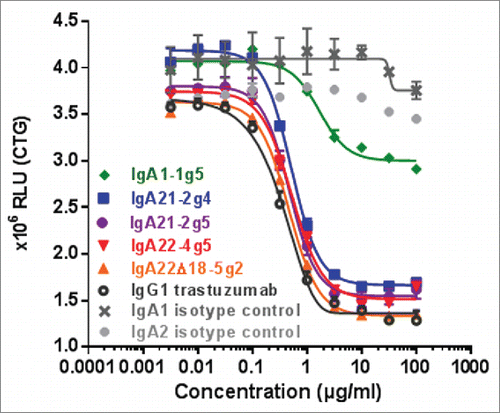
Figure 6. ADCC assays with IgA1-1g5 and IgA22-4g5 purified as described in , IgG1 trastuzumab and isotype controls using human whole blood as effector cells and BT-474 (A) or SK-BR-3 (B) as target cells. Cell lysis was measured after 24 hours. Representative graphs of data from one donor per target cell are depicted. n = 3 replicates; ± SEM.
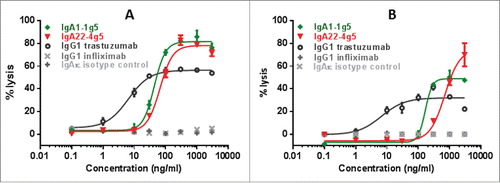
Figure 7. ADCC assays with IgA1-1g5 and IgA22-4g5 purified as described in , IgG1 trastuzumab and isotype controls incubated at 1 µg/mL using isolated human PMN and MNC as effector cells and SK-BR-3 as target cells in a 25:1 ratio. Cell lysis was measured after 24 hours. The graph represents typical data from one of 3 different donors that were tested. n = 3 replicates; ± SEM.
Pharmacokinetic (PK) analysis
After 10 mg/kg intravenous dosing in wild type BALB/c mice, the PK properties of the different IgA antibodies were highly dependent on the glycosylation status (). The highly sialylated IgA1-1g5 and IgA22-4g5 showed similar PK with an AUClast of 1391 and 1827 h*ug/mL, a clearance of 6.0 and 4.8 mL/h/kg and a terminal half-life of 32.2 and 27.4 h, respectively (). In contrast, the poorly sialylated IgA21-2g5 and −2g4 from PER.C6 cells and IgA22-4g2 and IgA22Δ18-5g2 from HEK293F showed a 3-60-fold higher clearance compared to IgA1-1g5. Intraperitoneal dosing of the highly sialylated IgA1-1g5 and IgA22-4g5 resulted in similar exposure, as expected (Fig. S7). Snapshot PK sampling in xenograft studies, 24 hours after the 10th daily IgA dose showed that IgA1-1g5 and IgA22-4g5 reached plasma concentrations that were at least 10-fold higher at a 25 mg/kg IgA dose than the level of the corresponding IgG1 at a single 5 mg/kg dose (Table S6). Multiplying the IgA exposure by 10 for the multiple dose and by 2.5 to compensate for the dose difference, results in a theoretical IgA22-4g5 AUC of 45675 h*ug/mL, compared to published trastuzumab AUC of 9761 h*ug/mL.Citation20
Figure 8. PK analysis of IgA1-1g5, IgA21-2g4, IgA21-2g5, IgA22-4g5, IgA22-4g2 and IgA22Δ18-5g2 purified as described in . 200 µg of the IgA antibodies were dosed by intravenous bolus injection into the tail vein.
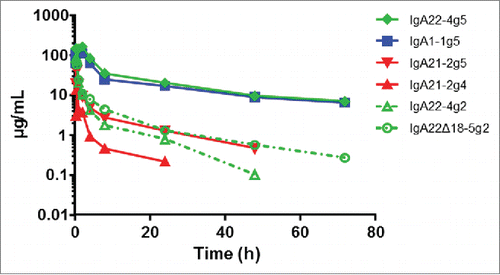
Table 2. PK parameters of the different IgA preparations.
Xenograft studies
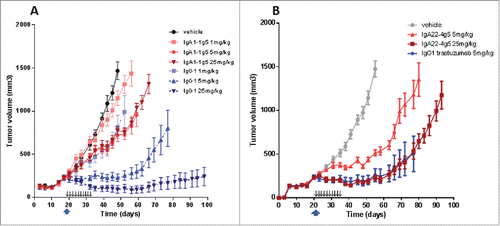
In the BT-474 xenograft studies in FcαRI-transgenic SCID mice, single dose treatments with trastuzumab were compared to multiple dosing with the short-lived IgAs to compensate for large differences in exposure levels. The different dose levels of trastuzumab resulted in a dose-dependent decrease of tumor growth (). Already at 1 mg/kg, tumor growth was delayed for 10 – 20 days, and at 5 mg/kg the IgG1 induced tumor stasis for almost 40 days, whereas actual tumor regression occurred at 25 mg/kg. In contrast, IgA1-1g5 administered on 10 consecutive days clearly was much less efficacious. Unlike trastuzumab, the low-dose regimen at 1 mg/kg hardly affected tumor growth. A similar delay in tumor growth of about 20 days was seen at both 5 and 25 mg/kg (), which was surprising in view of the clear difference in plasma exposure between them (Table S6). In the BT-474 xenograft study shown in , trastuzumab was dosed once at the intermediate 5 mg/kg single dose; it demonstrated tumor reduction comparable to that in the study shown in Fig. 9A. In contrast to the results obtained with IgA1-4g5, the multiple doses of IgA22-4g5 did achieve a clear dose-dependent reduction of tumor growth at 5 mg/kg, even leading to tumor stasis at 25 mg/kg, i.e., comparable to the efficacy of a single 5 mg/kg dose of trastuzumab. Likewise, in a HER2-overexpressing A431 intraperitoneal tumor model, but with IgA22-4g5 administered directly to the peritoneum, this IgA showed tumor growth inhibition in FcαRI-transgenic SCID mice (Fig. S8). In general, IgA antibodies dosed in the same tumor model, but using non-transgenic SCID mice, displayed no significant tumor growth inhibition (data not shown).Citation13
Figure 9. In vivo activity of IgA1-1g5 (A), IgA22-4g5 (B) purified as described in and IgG1 trastuzumab (A, B) in a BT-474 xenograft model in female FcαRI-transgenic SCID mice. Treatment was started at a tumor volume of 200-300 mm3 using single-dose trastuzumab (upward arrow) or multiple dosing of the IgA antibodies (small downward arrows). n = 8 animals per group; ± SEM.
