Abstract
Fusion to an IgG Fc region is an established strategy to extend the half-life of therapeutic proteins. Most Fc fusion proteins, however, do not achieve the long half-life of IgGs. Based on findings that scFv-Fc fusion proteins exhibit a shorter half-life than the corresponding IgG molecules, we performed a comparative study of different antibody-derived Fc fusion proteins. We could confirm that fusion of single-chain Fv (scFv) and single-chain diabody (scDb) molecules to an Fc region yields in fusion proteins with substantially extended half-lives compared with the single-chain versions. However, even fusion proteins with a size similar to that of IgG, e.g., scDb-Fc, did not have a half-life as long as an IgG molecule. Binding to the neonatal Fc receptor (FcRn) under acidic and neutral conditions was similar for IgG and all Fc fusion proteins. However, we observed differences between IgG and the Fc fusion proteins for dissociation of FcRn-bound proteins induced by shifting from acidic to neutral pH, reflecting the physiological release mechanism, further supporting a contribution of the kinetics of pH-dependent release from FcRn to the pharmacokinetic properties of IgG and Fc fusion proteins.
Abbreviations
| AUC | = | area-under-the curve |
| CEA | = | carcinoembryonic antigen |
| Fc | = | fragment crystallizable |
| IgG | = | immunoglobulin G |
| QCM | = | quartz crystal microbalance |
| scFv | = | single-chain Fragment variable |
| scDb | = | single-chain Diabody |
| VH | = | antibody heavy chain variable domain |
| VL | = | antibody light chain variable domain. |
Introduction
The fusion of therapeutic proteins to the immunoglobulin Fc is a well-established strategy to improve bioactivity through dimerization, and to extend the plasma half-life by both increasing the size and implementing rescue from intracellular degradation by the neonatal Fc receptor (FcRn)-mediated recycling process.Citation1-4 Examples of approved Fc fusion proteins include etanercept (Enbrel®), belatacept (Nulojix®) and romiplostim (NPlate®), which are composed of Fc regions fused with tumor necrosis factor receptor 2, the extracellular region of CTLA-4 and a TPO-mimetic peptide, respectively.Citation5 The Fc region has also served as the basis of targeted IgG-like therapeutics and imaging molecules by fusing, for example, an scFv fragment to the N-terminus of the Fc fragment and adding a further therapeutic compound to the C-terminus.Citation6-8
Fc fusion proteins such as scFv-Fc are similar in size to IgG molecules (100 kDa versus 150 kDa), and both are capable of utilizing Fc-mediated recycling, resulting in a substantially extended half-life in mice.Citation9 Nevertheless, several studies demonstrated that Fc fusion proteins have a shorter plasma half-life in mice than IgG molecules.Citation3,10,Citation11 While differences in affinity for FcRn was proposed as a possible mechanism responsible for the shorter half-lives of Fc fusion proteins in humans,Citation3,12 others failed to find a correlation between affinity for FcRn and the serum half-life for a family of antibodies investigated in mice and humans.Citation13
Here, we generated various carcinoembryonic antigen (CEA)-targeted IgG-like fusion proteins and compared their biochemical and pharmacokinetic properties to a chimeric IgG1 molecule. These IgG-like molecules included an scFv-Fc, a bispecific single-chain diabody Fc-fusion protein (scDb-Fc), as well as a single-chain version of an IgG molecule generated by fusing an scFv to a single-chain CL-CH1 (scCLCH1) moiety further fused to the Fc region (scFv-scCLCH1-Fc). In addition, an scDb-CH2 fusion protein was included in these studies. All proteins were analyzed for stability, binding activity, binding to the FcRn at acidic and neutral pH, and their pharmacokinetic properties in mice.
Results
IgG and scFv-Fc fusion proteins
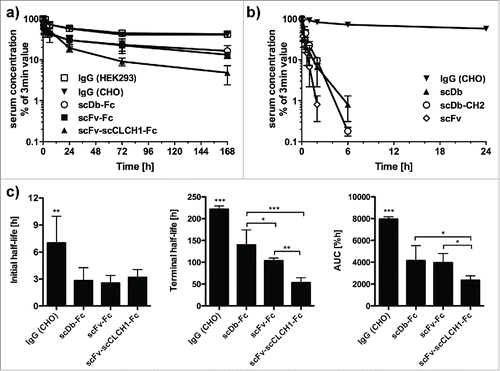
A chimeric anti-CEA IgG1 was generated by fusing the VH and VL domain of antibody MFE-23 to the human constant domains,Citation14,15 and a corresponding anti-CEA scFv-Fc fusion protein was generated by fusing the scFv MFE-23 to the γ1 hinge region of the human Fc fragment (). Both proteins were produced in stably transfected HEK293 cells and purified by protein A chromatography. In addition, the anti-CEA IgG1 was also produced in CHO-K1 cells. Proteins were purified by protein A affinity chromatography. Analysis of the pharmacokinetic properties of the IgG and scFv-Fc fusion protein in CD1 mice showed an ∼2-fold longer terminal half-life and a 2-fold increased AUC for the IgG1 molecule compared to the scFv-Fc fusion protein (, ). No differences were observed between the HEK293 and CHO-K1 produced IgGs. Similar results were also obtained for the chimeric anti-epidermal growth factor receptor (EGFR) IgG1 cetuximab compared to an scFv-Fc derivative thereof generated from a humanized version of cetuximab (scFv-hu225) (, ).
