ABSTRACT
We investigated the evolution process of collaborative inter-organizational network of the research and development (R&D) on monoclonal antibody (mAb) over the past 30 y. The annual detection of the collaboration network provides dynamics on network structures and relationship changes among different organizations. Our research showed continuous growth of the network's scale and complexity due to the constant entry of new organizations and the forging of new partnering relationships. The evolving topological features reveal a core-periphery structure that became clearer over time and an increasing heterogeneity within the collaborative mAb R&D network. As measured by the number of network participants, dedicated biotechnology firms (DBFs) were the dominant organization form in the field of mAb development, but their average centrality was reduced during the period of 2004–2009, when pharmaceutical companies took over the positions of DBFs. Along with the network evolution, 2 waves of substitution on the leading positions were driven by technological innovations and mergers and acquisitions (M&A). In addition, this study analyzed organizational-level behaviors to help understand the evolution of network structures over the field of mAb development across the different technologically innovative or economic contexts.
Abbreviations
| DBF | = | dedicated biotechnology firm |
| M&A | = | mergers and acquisitions |
| mAbs | = | monoclonal antibodies |
| PRO | = | public research organization |
| R&D | = | research and development |
| US. FDA | = | US. Food and Drug Administration |
Introduction
The emergence of biotechnological innovations since the mid-1970s has revolutionized the current trajectories of drug discovery,Citation1,2 and the business philosophy of research and development (R&D) activity has also been substantially modified.Citation3,4 Actors besides pharmaceutical companies, such as universities, research institutions, and newly founded biotechnology firms, now play pivotal roles in the drug R&D process, especially in therapeutic strategies largely derived from biotechnological innovations. Large pharmaceutical companies have rebuilt borders to facilitate the R&D processes by strategically harnessing their external sources of innovation, including ideas, technologies, and R&D projectsCitation5,6 that could not be generated internally, and have particularly intensified relationships with biotechnology. Biotechnology firms, which denote profit-seeking firms focusing on researching, developing, and commercializing biotechnologies, need certain resources to bring biotech-derived products to market, and are therefore involved in intricate relationships with pharmaceutical companies, universities, and research institutions.Citation7 Typically, collaborative R&D plays a critical role in allowing organizations handling diverse connections to gain knowledge and information about innovative technologies, investing, and markets.Citation8,9 The mutual and frequent collaborations among the different actors can be mapped as a complex “network” through which they may gain access to resources such as capital, power, and influence.Citation10-12
Networks of organizations are commonly understood as a phenomenon of organizational life, and have been studied extensively using various theoretical approaches, such as resource dependence theory, transaction cost economics, and dyadic relationships focusing on 2 organizations.Citation13,14 When social network analysis was applied to perceive the specific structure between organizations, the conceptualizations of networks were gradually distinguished from those interactions across organizational boundaries that were summarized as an analytical perspective with quantifiable features or a description of interaction among organizations.Citation15 Social network analysis containing a set of nodes linked by a set of specified relationships could provide whole network examinations and uncover the social structures, opportunities, as well as constraints beyond the organizational level.Citation16 Analytical network investigation was conducted in this study to present the inter-organizational relations.
The general concerns of network analysis, including a fair number of studies in health-relevant sectors, lie in structural features and position measures of participants or relationships.Citation16,17 Given the high levels of dynamics and changes in technologies, products, and markets, organizations must adjust themselves and their relationships with others, such as by becoming more specialized and intensifying activities between organizations,Citation18 just as organizational relationship changes in the pharmaceutical industry interact with biotechnology innovations.Citation9 Consequently, networks evolve in response to these dynamics in terms of both organizational-level activities and environmental changes. On the other hand, changes on organizational network positions also influence the subsequent performance of participants or even the network development.Citation19,20
A longitudinal study on the inter-firm R&D partnering in the pharmaceutical biotechnology industry identified the particular growth patterns of the network encouraged by the desire of large pharmaceutical companies to access new biotechnological knowledge, and the changes of dominance in the network that occurred between biotechnology firms and large pharmaceutical companies in the 1990s.Citation21 Other important research investigated a 12-year evolution of biotechnology firms' collaboration network and demonstrated the mechanisms for affiliation that shaped the network evolution with respect to diverse partnering.Citation11 Accordingly, researching the changes of inter-organizational networks is critical to understand how the whole network structure evolves, how relationships are affected, and what drives the evolution along with the continuous adaption of the sustaining innovations and specific developments within a field. In this context, our study attempts to comprehensively investigate the evolution of collaborative inter-organizational networks, taking well-developed biotechnological monoclonal antibody (mAb)-based clinical products as a specific example to detect network structural dynamics, relationship changes, and the relevant determinants in the development of the field.