Discussion
One of the reasons for a paucity of research into the potential value of anti-cancer IgAs in oncology might be due to the belief that it would be difficult to produce high levels of antibody with consistent quality. Here, we observe that titers of the anti-HER2 IgA antibodies produced in pools of stable, transfected PER.C6 cells used in this study were similar to that of trastuzumab (data not shown). However, the final yield of monomeric IgA from the large scale production runs was relatively low due to the fact that a large amount of IgA is produced in the form of dimers and polymers instead of monomers, despite the absence of a J-chain.Citation21 This is in agreement with earlier research showing that J-chain incorporation is not required for polymerization of antibodies containing the IgA tailpiece.Citation22 As expression of an anti-HER2 IgA2m2 lacking the penultimate cysteine or even the entire heavy chain tailpiece yielded mostly monomeric species, it can be concluded that dimers and polymers were primarily due to tailpiece interactions probably involving disulfide bridge formation between the penultimate cysteines.Citation23 The non-reducing SDS-PAGE revealed that especially the IgA2m1 preparation seemed to be unstable as the profile displayed a variety of protein species in addition to the full-length protein, species most likely representing antibodies lacking one light chain or species consisting of homodimers of heavy and light chains.Citation21 However, SEC proved conclusively that the anti-HER2 IgA antibodies were intact. The low level of fully assembled IgA2m1 and the abundance of light chain dimers and free light chain was probably due to inefficient formation of disulfide bonds between heavy and light chains.Citation24 A similar phenomenon was also observed with IgA2m2 produced in Chinese hamster ovary (CHO) cells, where it was associated with the presence of free cysteine residues leading to gel electrophoresis artifacts.Citation25
The role of the free thiols in the appearance of additional bands during non-reducing SDS-PAGE was studied in more detail. Since blocking the free thiols with NEM before gel electrophoresis strongly suppressed the formation of the additional bands, a series of mutated proteins was made in which the free thiols at positions 320 and 480 were separately or together replaced by serine, or in which the complete tailpiece was deleted. In secretory IgA, one of the 4 heavy chains is covalently bound to the secretory component through a cysteine corresponding to position 320 in the IgA2m2 mutant series.Citation26 This position corresponds to position 309 in the IgA numbering used by Smith and Morrison.Citation27 Interestingly, these authors observed that engineering a leucine to cysteine mutation at the corresponding position in an IgG3 triggered apparent polymerization of the otherwise monomeric IgG3 antibody, which clearly illustrates the potential reactivity of the cysteine in the Cα2 domain possibly resulting in gel artifacts. The four penultimate cysteine residues, which also seem to play an important role in causing gel artifacts, connect the different monomeric IgAs in secretory IgA by binding to J-chain and probably to each other.Citation28 Clearly, when both types of free thiols were absent – either through mutation or deletion – most of the gel artifacts were gone. More importantly, it is likely that these combined mutations will also greatly facilitate commercial manufacture of a consistent, homogeneous product.
Earlier publications on plasma clearance showed that human IgA was cleared rapidly in mice with half-lives between 10 to 14 hours, whereas human IgG1 half-life in mice is generally reported to be around 10 days.Citation29,30 Hence, we anticipated that repetitive IgA dosing would be essential to maintain adequate and equivalent exposure levels of IgA and IgG1. The fact that IgA does not bind to FcRn – neither in mice nor in humans – leads to a much shorter half-life for IgA than for IgG1, particularly in mice. In humans, the clearance rates of IgG1 and IgA do not differ as strikingly as in mice (IgG1 half-life is ∼21 days and IgA half-life is ∼5 days in humans).Citation31,32,33 Our preliminary PK studies with IgA from HEK293 cells transiently expressing the antibodies confirmed the published data on poor half-life in mice and prompted the step toward glyco-engineering (data not shown).Citation29
The earlier PK studies on human mIgA clearance in mice not only demonstrated rapid clearance, but they also showed that it was probably uptake by the liver asialoglycoprotein receptor (ASGPR) that was responsible for the high clearance rate.Citation13,29,34 The involvement of the ASGPR implied that the extent of IgA glycan sialylation would control the antibodies' fate in serum. In human serum IgA1, 64% of the terminal N-linked galactose residues are sialylated.Citation18 In view of the apparent relevance of high sialylation, it was decided to produce the different mIgA types in PER.C6 cells that co-expressed a sialyltransferase gene to augment capping of the exposed galactose residues on N-glycans.Citation35 Since mouse ASGPR also seems to bind to α2,6-sialylated glycans in addition to ß-linked galactose and N-acetylgalactosamine, the human α2,3-sialyltransferase ST3GAL4 - and not an ST6GAL1 - was selected for increasing the level of antibody sialylation.Citation36,37 In contrast, in human serum IgA1 more than 95% of sialic acids were linked in the α2,6-configuration.Citation18
The N-glycan profiles of each of the IgA antibodies were determined using a novel, robust MALDI-TOF MS method following glycan ethyl esterification, which not only avoids the usually occurring loss of sialic acid residues in MALDI-TOF analysis, but also allows determination of their linkage type.Citation38 N-glycans from the antibodies IgA1-1g5 and IgA22-4g5 were highly α2,3-sialylated, whereas N-glycans from antibodies IgA21-2g4 and −2g5 exhibited low levels of both α2,3- and α2,6-sialylation and very high levels of Lewis A or X epitope (not distinguished by MS). These two IgA2m1 antibodies displayed very similar N-glycan profiles, with IgA21-2g5 displaying a moderately higher level of not only α2,3-sialylation, but oddly enough, also of α2,6-linked sialic acid residues, despite being generated in the presence of ectopically expressed α2,3-sialyltransferase. Perhaps, extensive sialylation of the exposed LacNAc termini by endogenous or co-expressed sialyltransferases was prevented by prior α1,3-fucosylation.Citation39 N-glycan profiles of the antibodies IgA22-4g2 and IgA22Δ18-5g2 produced in transiently transfected HEK293F cells were remarkably different from those produced in stably transfected PER.C6 cells, including IgA22-4g5, i.e., the same IgA2m2 sequence as IgA22-4g2, but produced in PER.C6 cells. The two preparations also differed from each other in that IgA22Δ18-5g2 – lacking the tailpiece – hardly contained high-mannose N-glycans in contrast to IgA22-4g2. A analogous phenomenon was noticed by Brunke et al. when comparing N-glycans from 2 IgA2m1 antibodies produced in CHO-K1, one a wild-type sequence and the other a mutated version in which the cysteine in the tailpiece had been deleted.Citation23 Here as well, the IgA with a tailpiece mutation contained significantly lower high-mannose N-glycans compared to the wild-type IgA. It is quite conceivable, as suggested by these authors, that interfering with intracellular tailpiece interaction by deletion of the penultimate cysteine or even the complete tailpiece may alter the intracellular fate of the IgA molecule. The reduction of IgA polymerization that we observed following deletion of the tailpiece in IgA22Δ18-5g2 might facilitate trafficking through the secretory pathway, with the high-mannose N-glycans in IgA22-4g2 being indicative of retention in early endoplasmic reticulum (ER) and Golgi compartments. The sialylation levels of the IgA antibodies produced in HEK293F were as low as those of IgA21-2g4 and −2g5.