Figure 1. Schematic composition of the various proteins. Carbohydrates are shown as hexagonal gray symbols.
Figure 2. Pharmacokinetics of IgG1 and scFv-Fc fusion proteins. a) Anti-CEA antibody molecules. b) Anti-EGFR antibody molecules. CD1 mice received a single injection of 25 µg protein and serum concentrations were determined by ELISA.
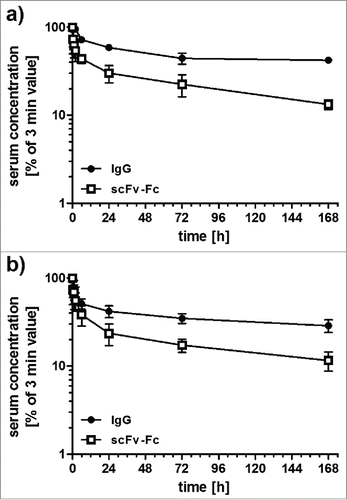
Table 1 Biochemical and pharmacokinetic properties
Anti-CEA IgG-like fusion proteins
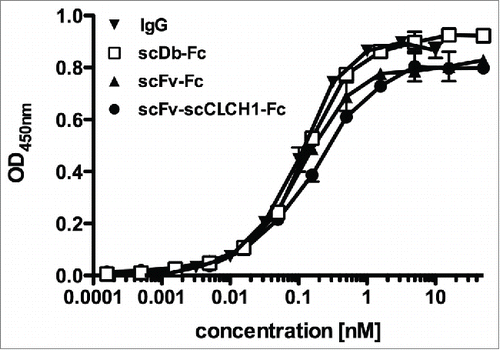
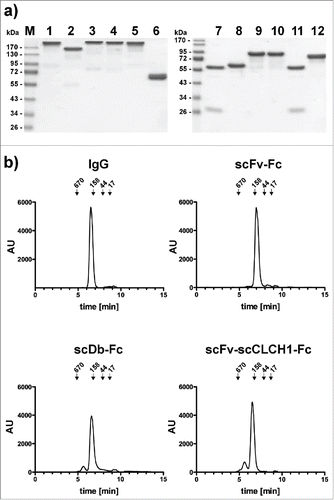
To further investigate the influence of molecule size on pharmacokinetic properties, we generated a fusion protein, (scFv-scCLCH1-Fc) in which the anti-CEA scFv fragment was fused to a single-chain version of the CL and CH1 heterodimer, with the CH1 domain connected to the human γ1 Fc region (). In addition, we generated a bispecific IgG-like fusion protein by fusing a bispecific single-chain diabody (scDb) directed against CEA and CD3Citation16 to the human γ1 Fc region. Both fusion proteins included the complete hinge region. ScFv-scCLCH1-Fc and scDb-Fc exhibit a calculated molecular mass similar to that of IgG1 (see ). All proteins were produced in stably transfected HEK293 cells. As a further approach, we included an scDb-CH2 fusion protein based on reports that the CH2 domain might be useful as half-life extension module.Citation17,18 This scDb-CH2 fusion protein was endowed with a C-terminal hexahistidyl-tag for purification, while all other fusion proteins were tag-less. SDS-PAGE analysis as well as size-exclusion chromatography confirmed integrity and correct assembly of the fusion proteins (). The Stokes radii of scFv-scCLCH1-Fc and scDb-Fc were identical to that of the IgG molecule (5.5 to 5.6 nm), while a Stokes radius of 4.8 nm was determined for the scFv-Fc fusion protein (). These Stokes radii are much larger than that of the anti-CEA scFv (2.5 nm) and the scDb CEAxCD3 (2.6 nm) (). Correct assembly was further confirmed by ELISA with immobilized CEA. Here, all Fc-fusion proteins bound with similar sub-nanomolar EC50 values to CEA ().