Over the 3 decades since the first therapeutic mAb (Orthoclone OKT3) was approved by the US. Food and Drug Administration (FDA), antibodies in clinical application made dramatic advancementsCitation22 and have dominated the biologics marketplace,Citation23 especially after humanized and human version of antibodies were fully developed.Citation24 In 2013, 6 of the top 10 best-selling drugs were antibodies. The continued approval of antibody drugs, and the sale growth of current products would drive the global sales of these therapeutics to almost $125 billion by 2020.Citation25 Supplementary Table 1 lists the top-selling mAbs in 2013. Almost all of these blockbuster therapeutic mAbs were initially developed by biotechnology firms, while the subsequent commercialization involved pharmaceutical companies. This highlights the crucial relationships between biopharmaceutical firms and pharmaceutical companies in antibody drug development. Certainly, the positions and influence of organizations and their collaborative relationships in networks should reflect or adjust to accommodate the contextual changes in drug development and specific field developments, such as continuous technological innovations, growing complexity, and increasing specialization of organizations.
Table 1. Topological properties in 6 static snapshots of the collaboration network.
Previous studies compared 2 different periods of alliance networks in the mAbs sector (1990–1996 vs. 1997–2004),Citation26 but failed to depict the continuum of network evolution and lacked analysis on the different participants and the varying relationships among them. This has further limited conclusions about specific developments in the field. Therefore, our study analyzes the continuous dynamics of collaboration networks in relation to clinical R&D on mAb over the past 3 decades, attempting to elucidate the evolution of the whole network topology and the changes of different organizations and their connections, and subsequently illuminate the underlying determinants. Furthermore, it also analyzes organizational-level behaviors to help understand the evolution of network structures over the field development across different innovative or economic contexts.
Results
We investigated the topology and dynamics of the mAb R&D collaboration network to identify the determinants that are crucial to understand the relevant evolving processes. The collaboration networks are generated from the R&D projects that involve partnership information relating to drug development progress. The sample projects were collected from IMS R&D Focus database. MAbs and their derivatives for disease diagnosis and therapy, such as antibody-drug conjugates and bispecific antibodies, were included.
Collaboration level
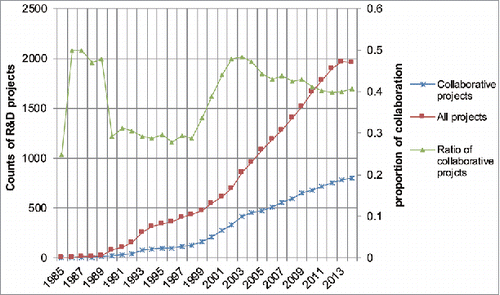
From 1985–2014, a total of 2643 mAb-related R&D projects were identified, including 1082 collaborative projects and 1561 non-collaborative projects. shows the distribution of the collaboration level of mAb R&D projects over the 3 decades. The 853 organizations were involved in the collaborative projects through diverse modes of cooperation with others, accounting for a large proportion of all the participants in the field of mAb development. Only a relatively small subset of organizations has no connections with others. The annual relative number of the subset of non-connected organizations, compared with the entire set of organizations, is exhibited as Supplementary Fig. 1, which shows the ratio of non-connected organizations remained relatively low and steady.