As anticipated, the plasma exposure levels of the IgA antibodies with low sialylation were much lower than of those with high sialylation. Apparently, the high rate of capping of terminal galactose with sialic acid in IgA1-1g5 and IgA22-4g5 indeed protected these antibodies from rapid clearance via the ASGPR. The high incidence of LewisA/X epitopes on both IgA21-2g4 and −2g5 may have contributed to their fast clearance through the scavenger receptor C-type lectin, for example.Citation40,41 Likewise, high-mannose N-glycans may enhance antibody clearance.Citation42
All IgA antibodies, except IgA1-1g5, displayed efficacy and potency similar to trastuzumab in the proliferation inhibition assay with BT-474 breast carcinoma cells. It is unclear why this IgA showed only partial inhibition, especially since SPR and cellular binding analysis revealed that it has HER2 affinity comparable to the other IgA antibodies. In contrast, performance of all IgA antibodies was quite similar in the ADCC assays. These assays confirmed that IgA could be superior to IgG1 with regard to the level of cell lysis when using human whole blood or granulocytes as effector cells, although this result was quite donor dependent in case of human whole blood. On the other hand, the potency of trastuzumab is better (e.g., > 7-fold lower IC50 in a 24 hours ADCC assay using human whole blood) and would in theory require lower exposure levels compared to the IgA antibodies to be efficacious. The lower IgA potency might be compensated in vivo by reaching a higher (e.g., tenfold) IgA plasma exposure.
The question as to which mouse model is most likely to provide a fair comparison of the therapeutic value of the different mIgA isotypes is difficult to answer, especially since it concerns a comparison of human IgG1 and human IgA with identical variable domains that utilize different human Fc receptors. Hence, an inevitable methodological flaw arises from the fact that interactions of these antibodies with the effector cells in (transgenic) mice will be quite different from those in patients, because the type, distribution and density of antibody receptors across human and mouse effector cells are quite different. An added complication comes from the fact that mice lack FcαRI, which requires the use of FcαRI-expressing transgenic mice for testing Fc-mediated IgA effects. In mice, human IgG1 binds avidly to all mouse FcγRs and is able to induce ADCC/ADCP with mouse NK cells and macrophages.Citation43
In vivo assessment of antibody efficacy is usually performed in xenograft models in SCID mice and is thus restricted to analyses of innate mechanisms of cancer cell killing. Hence, possible antibody-mediated elicitation or enhancement of anti-tumor humoral and cellular responses is ignored, even though such potentially therapeutic responses have been observed in patients treated with trastuzumab or cetuximab.Citation44,45,46 As FcαRI-dependent internalization of IgA-immune complexes and ensuing presentation has been clearly demonstrated, it is quite conceivable that IgA could also induce adaptive anti-tumor immunity.Citation47,48 On the other hand, Otten et al. reported that antigen presentation on DC via the FcαRI is low.Citation49 Nevertheless, if a differential effect of IgA versus IgG1 with regard to initiation of adaptive immunity does exist, our studies will not detect it.
Thus far, in vivo experiments with IgA antibodies were hampered by the fast clearance of IgA via the ASGPR. We have been able to obtain IgA preparations with sufficiently high N-linked sialylation to allow a direct comparison to an IgG1, albeit with repetitive dosing for the IgAs vs. a single IgG1 dose to obtain comparable exposure levels. In a BT-474 xenograft study, IgA1-1g5 displayed only limited efficacy compared to trastuzumab, but, using a similar dosing regimen, IgA22-4g5 showed impressive, dose-dependent tumor growth inhibition. The clear in vivo efficacy difference between IgA1-1g5 and IgA22-4g5 concurred with the results of the in vitro proliferation inhibition assays, but not with the ADCC data, which showed equivalent efficacy of the 2 antibodies with human whole blood and PMN from FcαRI-expressing transgenic mice as effector cells. Preliminary immunohistochemistry analysis indicated that both IgA antibodies reached and penetrated the tumors at least as well as trastuzumab (data not shown). Thus, with the proliferation inhibition activity being the only tangible in vitro efficacy trait differing between IgA1-1g5 and IgA22-4g5, we consider that ADCC/ADCP may not be a dominant mode-of-action in this in vivo model, implying that the superior therapeutic effect of IgA22-4g5 might be purely Fab-mediated. Then again, it has been shown unequivocally that (relatively high-dose) trastuzumab treatment in another BT-474 model in athymic nude BALB/c mice requires FcγR functions of effector cells.Citation2 Furthermore, in the A431 tumor model, efficacy is only observed in FcαRI-expressing transgenic mice. Here as well, the efficacy of a single dose trastuzumab was higher than that of multiple doses of IgA22-4g5. We conclude that the in vivo tumor cell killing capacity of IgA antibodies appears to be much less potent than that of an equivalent IgG1 in the subcutaneous BT-474 model used in this study. Consequently, even though the manufacture of high quality IgA antibodies may be perfectly feasible, one would need to identify tumor types, combination therapies or patient populations that would merit taking an IgA-based approach instead of one using IgG1. As reported recently, hybrid IgG1/IgA antibodies might be a promising way to create anti-tumor molecules efficiently employing a broad repertoire of effector cells with adequate plasma exposure.Citation50
Materials and Methods
Cell Lines
Human breast carcinoma cell lines SK-BR-3 and BT-474 were obtained from American Type Culture Collection (Rockville, MD). SK-BR-3 were cultured in McCoy's 5A medium (Lonza, BE12-688F ) supplemented with 10% v/w fetal bovine serum (FBS; Invitrogen, 10100-147), Heat-inactivated (HI), and BT-474 cells in RPMI-1640 (Lonza, BE12-702F) containing 2 mM L-glutamine (Lonza, BE17-605E) supplemented with 10% FBS (Invitrogen, 10100-147), at 37°C in a humidified incubator containing 5% CO2.