Figure 3. a) SDS-PAGE analysis of IgG and Fc fusion proteins (lanes 1 and 7, IgG1 produced in CHO cells; lanes 2 and 8, scFv-Fc; lanes 3 and 9, scDb-Fc; lanes 4 and 10, scFv-scCLCH1-Fc; lanes 5 and 11, IgG1 produced in HEK293 cells; lanes 6 and 12, scDb-CH2) analyzed under non-reducing (1–6) or reducing (7–12) conditions. Proteins were stained with Coomassie blue. b) Size-exclusion chromatography of IgG and Fc fusion proteins as indicated.
Stability of Fc-fusion proteins
Thermal stability of the proteins was analyzed by dynamic light scattering (, ). The scFv showed a melting point of 46°C. For the scFv-Fc fusion protein, we could determine 2 melting points of 46°C and 76°C, the latter probably reflecting melting of the Fc region. The IgG molecule also exhibited 2 melting points of 58°C and 76°C, indicating that conversion of the scFv into a Fab fragment increases stability. A stabilizing effect was also observed for the scFv-scCLCH1-Fc molecule, which had melting temperatures of 54°C and 76°C. The scDb-Fc fusion protein showed a prominent melting point at 51°C, identical to the unfused scDb. Similar results were obtained for scDb-CH2 fusion protein (not shown).
Figure 5. a) Thermal melting points of IgG and Fc fusion proteins determined by dynamic light scattering using 1°C intervals and 2 min equilibration time. A drastic increase in mean count rates is defined as melting point and indicated by dashed lines. b) In vitro serum stability of IgG and Fc fusion proteins. Proteins were incubated with mouse serum at 37°C for up to 7 d and active protein was determined by ELISA (IgG and Fc fusion proteins with an anti-Fc antibody, scDb-CH2 either with an anti-His-tag antibody or protein L).
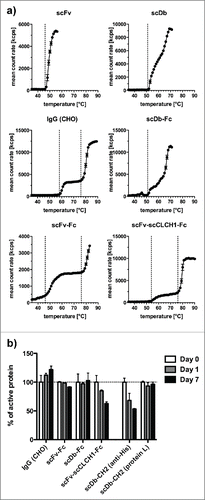
Serum stability of the proteins was tested with mouse serum at 37°C. No significant loss of antigen binding activity was detected after 7 d for IgG, scFv-Fc and scDb-Fc, while the scFv-scCLCH1-Fc molecule showed an ∼35% reduction until day 7. The scDb-CH2 molecules showed a more than 45% reduction until day 7 using detection with an anti-His-tag antibody recognizing the C-terminal His-tag, while with protein L, binding to the VL domains of the scDb moiety, no reduction was observed, indicating that either the His-tag or the CH2 domain were partially cleaved from the fusion protein ().
Binding kinetics for mouse and human FcRn
Affinities of the IgG and the Fc fusion proteins for mouse FcRn were determined by quartz crystal microbalance measurements using biotinylated FcRn immobilized on the sensor chip. Measurements performed at pH 6.0 revealed very similar biphasic binding curves for all 4 proteins, with KD values of ∼100 nM for monovalent binding (), and a negligible binding at pH 7.4, with no obvious differences between IgG1 and the Fc fusion proteins (). The scDb-CH2 showed only a very weak interaction at pH 6.0 and, interestingly, an increased binding at pH 7.4.
Figure 6. a) QCM measurements of binding of IgG and Fc fusion proteins to immobilized mouse FcRn (at a density of 50 Hz) at pH 6.0 or pH 7.4. For comparison, binding curves are shown for 100 nM protein, except for scDb-CH2 which was analyzed at 1 µM. b) Analysis of dissociation of proteins bound to mouse FcRn (at pH 6.0) by shifting pH to 7.4. c) Analysis of dissociation of IgG and scFv-Fc fusion protein to human FcRn at pH 6.0 by shifting pH to 7.4.