MAbs were first developed for human disease diagnosis and therapy in the late of 1970s; however, the number of mAbs in clinical study was low until the late 1980s. After that, there was a gradual increase in the number of drug development projects, which was directly driven by the constant increase in the number of newly started projects each year and the dramatic increase of relevant patent applications over the past decades (more details on the increase of new projects and patent applications can be found in 2 published articles.Citation27,28). This growth was propelled by the increasing promise of mAbs as beneficial and profitable products.
The collaboration proportions we examined can be divided into 4 stages: an uncertain stage, a stable stage at low level, a rapid promotion stage, and a stable stage at high level. The uncertain stage, which occurred during the late 1980s, exhibited substantial fluctuation on the level of collaboration, which might have been affected by the low number of projects number and the shift in financial support for biotechnology firms.Citation21 The next stage was one of stabilization that lasted for several years in which the level of collaboration stabilized at around 30%. From the last few years of the 1990s to the early 2000s, there was a stage of rapid promotion of collaboration level. Large numbers of new mAb developers entered into the network through proprietary platform-based collaboration in this period. Since then, the collaboration level for mAb R&D has decreased slightly to around 40%, marking the fourth stage of the evolution of collaboration level in the field. The decrease might be due to the combination of continuous applications of platforms and the concomitance of changing collaborative relationships after acquisitions. At this point, it is important to note that, for almost 10 years, the number of mAb R&D projects with collaborative relationships has been maintained and represents a relatively substantial and steady proportion of the total number of projects.
Collaboration network topological properties
Next, we provide an overview of the major changes in the mAb R&D collaboration network topological properties over time by examining several general structure properties.
The collaborative data over 3 decades was collected and used to construct a representation of the network in each year. The evolving collaboration network from 1985–2014 is visualized using a dynamic display, which makes a comprehensive presentation of mAb R&D partnerships over a large time span possible. To better describe the evolution process, 6 static snapshots of the collaboration network (1989, 1994, 1999, 2004, 2009, and 2014), with 5-y intervals between the years represented, are shown in , and the general network structure properties are listed in . The 6 snapshots are like windows by which we can snoop the evolving process of collaboration network. displays the whole network, including several small components that denote sub networks with the low number of member nodes. only show the largest connected component; other small components are not exhibited due to the dominance of the largest connected component in the network.
Figure 2. Collaboration network in the years: (A) 1989; (B) 1994; (C) 1999; (D) 2004; (E) 2009; (F) 2014. The main component of the network at each time point is displayed, except the one in 1989 (A) that shows the whole network. In the network, nodes denote the organizations involved in collaborative relationships and are represented as edges. Node size is scaled to the number of edges an organization has (only comparable within the network at a specific time point). The thickness of edges represents the frequency between 2 nodes. Loops, referring to edges that share the head and the end, are eliminated from data collection. The colors of nodes represent different forms of organizations: the purple represents pharmaceutical companies; the green represents DBFs; the yellow represents PROs; and the gray represents other forms. The edges are also colored according to the collaboration modes between nodes in terms of their specific organizational forms, with magenta between 2 pharmaceutical companies; blue between a pharmaceutical company and a DBF; light cyan between 2 DBFs; orange between a pharmaceutical company and a PRO; brick red between a DBF and a PRO; and gray between others.
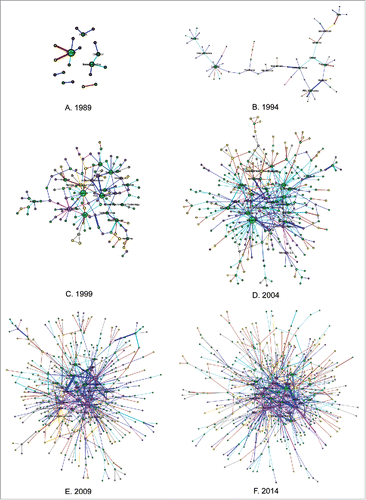
Clearly, the collaboration network experienced a continuously growing trend, based on both the network's scale and complexity. By 2014, the network contained 537 nodes and 760 weighted ties. This can be largely attributed to the constant entry of new organizations and the forging of new partnering relationships on mAb R&D.