IgA production and purification
The heavy and light chain sequences for the antibodies and the human α2,3-sialyltransferase (ST3GAL4) gene were synthesized by GeneArt. Stable PER.C6 pools producing IgA were made by transfecting 4 µg of pcDNA-based vectors containing the variable regions of trastuzumab combined with the constant domains of the human IgA1, IgA2m1 and IgA2m2 heavy chains and the human kappa light chain. In three out of 4 transfections, PER.C6 cells were co-transfected with 4 μg of a pcDNA3.1-Hyg-based vector (Invitrogen, V870-20) containing the ST3GAL4 gene. Stable PER.C6 pools were cultured in CDM4PERMAb growth medium (Hyclone, SH30871.02) with 3 mM L-glutamine (Lonza, BE17-605F), 0.05% Pluronic F68 (Sigma-Aldrich, P5556), 62.5 μg/mL G418 (Life Technologies, 10131-027), and 6.25 μg/mL hygromycin B (Life Technologies, 10687-010). The four stable pools were expanded to a 10 L working volume in CDM4PERMab without selection pressure in a 20 L culture bag on an orbital shaker and a batch run of 7 days was performed. Product titers were determined using a human IgA AlphaLISA kit (PerkinElmer, AL262 C/F) according to the manufacturer's instructions. The batch cultures were harvested and filtered and the antibodies were purified by affinity chromatography using KappaSelect chromatography resin (GE Healthcare, 17-5458-01). The bound product was eluted with 0.1 M glycine (Sigma-Aldrich, 15527) pH 2.5 followed by immediate neutralization to pH 7 using 1M Tris buffer (Sigma-Aldrich, T1378). As a second purification step, SEC was performed using a HiPrepTM 26/60 SephacrylTM S-300 HR column (GE Healthcare, 17-1196-01) equilibrated with phosphate-buffered saline (PBS) pH 7.4 (Sigma-Aldrich, P5368). In each run, 10 to 15 mL partially purified IgA (KappaSelect eluate, pH adjusted) was loaded to the column using PBS pH 7.4 as running buffer. Fractions were collected in 96 deep-well plates. To prevent cross contamination, the SEC column was cleaned with 2 column volumes of 0.2 M NaOH in between each run followed by re-equilibrated with PBS, pH 7.4. The preparations were analyzed for aggregates, dimers and monomers by means of SDS-PAGE and UPLC-SEC analysis using ACQUITY UPLC BEH200 SEC 1.7 µm (Waters, 186005225). Protein concentrations were determined using A280 absorbance readings obtained with a Nanodrop 1000 spectrophotometer. In addition, antibodies were produced using transient transfection of HEK293F cells, using similar IgA expression vectors as described above. Mutations were introduced by site-specific mutagenesis. Transient transfections were conducted using the FreeStyle 293 expression system (Life Technologies) according to the manufacturer's instructions.
SDS-PAGE
Non-reducing SDS-PAGE was performed by mixing 25 μL sample with 25 μL Laemmli sample buffer (BioRad, 161-0737) and subsequent loading on 4-20% polyacrylamide gel (Criterion TGX Stain free, Biorad, 567-8094). Protein bands were visualized by means of UV using a GelDoc EZ stainfree imager (BioRad) followed by Krypton staining (Thermo Scientific, 46630) according to the manufacturer's instructions. In some samples, N-ethylmaleimide (Sigma Aldrich, E3876) was added to the mixture in a final concentration of 28 mM prior to addition of sample buffer.
N-glycan analysis
N-glycans of purified IgAs were analyzed by MALDI-TOF MS as described previously, with some minor modifications.Citation37 Briefly, 100 µL 2% SDS (Merck, 8170341000) was added to 50 µL of IgA1-1g5 (1.12 mg/mL), IgA2m1-2g4 (0.96 mg/mL), IgA2m1-2g5 (1.01 mg/mL), IgA2m2-4g5 (0.98 mg/mL), IgA2m2-4g2 (1.20 mg/mL) and IgA2m2Δ18-5g2 (1.03 mg/mL), which was then denatured 10 min at 60°C. N-glycan release was performed by adding 100 µL release mix (containing 2% NP-40 substitute (Sigma-Aldrich, 74385) and 2.5 mU PNGase F (Roche Diagnostics, 11365177001) in 2.5x PBS (Merck)) and incubating 16 h at 37°C. Then, in quadruplicate, the released glycans were ethyl esterified at the α2,6-linked sialic acids, and lactonized at the α2,3-linked sialic acids, by adding 5 µL released sample to 35 µL 0.25 M 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (Fluorochem, 024810) and 0.25 M 1-hydroxybenzotriazole (Sigma-Aldrich, 54802) in ethanol (Merck, 1009831000), and incubating 1 h at 37°C. The reaction was stopped by adding 40 µL acetonitrile (ACN) (HPLC Supra gradient, 012035) and incubating 15 min at −20°C. The derivatized glycans were extracted from the mixture by hydrophilic interaction liquid chromatography using cotton as stationary phase, and eluted in a 10 µL volume of deionized water.Citation51 Two µL of the eluted samples was transferred to a MTP AnchorChip 800/384 TF MALDI target (Bruker Daltonics, 209514), and mixed on plate with 1 µL 5 mg/mL 2,5-dihydroxybenzoic acid (Bruker Daltonics, 201346) 1 mM NaOH (Sigma-Aldrich, 71686) in 50% ACN. After letting the spots dry by air, recrystallization was performed by addition of 0.2 µL ethanol.