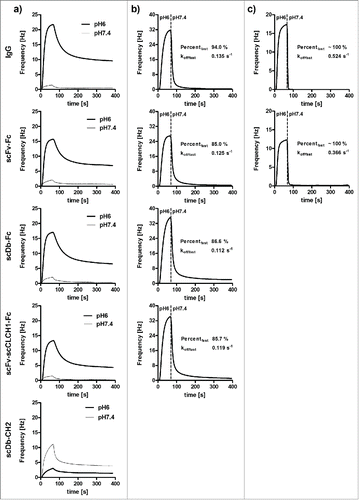
Table 2 Binding to mouse FcRn
To further investigate the kinetics of dissociation of FcRn-bound molecules at neutral pH, IgG and the Fc fusion proteins were bound to mouse FcRn at pH 6 followed by a change to pH 7.4. Here, compared to the Fc fusion proteins, a faster dissociation from FcRn was observed for the IgG (). Using a biphasic fitting algorithm, the fast dissociating fraction was calculated to be 94% for IgG compared to 85–87% for the 3 Fc fusion proteins, which correlated with a slightly faster dissociation rate constant of this fraction of the IgG (0.135 s−1) compared to the Fc fusion proteins (0.113 - 0.125 s−1). The IgG and scFv-Fc fusion protein were also analyzed for their binding kinetics using immobilized human FcRn (). For both proteins, we observed a rapid dissociation with a dissociation rate constant of 0.524 s−1 for IgG and an approximately 70% slower dissociation for scFv-Fc (dissociation rate constant of 0.366 s−1).
Pharmacokinetic properties
Pharmacokinetics were determined in CD1 mice after a single injection of 25 µg protein into the tail vein. Plasma concentrations were determined by ELISA with immobilized CEA, thus measuring the concentration of active protein. A terminal half-life of ∼9.5 d was determined for the IgG1 (, ). No significant differences were found for IgG1 produced in CHO and HEK293 cells. Reduced initial and terminal half-lives were observed for scFv-Fc and scDb-Fc. The shortest half-life of the Fc-fusion proteins was determined for scFv-scCLCH1-Fc. The reduced half-life was also reflected by a reduced AUC (). However, all Fc-fusion proteins showed a dramatically prolonged half-life and AUC compared with the scFv and scDb molecules. In contrast, the scDb-CH2 molecule had pharmacokinetic properties similar to the unmodified scDb.
Figure 7. a) Pharmacokinetics of IgG and Fc fusion proteins. b) Pharmacokinetics of scFv as well as scDb and scDb-CH2. CD1 mice received a single injection of 25 µg protein and serum concentrations were determined by ELISA. c) Statistical analysis of terminal half-lives and AUCs of the IgGs and Fc fusion proteins.
Discussion
Our comparative study of different anti-CEA molecules confirmed that fusion of different antibody fragments (scFv, scDb, scFv-scCLCH1) to an Fc region results in strongly increased plasma half-lives. None of the Fc-fusion proteins, however, reached the long half-life of a chimeric IgG directed against the same target. This was also observed for an anti-EGFR IgG compared to an scFv-Fc fusion protein. The faster clearance might be due to a lower molecular mass and hydrodynamic radius of the scFv-Fc fusion proteins. However, analyzing an scDb-Fc and scFv-scCLCH1-Fc fusion protein with a size and hydrodynamic radius similar to that of IgG molecules did not improve the pharmacokinetic properties compared to scFv-Fc fusion protein, which was reflected by a faster initial and terminal half-life. Here, the scFv-scCLCH1-Fc fusion protein, which was also included in this study to analyze possible effects of the first constant domains on pharmacokinetics, had a similar initial half-life as the scDb-Fc fusion proteins (∼3 h), while the terminal half-life was much shorter (53 h vs. 140 h). This is probably due to a reduced serum stability observed for the scFv-scCLCH1-Fc fusion protein but not for the other Fc fusion proteins.
A multitude of factors has been proposed to contribute to differences of half-lives of IgG and Fc fusion proteins.Citation19 This includes differences in interaction with FcRn at acidic and neutral pH, steric hindrance between the Fc-domain and the fusion partner influencing protein stability and interaction with FcRn, alternative clearance pathways dependent on the fusion partner, and a contribution of the Fab arms or the molecular architecture to FcRn binding and recycling.Citation4,20,Citation21 Furthermore, differences in glycosylation, e.g., caused by using different expression systems, might influence half-life.Citation22 Our finding that IgGs produced in CHO-K1 or HEK293 cells exhibit identical pharmacokinetic properties in mice indicates that the production system is not responsible for the reduced half-life of our Fc fusion proteins produced in HEK293 cells. We can also exclude possible differences due to target-mediated clearance because of the absence of CEA in mice and lack of cross-reactivity of the anti-EGFR antibodies with mouse EGFR and the CD3-binding bispecific antibodies with mouse CD3.