In the early years of its development, the network consisted of several isolated network components. By 1994, a largely connected network component that contained a large fraction of the participants and partnerships and further formed a giant collaborating cluster in the field of mAb development had emerged. The structure of the main component substantially changed as its internal connections intensified in the 1994–2004 period. Starting in 2005, the network topology became stationary due to the high and stable share of main components (, Fig. S2). This indicates that the new entrants and new partnerships tend to become part of the largest component. For at least the foreseeable future, the main component in the collaboration network will be maintained and is unlikely to fall apart, since the organizational changes triggered by technological advances or other aspects happen more likely with the involvement of the main component.
The average degree, referring to the average number of partners per organization, increases with time for the collaboration network, indicating the gradual increase of the network interconnectedness. The increasing tendency is the same with respect to the average weighted degree that refers to the strength of partnerships. However, the pace of the growth of the average degree decelerated in the past 10 y due to the relatively large size of the network, and the collaboration system entered the period of stable growth.
The value of average path length reveals the average ability of 2 nodes in the network to communicate with each other. In the organizational network, a linkage is also a channel of knowledge communication between 2 organizations with the immediate neighbors or indirect contacts (with distance of larger than 1).Citation29 From the perspective of organizational network theory, the shorter average path length means that a node needs fewer steps on average to transport information to others in the network. The merging of the 2 largest components with similar size identified in the network in 1993 expanded the average distance and the diameter of the network at 1994 to the highest levels (). After that, the interconnectivity in the main component substantially expanded, and therefore the participants could traverse shorter paths to connect. The subsequent convergence of average distance to a steady-state (4 or below) could be seen as regular growth on an increasingly large-scale network base.
The clustering coefficient can quantitatively measure the clustering phenomena in a real-world network. A high clustering coefficient of a node in a collaboration network represents the high willingness of the node's partners to connect with each other. The clustering coefficient of the whole network can be evaluated by the average value of all nodes in the network. As shown in , there was no clustering in the early years of the construction of the collaboration network. This situation changed when the internal links increased in the main component after 1994. However, as the collaboration network was relatively modest, the average clustering coefficient could fluctuate easily with small changes in the number of neighbors' connections. The value of the average clustering coefficient dropped to a very low level (0.055) after 2004. The stably increasing collaboration density therefore promoted the slightly increasing network clustering coefficient.
The node degree characterizes the centrality of individual actors in the network, while the degree distribution is defined as quantifying the degree diversity of the whole network, which has been recognized as an important measure of the network's topological features. Given the probability that a node randomly selected from the network has k links, p(k), the most popular degree probability distribution in real world networks is the power-law distribution (k−γ dependence) with a long tail, as it decays for large degree k. The fitness results in a log-log scale showed that a clear power-law degree distribution developed from 1994–2014 (Fig. S3), indicating the heterogeneity of degree distribution in the collaboration network. Only a small number of organizations have many collaborators, while most interact with just a limited number of other organizations. The network, coherently, discloses a clear core-periphery structure, with a core of high-degree nodes surrounded by nodes with lower degrees. Moreover, a linear least squares fitness analysis revealed the exponent γ was in the range of 1.83–1.48, with a gradually declining trend over the years. The fitting slope was stretched to the right by the growing group of nodes with the highest degree (Fig. S3), and the core of the network, mainly consisting of these nodes, was further correspondingly corroborated (). At the same time, the collaboration network appeared more heterogeneous, as indicated by the smaller value of γ.