MALDI-TOF MS measurements were taken using reflectron positive mode of an UltraFlextreme with Smartbeam-II laser (Bruker Daltonics), controlled by Flexcontrol 3.4 Build 119 (Bruker Daltonics). After 140 ns delayed extraction, acceleration was performed at 25 kV. Laser power was set as high as possible to still allowing baseline separation of isotopic peaks. Using these settings, the instrument was calibrated using a peptide calibration standard (Bruker Daltonics, 206195). Sample spectra were acquired by summing 25000 laser shots at a frequency of 2000 Hz, using a window from m/z 1000 to 5000 with suppression up to m/z 950. As shooting pattern, complete sample random walk was used with 200 shots per raster spot. To confirm the structures of the most abundant peaks, tandem mass spectrometry (MALDI-TOF/TOF MS/MS) was performed using laser-induced disassociation.
Obtained spectra were processed in flexAnalysis 3.3 build 65 (Bruker Daltonics), by first smoothing by Savitzky-Golay filter (width 0.2, cycles 1), followed by a Top-hat filter for baseline subtraction. Peaks were picked by Snap algorithm with a minimum signal-to-noise (S/N) threshold of 6, after which the spectrum was internally calibrated. Peak lists were exported with S/N, intensity and area, and further analysis was performed in Excel.
Data from 4 independent ethyl esterification procedures were compared per sample and peaks were selected for further analysis if occurring in all spectra above a S/N threshold of 6. Then, based on the average m/z values, the most likely N-glycan compositions were assigned. Signals were excluded from further analysis if they could not be assigned to N-glycan compositions within ± 0.05 Da. For the remaining glycan compositions, the areas were calculated relative to the sum of all areas within a spectrum, and these values were also averaged for the 4 independent measurements. These average relative areas were used for further calculation.
Percentages of fucosylation, sialylation, α2,3-linked sialylation, α2,6-linked sialylation and high mannose type glycans were calculated for a sample by summing the relative ratios of compositions meeting the required criteria. Fucosylation, α2,3-linked sialylation and α2,6-linked sialylation were summed if any respective number of fucoses, lactonized or ethyl esterified sialic acids were found, while sialylation was summed if any type of sialic acid was found. High mannose was summed if the amount of found hexoses was higher than 5, while the amount of N-acetylhexosamines did not rise above 2. The average amount of fucoses, sialic acids or mannoses in the fucosylated, sialylated and high mannose type glycan fractions could be calculated by multiplying the relative areas by the number of relevant monosaccharides found.
Figures were assigned with structures following the CFG notation, made in GlycoWorkbench 2.1 build 146.Citation52
Proliferation inhibition
Cells in complete growth medium were plated in 96-well plates (90 µL/well) and incubated overnight at 37°C, 5% CO2 at the following cell densities of 6,500 SK-BR-3 and 10,000 BT-474 cells. Serial dilutions of each antibody were made in culture medium. Human IgA1 kappa and IgA2 kappa isotype control antibodies were obtained from Meridian Bioscience, Inc.. (A50555H and A50166H), human IgA kappa isotype control antibody from SouthernBiotech (0155k-01), and human IgG1 Remicade® (infliximab) from Johnson & Johnson. 10 µL of each antibody concentration was added to the 96-well plate. Cell viability was assessed after 6 days using the CellTiter-GloTM (CTG) luminescent assay kit from Promega Corporation (G7572) according to the manufacturer's instructions. Survival percentage was calculated by dividing the measured luminescence for each mAb concentration with the average mean of the control wells (no antibody added, only growth medium) multiplied by 100.
Antibody-dependent cell-mediated cytotoxicity assay
ADCC was measured by lactate dehydrogenase (LDH) release of SK-BR-3 and BT-474 breast carcinoma cells as target cells. Serial dilutions of each antibody were made in complete growth medium. 50 µL of each antibody solution was incubated for 30 minutes at 37°C, 5% CO2 with 50 µL of SK-BR-3 (10,000 cells/well) or BT-474 cells (15,000 cells/well) in 96-well round-bottom plates.
Human whole blood from healthy donors was used to isolate monocytes (MNC) and PMN using Density Gradient Centrifugation with Histopaque®-1119 (Sigma-Aldrich, 11191) and Lymphocyte Separation Medium (Lonza, 17-829F) according to the manufacturer's instruction. 50 µL of 2.5 × 105 PMN or MNC were added to 104 of the antibody-treated SK-BR-3 or BT-474 cells at an effector-to-target ratio of 25:1. Unseparated human whole blood from healthy donors was exposed to hypotonic lysis to remove remaining erythrocytes before 50 µL of human whole blood was added to the antibody-treated target cells. After 4 hours of incubation at 37°C, 5% CO2, cell supernatant was transferred to a 96-well plate to determine the amount of released LDH using a colorimetric assay (Roche, 11644793001). Percent cytotoxicity was calculated as follows: percent cytotoxicity = (“experimental” – “effector plus target spontaneous”) / (“target maximum” – “target spontaneous”) x 100%, where “experimental” corresponds to the signal measured in a treated sample, “effector plus target spontaneous” corresponds to the signal measured in the presence of PMN or MNC and tumor cells alone, “target maximum” corresponds to the signal measured in the presence of detergent lysed tumor cells (Triton X-100, Sigma Aldrich, X100), and “target spontaneous” corresponds to the signal measured in the presence of tumor cells alone.
Mice
Human FcαRI transgenic mice were generated at the UMC Utrecht, and were backcrossed to the immunodeficient SCID background (CB17/lcr‐Prkdcscid/ lcrlcoCrl, Charles River) and maintained as hemizygotes.Citation14 Transgene-negative littermates, WT BALB/c or C57BL/6 mice (Janvier) were used as controls. All animal studies were approved by local animal ethical committees and performed according to local ethical guidelines of animal experimentation.
In vivo pharmacokinetics
The IgA antibodies were dosed to 7 weeks old female mice (CB-17/lcr-Prkdc SCID) via an intravenous bolus injection into the tail vein. Blood samples were taken via terminal intracardiac puncture under general anesthesia (isoflurane-O2 mixture) in different mice at several time points after dosing and processed to K2-EDTA plasma. Plasma samples were stored at −80°C until bioanalysis. Based on the reported plasma levels, PK parameters were calculated in WinNonlin version 5.3 using the non-compartmental analysis for single intravenous bolus injection.