Another factor that might influence the elimination of proteins is the net charge of the protein. For example, a comparison of IgG4 antibodies with variable domains of different isoelectric points showed that molecules with a lower isoelectric point had a longer half-life.Citation23 A recent study confirmed this finding for antibodies with identical Fc regions but different Fab arms, which was attributed to differences in interaction with FcRn.Citation20 It was postulated that fractions of antibodies with slower off-rates stay bound to FcRn during recycling to the plasma membrane and are not released back into circulation. This was also described in a recent study comparing 2 anti-IL-12/IL-23 antibodies, which showed that the charge distribution in the VH domain contributes to excessive FcRn binding, preventing efficient FcRn-IgG dissociation under physiological pH.Citation21 In this context, 2 factors would contribute to efficient recycling and long half-life: 1) strong and fast binding at acidic pH, and 2) weak binding and fast release from FcRn at neutral pH.Citation19 This assumption is in accordance with findings that antibodies engineered to exhibit increased binding to FcRn at neutral pH are rapidly cleared from circulation.Citation24,25 This might also be the reason for the short half-life of the scDb-CH2 fusion protein, which showed increased binding to FcRn at neutral pH compared to acidic pH. No obvious differences in binding at acidic and neutral pH were observed for the other fusion proteins and IgG. However, when analyzing the dissociation of the FcRn-bound proteins by changing the pH from 6.0 to 7.4, differences between the IgG molecule and the 3 Fc-fusion proteins became evident.
In accordance with the above described mechanism, the IgG molecule showed a faster dissociation than the Fc-fusion proteins, resulting in a lower amount of IgG being retained. We assume that the differences in dissociation are due to differences between the Fab arm of the IgG and the fusion partner in the Fc fusion proteins, because all proteins exhibit the same Fc region, including a complete hinge region. Also, all proteins comprise the same anti-CEA VH and VL domains. Thus, we can exclude a recently described charge-mediated influence of the antibody variable domains.Citation21 However, differences in the arrangement, distance, and positioning of the variable domains, as well as the overall molecular structure, might influence interaction with and dissociation from FcRn. Since the scFv-scCLCH1-Fc fusion protein also showed a slower dissociation from FcRn, a contribution of the first heavy and light chain constant domains is rather unlikely, although the additional linkers that were introduced into this Fab arm-like portion might affect the dissociation process. Finally, differences might already occur during endocytosis via non-specific fluid-phase pinocytosis and intracellular sorting by separate recycling pathways.Citation21,26-28
In summary, our results further underline the unique pharmacokinetic properties of IgG in comparison to different Fc fusion proteins comprising an identical Fc region and also identical variable domains, and for some of the molecules that are also similar in size. Differences in dissociation from bound FcRn at neutral pH can be correlated with a reduced half-life. However, further studies are required to unravel the molecular mechanisms responsible for these differences, e.g., performing a mutational screening study. A recent study also considered, in addition to the dissociation rate at pH 7.5 the association rate at pH 6.0 to determine FcRn binding rates predictive for in vivo half-life,Citation19 which might be further investigated for Fc fusion proteins. Furthermore, studies might be extended evaluating the pharmacokinetic properties in transgenic mice endowed with the human FcRn.Citation29 Binding studies with human FcRn indicate also a slower dissociation of the Fc fusion proteins supporting findings by others that Fc fusion proteins compared to IgG molecules exhibit shorter plasma half-lives in human.Citation12
Materials and Methods
Materials
Horseradish peroxidase (HRP)-conjugated anti-human IgG (Fc-specific) antibody was obtained from Sigma and HRP-conjugated anti-His-tag antibody from SantaCruz Biotechnology (Santa Cruz, CA, USA). Cetuximab was provided by IKP (Stuttgart, Germany). CEA was purchased from Antikoerper-online.de (Aachen, Germany). Biotinylated mouse and human FcRn was produced as described elsewhere.Citation30 CD1 mice were purchased from Charles River (Sulzfeld, Germany). Protein A beads were obtained from Toyopearl (Tessenderlo, Belgium) and Ni-NTA beads from Macherey-Nagel (Düren, Germany).
Cloning and production
The anti-CEA IgG1 was generated by fusion of the VH and VL domain of antibody MFE-23Citation14 to the human heavy and light chain constant domains, respectively, and cloning into the Lonza expression vectors pEE14.4 and pEE6.4. Both plasmids were combined and stably transfected into either CHO-K1 cells or HEK293T cells. DNA encoding the different anti-CEA antigen-binding fragments (scFv, scDb, scFv-scCLCH1) as well as a humanized anti-EGFR scFv (hu225) was cloned either as SfiI-NotI or AgeI-NotI fragment into pSecTagA (Invitrogen), further comprising a human γ(Invitrogen), further comprising a human a human rising a human a human man a human ), further comprising a human man K293T cells.ant by protein A chromatography as described previously.Citation15 Protein concentrations were determined using the calculated molar extinction coefficients. The scDb-CH2 domain was also cloned into pSecTagA and contained a C-terminal hexahistidyl-tag for purification by immobilized metal affinity chromatography (IMAC) as described.Citation31
Size-exclusion chromatography
Purity and Stokes radii of the recombinant fusion proteins were determined by HPLC size-exclusion chromatography with a TSKgel G3000 SWXL column (Tosoh, Stuttgart, Germany). Proteins (25 µg, 0.5 mg/ml) were injected at a flow rate of 0.5 ml/min. The protein standard was obtained from BioRad, including thyroglobulin (669 kDa), bovine γglobulin (158 kDa), ovalbumin (44 kDa), myoglobin (44 kDa) and vitamin B12 (1 kDa).