Network dynamics over the field of mAb development
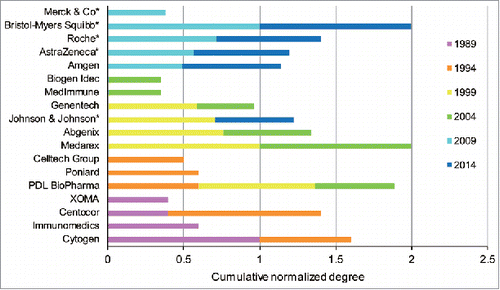
As mAbs first began to enter clinical studies in the 1980s, new and small biotechnology firms played an essential role in the inter-organizational network as the pioneers of these new therapeutics, although the number of pharmaceutical companies was higher than dedicated biotechnology firms (DBFs; , ). depicts the leading organizations over the network evolution, which can be observed generally from the specific representations at the 6 time points in the . The normalized degree is used to recognize the leading roles, with a separate maximum value of 1 for each time point. As depicted in , during the early years, the leading positions in the collaboration network were occupied by DBFs. In 1989, 4 DBFs, Cytogen, Immunomedics, Centocor and XOMA, which were founded in the late 1970s or early 1980s, emerged from isolated components and formed more than one partnership in the budding collaboration network (). In 1994, Cytogen and Centocor maintained their advantage in terms of the scale of partnerships in the network, although the first position switched from the former to the latter. All the leading participants were located in the main component, which contributed to the dominance of this component in later years. Generally, pharmaceutical companies, including several large ones, centered around the leading participants by leveraging complementary assets such as R&D progress or by in-licensing candidates or technologies. This explains the high proportions of the partnerships between pharmaceutical companies and DBFs in the early years (, ).
Figure 3. Distributions of different kinds of participants and varying relationships among them over time.
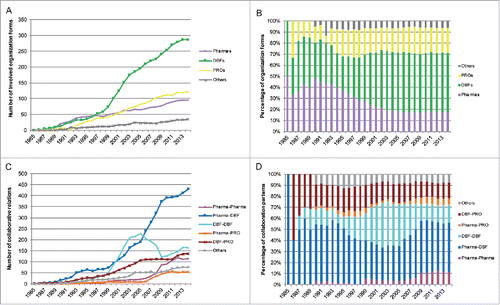
Figure 4. The cumulative normalized degree of the top 5 players with the highest degree over the 6 snapshots of the collaboration network. Organizations with an asterisk are large pharmaceutical companies.
Due to the technological limitations of murine-derived and chimeric antibodies, the field experienced a period of tentative clinical development from the late 1980s to the early 1990s, which left the scale of collaboration and new entrants in a low state. Since the use of the first approved mAb, Orthoclone OKT3 (muromonab-CD3), was limited due to reported side effects, such as a human anti-mouse antibody response,Citation36 the early clinical and commercial success of mAbs was relatively hampered. Although there were some trials of humanized mAbs in the early 1990s (), pharmaceutical companies still trailed DBFs in developing these new therapeutics due to technological uncertainties and gloomy market predictions. The proportion of DBFs and the partnerships between DBFs began to increase gradually at the early 1990s (, ).
Figure 5. (A) The number of collaborative projects with identified technological profiles that launched every 2 y. (B) The number of relationships changed by M&A activities every 2 y.
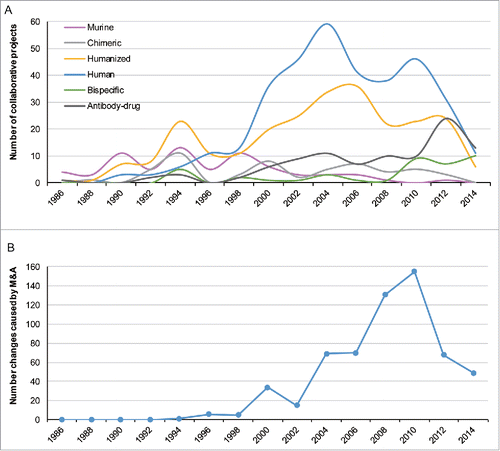
This situation continued in 1999, while the leading positions in the collaboration network were taken over by previously unassuming players, such as Medarex, Abgenix, Genentech, and Johnson & Johnson. What made them so influential in the network at this time was quite different. Genentech developed many partnerships by licensing agreements under which Genentech in-licensed drug candidates from other originators, mostly other DBFs, or supported further clinical research. The sudden spurt of Johnson & Johnson was ascribed to the acquisition of the top incumbent, Centocor. Medarex, Abgenix, and PDL BioPharma gained prominence with discrete technological innovations whereby murine and murine–human chimeric antibodies were substituted by generating qualified humanized or human mAbs. In fact, several key scientific breakthroughs concerning mAb humanization led by some DBFs were successfully commercialized in the late 1990s, including the applications of mAb-related technological platforms (). The technological progress promoted by DBFs resulted in the increase of network interconnectivity and the emerging dominance of the main component of the network. Humanized and human mAbs designed to reduce mAb immunogenicity resulting from murine-derived sequences had become the focus of collaborative projects in clinical development each year after 1998 ().