PK ELISA
An ELISA-based method was used for the determination of trastuzumab and anti-HER2 IgA antibody plasma concentrations. Study samples were diluted in appropriate buffers in order to be able to estimate the antibody concentrations within the analytical range. The solid phase consisted of an anti-idiotypic mini antibody (AbD Serotec, AbD15916) coated on high binding micro titer plates (Greiner, 655081). Detection of antibody captured on the solid phase was achieved by a biotinylated anti-idiotype mini antibody, followed by a streptavidin-HRP (R&D Systems, DY998) incubation step and TMB (TeBu-Bio, TMB100) incubation. The color reaction was stopped with H2SO4 and the plate was read at 450 and 630 nm. Each analytical run included appropriate calibrators and quality-control samples. Antibody concentrations of study samples were back calculated on the calibrator curve.
BT-474 cell-line xenograft studies
BT-474 cell-line xenograft studies were conducted at Oncodesign, Dijon, France. Female FcαRI-transgenic SCID mice were housed in polycarbonate cages (Tecniplast) that are equipped to provide food and water and contain environmental enrichment in a specific-pathogen-free (SPF) animal care unit.Citation14 Animal food (DIETEX) and water are provided ad-libitum. BT-474 tumors were induced in mice at the age of 35–46 weeks by a subcutaneous injection of 2 × 107 BT-474 cells in 200 μL of RPMI 1640 containing Matrigel (50:50, v:v; BD Biosciences, 356237) into the right flank. Mice were randomized according to their individual tumor volume into groups of 8 animals. At start of treatment, the mean tumor volume (in between 200-300 mm3) of each group did not differ from the others (analysis of variance). Treatment was by a single intravenous bolus injection in the tail vein (trastuzumab) or by intraperitoneal injection 10 times once daily (IgA mAbs and vehicle). Individual animals were sacrificed when tumor sizes were 2000 mm3 or at the end of the study (typically between day 70-90).
Disclosure of potential conflicts of interest
GJA Rouwendal, MM van der Lee, J Schouten, G de Roo, DF Egging, GF Verheijden, R Ubink, WH Dokter, and M Timmers are Synthon Biopharmaceuticals B.V. employees. JHW Leusen and M Wuhrer received research funding from Synthon Biopharmaceuticals B.V.
Supplemental material
Supplemental data for this article can be accessed on the publisher's website.
Rouwendal et al Supplemental Data
Download PDF (400.1 KB)Acknowledgments
We gratefully acknowledge technical assistance from Karin de Laat, Jochem Eigenhuijsen, Jos Verhagen, Dirk Glaudemans, Erin Hendriks-Franssen Wever, Meng Liu, Kim Burgers, Daniëlle Jacobs, Tanja van Achterberg, and Ellen Santegoeds-Lenssen from Synthon Biopharmaceuticals B.V. and Maaike Nederend and Marco Jansen from Laboratory for Translational Immunology (UMC Utrecht).
References
- Clynes R, Takechi Y, Moroi Y, Houghton A, Ravetch JV. Fc receptors are required in passive and active immunity to melanoma. Proc Natl Acad Sci U S A 1998; 95:652-6; PMID:9435247; http://dx.doi.org/10.1073/pnas.95.2.652
- Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 2000; 6:443-6; PMID:10742152; http://dx.doi.org/10.1038/74704
- Nimmerjahn F, Ravetch JV. Translating basic mechanisms of IgG effector activity into next generation cancer therapies. Cancer Immun 2012; 12:13; PMID:22896758
- Valerius T, Stockmeyer B, van Spriel AB, Graziano RF, van den Herik-Oudijk IE, Repp R, Deo YM, Lund J, Kalden JR, Gramatzki M, et al. FcαRI (CD89) as a novel trigger molecule for bispecific antibody therapy. Blood 1997; 90:4485-92; PMID:9373259
- Huls G, Heijnen IAFM, Cuomo E, van der Linden J, Boel E, van de Winkel JGJ, Logtenberg T. Antitumor immune effector mechanisms recruited by phage display-derived fully human IgG1 and IgA1 monoclonal antibodies. Cancer Res 1999; 59:5778-84; PMID:10582699
- Stockmeyer B, Dechant M, van Egmond M, Tutt AL, Sundarapandiyan K, Graziano RF, Repp R, Kalden JR, Gramatzki M, Glennie MJ, et al. Triggering Fcα-receptor I (CD89) recruits neutrophils as effector cells for CD20-directed antibody therapy. J Immunol 2000; 16:5954-61; http://dx.doi.org/10.4049/jimmunol.165.10.5954
- Braster R, O'Toole T, van Egmond M. Myeloid cells as effector cells for monoclonal antibody therapy of cancer. Methods 2014; 65:28-37; PMID:23811299; http://dx.doi.org/10.1016/j.ymeth.2013.06.020
- Otten MA, Bakema JE, Tuk CW, Glennie MJ, Tutt AL, Beelen RH, van de Winkel JGJ, van Egmond M. Enhanced FcαRI-mediated neutrophil migration towards tumour colonies in the presence of endothelial cells. Eur J Immunol 2012; 42:1815-21; PMID:22535639; http://dx.doi.org/10.1002/eji.201141982
- Dechant M, Beyer T, Schneider-Merck T, Weisner W, Peipp M, van de Winkel JGJ, Valerius T. Effector mechanisms of recombinant IgA antibodies against epidermal growth factor receptor. J Immunol 2007; 179:2936-43; PMID:17709508; http://dx.doi.org/10.4049/jimmunol.179.5.2936
- Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002; 99:754-8; PMID:11806974; http://dx.doi.org/10.1182/blood.V99.3.754
- Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol 2003; 21:3940-47; PMID:12975461; http://dx.doi.org/10.1200/JCO.2003.05.013
- Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 2008; 26:1789-96; PMID:18347005; http://dx.doi.org/10.1200/JCO.2007.14.8957
- Boross P, Lohse S, Nederend M, Jansen JH, van Tetering G, Dechant M, Peipp M, Royle L, Liew LP, Boon L, et al. IgA EGFR antibodies mediate tumour killing in vivo. EMBO Mol Med 2013; 5:1213-26; PMID:23918228; http://dx.doi.org/10.1002/emmm.201201929
- van Egmond M, van Vuuren AJH, van de Winkel JGJ. The human Fc receptor for IgA (FcαRI, CD89) on transgenic peritoneal macrophages triggers phagocytosis and tumor cell lysis. Immunol Lett 1999; 68:83-7; PMID:10397160; http://dx.doi.org/10.1016/S0165-2478(99)00034-6
- Launay P, Grossetête B, Arcos-Fajardo M, Gaudin E, Torres SP, Beaudoin L, Patey-Mariaud de Serre N, Lehuen A, Monteiro RC. Fcalpha receptor (CD89) mediates the development of immunoglobulin A (IgA) nephropathy (Berger's disease). Evidence for pathogenic soluble receptor-Iga complexes in patients and CD89 transgenic mice. J Exp Med 2000; 191:1999-2009; PMID:10839814; http://dx.doi.org/10.1084/jem.191.11.1999
- Soua Z, Ghanem N, Ben Salem M, Lefranc G, Lefranc MP. Frequencies of the human immunoglobulin IGHA2*M1 and IGHA2*M2 alleles corresponding to the A2m(1) and A2m(2) allotypes in the French, Lebanese, Tunisian and black African populations. Nucleic Acids Res 1989; 17:3625; PMID:2566982; http://dx.doi.org/10.1093/nar/17.9.3625
- Toraño A, Tsuzukida Y, Liu YS, Putnam FW. Location and structural significance of the oligosaccharides in human IgA1 and IgA2 immunoglobulins. Proc Natl Acad Sci U S A 1977; 74:2301-5; http://dx.doi.org/10.1073/pnas.74.6.2301
- Mattu TS, Pleass RJ, Willis AC, Kilian M, Wormald MR, Lellouch AC, Rudd PM, Woof JM, Dwek RA. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-Glycosylation on Fcα receptor interactions. J Biol Chem 1998; 273:2260-72; PMID:9442070; http://dx.doi.org/10.1074/jbc.273.4.2260
- Tsuzukida Y, Wang CC, Putnam FW. Structure of the A2m(1) allotype of human IgA - a recombinant molecule. Proc Natl Acad Sci U S A 1979; 76:1104-8; PMID:286295; http://dx.doi.org/10.1073/pnas.76.3.1104
- Zhang N, Liu L, Dumitru CD, Cummings NR, Cukan M, Jiang Y, Li Y, Li F, Mitchell T, Mallem MR, et al. Glycoengineered Pichia produced anti-HER2 is comparable to trastuzumab in preclinical study. mAbs 2011; 3:289-98; PMID:21487242; http://dx.doi.org/10.4161/mabs.3.3.15532
- Beyer T, Lohse S, Berger S, Peipp M, Valerius T, Dechant M. Serum-free production and purification of chimeric IgA antibodies. J Immunol Methods 2009; 346:26-37; PMID:19427867; http://dx.doi.org/10.1016/j.jim.2009.05.002
- Yoo EM, Coloma MJ, Trinh KR, Nguyen TQ, Vuong LU, Morrison SL, Chintalacharuvu KR. Structural requirements for polymeric immunoglobulin assembly and association with J chain. J Biol Chem 1999; 274:33771-7; PMID:10559270; http://dx.doi.org/10.1074/jbc.274.47.33771
- Brunke C, Lohse S, Derer S, Peipp M, Boross P, Kellner C, Beyer T, Dechant M, Royle L, Liew LP, et al. Effect of a tail piece cysteine deletion on biochemical and functional properties of an epidermal growth factor receptor-directed IgA2 m(1) antibody. MAbs 2013; 5:936-45; PMID:24492345; http://dx.doi.org/10.4161/mabs.26396
- Chintalacharuvu KR, Yu LJ, Bhola N, Kobayashi K, Fernandez CZ, Morrison SL. Cysteine residues required for the attachment of the light chain in human IgA2. J Immunol 2002; 169:5072-7; PMID:12391223; http://dx.doi.org/10.4049/jimmunol.169.9.5072
- Chintalacharuvu KR, Gurbaxani B, Morrison SL. Incomplete assembly of IgA2m(2) in Chinese hamster ovary cells. Mol Immunol 2007; 44:3445-52; PMID:17467056; http://dx.doi.org/10.1016/j.molimm.2006.12.030
- Fallgren-Gebauer E, Gebauer W, Bastian A, Kratzin HD, Eiffert H, Zimmermann B, Karas M, Hilschmann N. The covalent linkage of secretory component to IgA. Structure of sIgA. Biol Chem Hoppe Seyler 1993; 374:1023-8; http://dx.doi.org/10.1515/bchm3.1993.374.7-12.1023
- Smith RI, Morrison SL. Recombinant polymeric IgG: an approach to engineering more potent antibodies. Biotechnology (N Y) 1994; 12:683-8; PMID:7764912; http://dx.doi.org/10.1038/nbt0794-683
- Sørensen V, Rasmussen IB, Sundvold V, Michaelsen TE, Sandlie I. Structural requirements for incorporation of J chain into human IgM and IgA. Int Immunol 2000; 12:19-27; PMID:10607746; http://dx.doi.org/10.1093/intimm/12.1.19
- Rifai A, Fadden K, Morrison SL, Chintalacharuvu KR. The N-glycans determine the differential blood clearance and hepatic uptake of human immunoglobulin (Ig)a1 and Iga2 isotypes. J Exp Med 2000; 191:2171-81; PMID:10859341; http://dx.doi.org/10.1084/jem.191.12.2171
- Petkova SB, Akilesh S, Sproule TJ, Christianson GJ, Al Khabbaz H, Brown AC, Presta LG, Meng YG, Roopenian DC. Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized FcRn mouse model: potential application in humorally mediated autoimmune disease. Int Immunol 2006; 18:1759-69; PMID:17077181; http://dx.doi.org/10.1093/intimm/dxl110
- Morell A, Terry WD, Waldmann TA. Metabolic properties of IgG subclasses in man. J Clin Invest 1970; 49:673-80; PMID:5443170; http://dx.doi.org/10.1172/JCI106279