ELISA
CEA or EGFR-Fc, respectively, was immobilized on ELISA plates at 3 µg/ml overnight at 4°C and the remaining binding sites were blocked with 2% (w/v) non-fat dry milk/phosphate buffered saline (MPBS). Purified recombinant proteins or serum samples were diluted in MPBS, titrated in duplicates and incubated for 1 h at room temperature. Detection was performed with HRP-conjugated anti-His-tag antibody (scDb-CH2) or anti-human IgG (Fc-specific) antibody using 100 µl 3,3′,5,5′tetramethylbenzidine (TMB) substrate (0.1 mg/ml TMB, 100 mM sodium acetate buffer pH 6, 0.006% H2O2). The reaction was stopped with 50 µl of 1 M H2SO4 and absorbance was measured at 450 nm. Data were fitted with GraphPrism software (La Jolla, CA, USA) from 3 independent binding curves. From these 3 individual EC50 values the mean and standard deviation were calculated.
Thermal melting points
Proteins (100 µg in 1 ml PBS) were applied in a quartz cuvette for thermal stability determination in a Malvern Zetasizer Nano ZS. Dynamic light scattering (mean count rate) was measured while the temperature was increased in 1°C intervals starting at 35°C with 2 minutes equilibration time for each temperature step. The melting point was defined as the temperature at which the mean count rate increased.
Plasma stability
The Fc fusion proteins were diluted to a concentration of 200 nM in 50% murine serum and incubated for 0, 1 or 7 d at 37°C and then stored at -20°C. The protein integrity was determined via a CEA-binding ELISA as described above. The proteins were diluted to their determined EC50 value and OD values at 450 nm were compared.
Affinity determination
Affinities of the anti-CEA IgG and the different fusion proteins for biotinylated mouse FcRn were determined by quartz crystal microbalance (QCM) measurements (A200 C-Fast system; Attana, Stockholm, Sweden). The biotinylated mouse FcRn was immobilized on a Biotin-sensor chip using tetrameric streptavidin according to the manufacturer's protocol at a density resulting in a signal increase of 50 Hz. Binding experiments were performed in PBST (0.1% Tween 20) at pH 6.0 or at pH 7.4 using a flow rate of 25 µl/min at 37°C. The pH-dependent dissociation of FcRn-bound molecules was analyzed using PBST, 0.1% Tween 20, pH 6.0 as loading buffer. After 60 seconds of association at pH 6.0, the buffer was changed back to pH 7.4, which was used as running buffer, and dissociation was measured over a period of 300 seconds. The chip was regenerated twice with PBST pH 7.4 for 30 seconds for the Fc-fusion proteins and 10 mM HCl pH 1.95 for the scDb-CH2. Before each measurement, a base line was measured which was subtracted from the binding curve. Proteins were injected in a random order in two-fold dilutions, starting at a concentration of 100 nM. Data were analyzed with the Attana evaluation software (Version 3.3.4) and TraceDrawer 1.6, using an oneto2 binding model to determine the binding parameters.
Pharmacokinetics
Animal care and all experiments performed were in accordance with federal guidelines and had been approved by university and state authorities. Female CD1 mice (6–12 weeks, 25–35 g) received an intravenous injection of 25 µg of the proteins in a total volume of 150 µl. In time intervals of 3 min, 30 min, 1 h, 2 h, 6 h, 1 day, 3 days, and 7 days, blood samples were taken from the tail and incubated on ice. Clotted blood was centrifuged at 13,000 g for 30 min at 4°C, and serum samples were stored at -20°C. Serum concentrations were determined by ELISA (as described above). For comparison, the first value (3 min) was set to 100%. Half-lives (t1\2α, t1\2β) and AUC were calculated with Excel. Terminal half-lives (t1\2β) were calculated using the last 4 serum concentrations. For statistics, onewayAnova was applied with Tukey's post-test. Results are shown as mean value ± standard deviation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by Deutsche Forschungsgemeinschaft Grant Ko1461/5.