Table 2. The basic technological innovation profiles of some DBFs.
The field gradually matured over the next years as critical innovations were widely applied. The DBFs established or solidified their dominance in the inter-organizational R&D partnering system exhibited in 2004 substantially based on their technological advances, which had been typically developed as technological platforms to generate candidates with specific properties. In 2004, 212 of the 568 collaborative relationships (37%) in the network were developed through relevant technological platforms. briefly summarizes the active technological platforms of some DBFs that were widely applied in mAbs development, mostly by forming partnerships with other developers. Specifically, Medarex's UltiMab® platform (based on HuMab-Mouse®) and Abgenix's XenoMouse® technology were the leading technological platforms using transgenic mouse strains to generate human antibodies. This resulted in the high centrality of Medarex and Abgenix in the collaboration network in 2004, which can be identified by the high involvement of the platforms in their partnerships. Another popular approach to obtaining human antibodies is the recombinant expression of human antigen-binding fragments, followed by antigen-driven selection.Citation30,31 The contribution of this approach to the collaboration network was partly assumed by 3 companies, Cambridge Antibody Technology, Dyax, and MorphoSys, that developed phage display libraries and technologies.
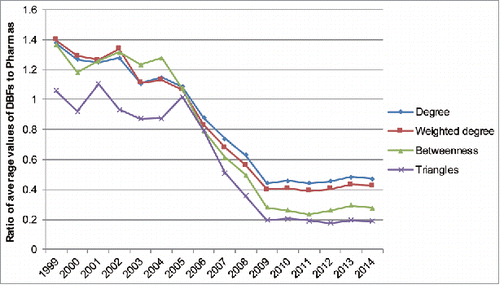
The spread of platforms based on applications to antibody-druggable targets lowered the technical barriers of mAbs development and directly led to the fastest expansion of the network. It also triggered the substantial increase of new DBF entrants and partnerships between DBFs and promoted the DBFs' centrality in the partnering network during 1999–2004 (). Moreover, in this period, the average centrality of DBFs, referring to the breadth of partnering (degree centrality), strength (weighted degree centrality), and intermediary capacity (betweenness centrality), was more influential in the network than that of pharmaceutical companies, as the ratio value was larger than 1 (). The heterogeneity of degree distribution was also significantly reinforced (Fig. S4). The proprietary technologies constituted the core competence of these DBFs and guaranteed their leading positions, as they possessed the ability to compete for connections to others. In this period, technological determinants had a major effect on the structural evolution of the collaboration network. These technological changes led to the replacement of some incumbents, and the initial leading participants faded away, likely because they did not make radical innovations or utilize their advantageous position to adapt to the radical technological changes,Citation32 which can be seen in .
As the relevance of the technologies and commercial potential of mAbs became more obvious, the pharmaceutical companies, especially “big pharma,” no longer hesitated to usurp the top positions in the R&D collaboration network. From 2004–2009, a wave of large mergers and acquisitions (M&A) in the field of mAbs, dominated by large pharmaceutical companies, was completed (). When M&A takes place, the active collaboration relationships of targeting organizations are taken over by the purchasers whose positions in the network are consequently upgraded. The high-frequency transition of partnerships caused by M&As () was observed in this period, which affected the network topological performance of several key participants.
Table 3. Important mergers and acquisitions influencing the position changes in the collaboration network.