- Blaese RM, Strober W, Levy AL, Waldmann TA. Hypercatabolism of IgG, IgA, IgM, and albumin in the Wiskott-Aldrich syndrome. A unique disorder of serum protein metabolism. J Clin Invest 1971; 50:2331-8; PMID:5096517; http://dx.doi.org/10.1172/JCI106731
- Delacroix DL, Elkom KB, Geubel AP, Hodgson HF, Dive C, Vaerman JP. Changes in size, subclass, and metabolic properties of serum immunoglobulin A in liver diseases and in other diseases with high serum immunoglobulin A. J Clin Invest 1983; 71:358-67; PMID:6401770; http://dx.doi.org/10.1172/JCI110777
- Moldoveanu Z, Epps JM, Thorpe SR, Mestecky J. The sites of catabolism of murine monomeric IgA. J Immunol 1988; 141:208-13; PMID:2454258
- Weikert S, Papac D, Briggs J, Cowfer D, Tom S, Gawlitzek M, Lofgren J, Mehta S, Chisholm V, Modi N, et al. Engineering Chinese hamster ovary cells to maximize sialic acid content of recombinant glycoproteins. Nat Biotechnol 1999; 17:1116-21; PMID:10545921; http://dx.doi.org/10.1038/15104
- Park EI, Mi Y, Unverzagt C, Gabius HJ, Baenziger JU. The asialoglycoprotein receptor clears glycoconjugates terminating with sialic acid α 2,6GalNAc. Proc Natl Acad Sci U S A 2005; 102:17125-9PMID:16286643; http://dx.doi.org/10.1073/pnas.0508537102
- Steirer LM, Park EI, Townsend RR, Baenziger JU. The asialoglycoprotein receptor regulates levels of plasma glycoproteins terminating with sialic acid α2,6-galactose. J Biol Chem 2009; 284:3777-83; PMID:19075021; http://dx.doi.org/10.1074/jbc.M808689200
- Reiding KR, Blank D, Kuijper DM, Deelder AM, Wuhrer M. High-throughput profiling of protein N-glycosylation by MALDI-TOF-MS employing linkage-specific sialic acid esterification. Anal Chem 2014; 86:5784-93; PMID:24831253; http://dx.doi.org/10.1021/ac500335t
- Kitagawa H, Paulson JC. Cloning of a novel α 2,3-sialyltransferase that sialylates glycoprotein and glycolipid carbohydrate groups. J Biol Chem 1994; 269:1394-1401; PMID:8288606
- Coombs PJ, Graham SA, Drickamer K, Taylor ME. Selective binding of the scavenger receptor C-type lectin to Lewisx trisaccharide and related glycan ligands. J Biol Chem 2005; 280:22993-9; PMID:15845541; http://dx.doi.org/10.1074/jbc.M504197200
- Graham SA, Antonopoulos A, Hitchen PG, Haslam SM, Dell A, Drickamer K, Taylor ME. Identification of neutrophil granule glycoproteins as LewisX-containing ligands cleared by the scavenger receptor C-type lectin. J Biol Chem 2011; 286:24336-49; PMID:21561871; http://dx.doi.org/10.1074/jbc.M111.244772
- Goetze AM, Liu YD, Zhang Z, Shah B, Lee E, Bondarenko PV, Flynn GC. High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans. Glycobiology 2011; 21:949-59; PMID:21421994; http://dx.doi.org/10.1093/glycob/cwr027
- Overdijk MB, Verploegen S, Ortiz Buijsse A, Vink T, Leusen JH, Bleeker WK, Parren PW. Crosstalk between human IgG isotypes and murine effector cells. J Immunol 2012; 189:3430-8; PMID:22956577; http://dx.doi.org/10.4049/jimmunol.1200356
- Disis ML, Calenoff E, McLaughlin G, Murphy AE, Chen W, Groner B, Jeschke M, Lydon N, McGlynn E, Livingston RB, et al. Existent T-cell and antibody immunity to HER2/neu protein in patients with breast cancer. Cancer Res 1994; 54:16-20; PMID:7505195
- Taylor C, Hershman D, Shah N, Suciu-Foca N, Petrylak DP, Taub R, Vahdat L, Cheng B, Pegram M, Knutson KL, et al. Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin Cancer Res 2007; 13:5133-43; PMID:17785568; http://dx.doi.org/10.1158/1078-0432.CCR-07-0507
- Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, López-Albaitero A, Gibson SP, Gooding WE, Ferrone S, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res 2013; 19:1858-72; PMID:23444227; http://dx.doi.org/10.1158/1078-0432.CCR-12-2426
- Shen L, van Egmond M, Siemasko K, Gao H, Wade T, Lang ML, Clark M, van De Winkel JGJ, Wade WF. Presentation of ovalbumin internalized via the immunoglobulin-A Fc receptor is enhanced through Fc receptor γ-chain signaling. Blood 2001; 97:205-13; PMID:11133762; http://dx.doi.org/10.1182/blood.V97.1.205
- Lang ML, Shen L, Gao H, Cusack WF, Lang GA, Wade WF. Fcα receptor cross-linking causes translocation of phosphatidylinositol-dependent protein kinase 1 and protein kinase Bα to MHC Class II peptide-loading-like compartments. J Immunol 2001; 166:5585-93; PMID:11313398; http://dx.doi.org/10.4049/jimmunol.166.9.5585
- Otten MA, Groenveld I, van de Winkel JGJ, van Egmond M. Inefficient antigen presentation via the IgA Fc receptor (FcαRI) on dendritic cells. Immunobiology 2006; 211:503-10; PMID:16920489; http://dx.doi.org/10.1016/j.imbio.2006.05.016
- Borrok MJ, Luheshi NM, Beyaz N, Davies GC, Legg JW, Wu H, Dall'Acqua WF, Tsui P. Enhancement of antibody-dependent cell-mediated cytotoxicity by endowing IgG with FcαRI (CD89) binding. MAbs 2015; 7(4):743-51
- Selman MHJ, Hemayatkar M, Deelder AM, Wuhrer M. Cotton HILIC SPE microtips for microscale purification and enrichment of glycans and glycopeptides. Anal Chem 2011; 83:2492-9; PMID:21366235; http://dx.doi.org/10.1021/ac1027116
- Ceroni A, Maass K, Geyer H, Geyer R, Dell A, Haslam SM. GlycoWorkbench: a tool for the computer-assisted annotation of mass spectra of glycans. J Proteome Res 2008; 7:1650-9; PMID:18311910; http://dx.doi.org/10.1021/pr7008252