References
- Kontermann RE. Strategies for extended serum half-life of protein therapeutics. Curr Opin Biotechnol 2011; 22:868-76; PMID:21862310; http://dx.doi.org/10.1016/j.copbio.2011.06.012
- Czajkowsky DM, Hu J, Shao Z, Pleass RJ. Fc-fusion proteins: new developments and future perspectives. EMBO Mol Med 2012; 4:1015-28; PMID:22837174; http://dx.doi.org/10.1002/emmm.201201379
- Levin D, Golding B, Strome SE, Sauna, ZE. Fc fusion as a platform technology: potential for modulating immunogenicity. Trends Biotechnol 2015; 33:27-34; PMID:25488117; http://dx.doi.org/10.1016/j.tibtech.2014.11.001
- Sockolosky JT, Szoka FC. The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy. Adv Drug Deliv Rev 2015; 91:109-24; PMID:25703189; http://dx.doi.org/10.1016/j.addr.2015.02
- Beck A, Reichert JM. Therapeutic Fc-fusion proteins and peptides as successful alternatives to antibodies. mAbs 2011; 3:415-6; PMID:21785279; http://dx.doi.org/10.4161/mabs.3.5.17334
- Hombach A, Heuser C, Abken H. Simultaneous targeting of IL2 and IL12 to Hodgkin's lymphoma cells enhances activation of resting NK cells and tumor cell lysis. Int J Cancer 2005; 115:241-7; PMID:15688386; http://dx.doi.org/10.1002/ijc.20829
- Gillies SD, Lan Y, Brunkhorst B, Wong W, Li Y, Lo K. Bi-functional cytokine fusion proteins for gene therapy and antibody-targeted treatment of cancer. Cancer Immunol Immunother 2002; 51:449-60; PMID: 12202906; http://dx.doi.org/10.1007/s00262-002-0302-6
- Wu AM. Engineered antibodies for molecular imaging of cancer. Methods 2014; 65:139-47; PMID:24091005; http://dx.doi.org/10.1016/j.ymeth.2013.09.015
- Kenanova V, Olafsen T, Crow DM, Sundaresanm G, Subbarayanm M, Carter NH, Ikle DN, Yazaki PJ, Chatziioannou AF, Gambhir SS, et al. Tailoring the pharmacokinetics and positron emission tomography imaging properties of anti-carcinoembryonic antigen single-chain Fv-Fc antibody fragments. Cancer Res 2005; 65:622-31; PMID:15695407
- Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol 2005; 23:1137-46; PMID:16151407; http://dx.doi.org/10.1038/nbt1141
- Hopp J, Hornig N, Zettlitz KA, Schwarz A, Fuß N, Müller D, Kontermann RE. The effects of affinity and valency of an albumin-binding domain (ABD) on the half-life of a single-chain diabody-ABD fusion protein. Protein Eng Des Sel 2010; 23:827-34; PMID:20817756; http://dx.doi.org/10.1093/protein/gzq058
- Suzuki T, Ishii-Watanabe A, Tada M, Kobayashi T, Kanayasu-Toyoda T, Kawanishi T, Yamaguchi T. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: a comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J Immunol 2010; 184:1968-76; PMID:20083659; http://dx.doi.org/10.4049/jimmunol.0903296
- Gurbaxani B, Dela Cruz LL, Chintalacharuvu K, Morrison SL. Analysis of a family of antibodies with different half-lives in mice fails to find a correlation between affinity for FcRn and serum half-life. Mol Immunol 2006; 43:1462-73; PMID:16139891; http://dx.doi.org/10.1016/j.molimm.2005.07.032
- Chester KA, Begent RH, Robson L, Keep P, Pedley RB, Boden JA, Boxer G, Green A, Winter G, Cochet O, Hawkins RE. Phage libraries for the generation of clinically useful antibodies. Lancet 1994; 343:455-6; PMID:7905958; http://dx.doi.org/10.1016/S0140-6736(94)92695-6
- Stork R, Zettlitz KA, Müller D, Rether M, Hanisch FG, Kontermann RE. N-glycosylation as novel strategy to improve pharmacokinetic properties of bispecific single-chain diabodies. J Biol Chem 2008; 283:7804-12; PMID:18211902; http://dx.doi.org/10.1074/jbc.M709179200
- Korn T, Müller R, Kontermann RE. Bispecific single-chain diabody-mediated killing of endoglin-positive endothelial cells by cytotoxic T lymphocytes. J Immunother 2012; 27:99-106; PMID:14770081; http://dx.doi.org/10.1097/00002371-200403000-00003