The leading positions of participants underwent drastic changes in this period, largely as a consequence of M&A (). In particular, Bristol-Myers Squibb, which acquired Medarex in 2009, dominated in place of Medarex in the former collaboration network. Other large pharmaceutical companies also gained power through M&A, such as AstraZeneca when it purchased 2 notable incumbents, Cambridge Antibody Technology (in 2006) and MedImmune (in 2007), Roche when it acquired Genentech (in 2009), and Merck & Co. when it merged with Schering-Plough (in 2009). Only one established biotechnology firm, Amgen, moved into a top-five position when it acquired Abgenix in 2006.
The M&A between pharmaceutical companies and DBFs also largely changed the partnering configuration of the collaboration network. More specifically, a significant decline of DBF–DBF partnerships and increase of collaboration between pharmaceutical companies and DBFs was seen in 2004–2009 (, ). The superiority of DBFs in the network collapsed, concomitant with a greatly lifting of the influence of pharmaceutical companies, with the ratio value of DBFs to pharmaceutical companies much smaller than 1 (). This governance position shift can also be observed in the top 5% organizations (Fig. S4).
As such, the new leading participants could espouse much more diversity in their collaborative activities, and the competitive relationships among their neighbors correspondingly might be mitigated, making it more possible for neighbors to connect. This could explain the increasing interconnectedness of the network during 2004–2014, as described previously.
During 2009–2014, the structure of partnering activities in the network became comparatively steady. It should be noted that the pharmaceutical companies that made up less than 20% of all organizations in the collaboration network participated in more than 60% of all collaborative development activities (, ). The set of pharmaceutical companies maintained the higher level of partnerships on average, compared with DBFs and public research organizations (PROs). This disclosed the superior capabilities of pharmaceutical companies to collaborate with others in the network. It could be determined based on their much higher average number of collaborators, stronger average connections, greater emphasis on intermediation across the network, and tenser interconnectedness involved when comparing with DBFs (). The leading participants mostly persisted in this period, while Johnson & Johnson retook its place in the top-five group after acquiring the DBF Crucell ().
Discussion
Collaboration is described as being important to alleviate some challenges in product R&D processes, and firms are opening up their doors to tap into complementary sources and benefit from innovation.Citation33,34 Our research addresses the evolution of network structures and collaborative behavior over the history of mAb commercial development in terms of the large-scale, longitudinal data on partnering relationships. It therefore provides insights into the dynamics of affiliations in the field.
Dynamic analysis from 3 perspectives, the overall network level, organizational groups, and the individual level, highlights a collaboration network structure in which the scale and interconnectivity increased with the continuous formation and dissolution of partnerships. The views on how participants get connected changed over the 30 y in question. The basic structure of the mAb R&D network has developed into a system containing one dominant component with a clear core-periphery structure. The heterogeneity of degree is demonstrated by the power-law distribution, as seen in many real-world networks.Citation35-37 Barabási and Albert proposed a linear preferential attachment model to explain the power-law degree distribution of networks, in which new nodes attached preferentially to already well-connected sites during the network formation.Citation38 In other words, for the collaboration network, the incumbent organizations with higher degrees would have a higher probability of establishing connections or partnerships with new entrants. Gay and Dousset's study adopted the model “fitter-gets-richer,” first proposed by Bianconi and Barabási,Citation39 to presume the field-specific attachment mechanism.Citation26 However, the interpretation may be inaccurate due to the limitation of non-consecutive data. The observed slopes (1.83–1.48) of the mAb collaboration network deviate from 3, as expected due to pure preferential attachment.Citation38 The attachment preference may be periodically detected for central participants that exhibited competitive advantages based on proprietary crucial technologies or complementary resources or both after fully adapting innovations. The preferential attachment is modified by the intrinsic quality of a node, defined as the node's fitness. The fitness of the node then determines the time dependence of the node's connectivity.Citation39 Similarly, Powell et al. also concluded in their empirical study on network evolution that the rich-get-richer hypothesis was weakened by multiprobability models as divergent rules of attachment.Citation11