- Gehlsen KR, Gong R, Bramhill D, Wiersma DA, Kirkpatrick SA, Wang Y, Feng Y, Dimitrov DS. Pharmacokinetics of engineered human monomeric and dimeric CH2 domains. mAbs 2012; 4:466-74; PMID:22699277; http://dx.doi.org/10.4161/mabs.20652
- Ying T, Gong R, Ju TW, Prabakaran P, Dimitrov DS. Engineered Fc based antibody domains and fragments as novel scaffolds. Biochim Biophys Acta 2014; 1844:1977-82; PMID:24792384; http://dx.doi.org/10.1016/j.bbapap.2014.04.018
- Souders CA, Nelson SC, Wang Y, Crowley AR, Klempner MS, Thomas W Jr. A novel in vitro assay to predict neonatal Fc receptor-mediated human Igg half-life. mAbs 2015; 7:912-21; PMID:26018774; http://dx.doi.org/10.1080/19420862.2015.1054585
- Wang W, Lu P, Fang Y, Hamuro L, Pittmann T, Carr B, Hochman J, Preksaritanont T. Monoclonal antibodies with identical Fc sequences can bind to FcRn differentially with pharmacokinetic consequences. Drug Metabol Disp 2011; 39:1469-77; PMID:21610128; http://dx.doi.org/10.1124/dmd.111.039453
- Schoch A, Kettenberger H, Mundigl O, Winter G, Engert J, Heinrich J, Emrich T. Charge-mediated influence of the antibody variable domain on FcRn-dependent pharmacokinetics. Proc Natl Acad Sci USA 2015; 112:5997-6002; PMID:25918417; http://dx.doi.org/10.1073/pnas.1408766112
- Solá RJ, Griebenow K. Effects of glycosylation on the stability of protein pharmaceuticals. J Pharm Sci 2009; 98:1223-45; PMID:18661536; http://dx.doi.org/10.1002/jps.21504
- Igawa T, Tsunoda H, Tachibana T, Maeda A, Mimoto F, Moriyama C, Nanami M, Sekimori Y, Nabuchi Y, Aso Y, Hattori K. Reducing elimination of IgG antibodies by engineering the variable region. Protein Eng Des Sel 2010; 23:385-92; PMID:20159773; http://dx.doi.org/10.1093/protein/gzq009
- Vaccaro C, Zhou J, Ober RJ, Ward ES. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat Biotechnol 2005; 23:1283-8; PMID:16186811; http://dx.doi.org/10.1038/nbt1143
- Swiercz R, Chiguru S, Tahmasbi A, Ramezani SM, Hao G, Challa DK, Lewis MA, Kulkarni PV, Sun X, Ober RJ, Mason RP, Ward ES. Use of Fc-engineered antibodies as clearing agents to increase contrast during PET. J Nucl Med 2014; 55:1204-7; PMID:24868106; http://dx.doi.org/10.2967/jnumed.113.136481
- Lencer WI, Blumberg RS. A passionate kiss, then run: exocytosis and recycling of IgG by FcRn. Trends Cell Biol 2005; 15:5-9; PMID:15653072; http://dx.doi.org/10.1016/j.tcb.2004.11.004
- Ward ES, Devanaboyina SC, Ober RJ. Targeting FcRn for the modulation of antibody dynamics. Mol Immunol 2015; 67:131-41; PMID:25766596; http://dx.doi.org/10.1016/j.molimm.2015.02.007
- Gurbaxani B, Dostalek M, Garnder I. Are endosomal trafficking parameters better targets for improving mAb pharmacokinetics than FcRn binding affinity? Mol Immunol 2013; 56:660-74; PMID:23917469; http://dx.doi.org/10.1016/j.molimm.2013.05.008
- Proetzel G, Wiles MV, Roopenian DC. Genetically engineered humanized mouse models for preclinical antibody studies. BioDrugs 2014; 28:171-80; PMID:24150980; http://dx.doi.org/10.1007/s40259-013-0071-0
- Magistrelli G, Maline P, Anceriz N, Desmurs M, Venet S, Calloud S, Daubeuf B, Kosco-Vilbois M, Fischer N. Robust recombinant FcRn production in mammalian cells enabling oriented immobilization for IgG binding studies. J Immunol Meth 2012; 375:20-9; PMID:21939661; http://dx.doi.org/10.1016/j.jim.2011.09.002
- Müller D, Karle A, Meissburger B, Höfig I, Stork R, Kontermann RE. Improved pharmacokinetics of recombinant bispecific antibody molecules by fusion to human serum albumin. J Biol Chem 2007; 282:12650-60; PMID:17347147; http://dx.doi.org/10.1074/jbc.M700820200