At the early period of our study, large pharmaceutical companies exhibited little interest in assuming the risks of mAb development due to the technological limitations. The extent of alliances among industries proved to be positively correlated with R&D intensity or the degree of technological sophistication of these industries.Citation40,41 When technological advances create technology discrete from existing know-how, previous incumbents could survive by embracing radical technological changes.Citation42 Otherwise, they would be replaced by new adaptors, following the Schumpeterian process of “creative destruction.”Citation43 Specifically, the technological advances in generating human or humanized antibodies enabled some explorers to stand out from previous incumbents and stimulated the restructuring of network positions, which led to the first wave of leading player substitutions in this field. The second wave of leading player substitutions was led by large pharmaceutical companies, following their realization of the value of the new therapeutics as relevant innovations were embraced in the late 2000s. In industries where technological know-how is vital, companies need to learn extensively from external sources by collaboration, besides sustaining their internal capabilities.Citation9,44,45 Large pharmaceutical companies internalized their external innovations, became more innovative in the field of mAbs, and took over the dominant positions in the collaboration network. The acquisitions made by pharmaceutical companies in the late 2000s were largely driven by strategic rationale and the desire to acquire the complementary technologies and potent drug candidates, rather than a mere desire to be “massive.”Citation46
Interestingly, throughout the evolution of the collaboration network, the network clustering coefficient seems much smaller than that in scientific collaboration networks, evaluated from the co-authorship,Citation37,47,48 and institutional perspectives.Citation49,50 Two possible causes contribute to this observed distinction. First, collaborating on an academic publication involving 3 or more parties is very common in the scientific collaboration system, which contributes to a high clustering coefficient by forming lots of local fully connected patterns in the network. However, for R&D partnerships, most collaborations are based on bilateral agreements, and few agreements involve 3 or more organizations. Second, the motivation to collaborate with partners' neighbors is very different. For scientific collaboration, the connections to partners' neighbors probably results in closer academic communication, more publications, and greater influence in a specific research field. In addition, the collaborative activities among neighbors are likely facilitated by common acquaintances. However, in the inter-organizational collaboration, the necessity for complementary resources is a typical driver. The lower possibilities of collaboration between 2 organizations with a same partner can be attributed to their potentially similar capacities in the drug development process. If an organization owns capacities that its partners does not possess, e.g., the capacities of commercialization of innovations, its partners would more likely be the organizations with complementary assets, like technological advantages. Pairs with non-complementary resources that have common partners are less likely to form new partnerships with each other than interdependent organizations that have complementary resources beneficial to the other.Citation29 This might explain in part the lower average clustering coefficient of organizational network.
The dynamic of the network continues as new discoveries and opportunities occur. The evolution of the network structure and participants shaped how new connections and entrants had been integrated into the network. The network centrality of participants depends not only on the network structure, but also on the industry or field context, and interactions between them are marked by strong associations that affect global settings of the observed field. These evolving profiles, analyzed mostly from a global perspective, examined the overall changes at different time points. However, this study did not analyze specific participants or relationships and their clinical performances over the field's development. The next question to address may be how the specific network structure and network centrality influence the actual innovative performance of organizations or collaborative relationships in the clinical R&D over the collaboration network evolution. The exploration of this question would help identify the specific evolving properties of organizations and dyadic relations that more likely lead to clinical success. This work requires reasonable performance measurements and theoretical assumptions based on specific properties in the field of clinical development of mAbs. Our future work may focus on the mentioned-above issues, and explore the arguments on the organizational performance within the collaboration framework. We believe this work on the evolution of network structure and positions is essential for understanding the collaboration profiles of mAb development and helps to open ways to capture some universal and particular determinants of network evolution.
Methods
Data retrieval
The study used the extensive and project-based database, IMS R&D Focus, which monitors and updates the progress of global pharmaceutical and biotechnology products through the R&D pipeline from the discovery phase to the marketing phase. It provides not only information about clinical progress but a commercial overview as well. Our focus is on the clinical project summary and relevant partnering details. Using the criteria “mechanism of action: monoclonal antibody,” we searched the database and identified 2643 projects from discovery and preclinical phases to marketed phase by April 2014. Among all the identified projects, 1082 (41%) included collaborative agreements during different phases in the development of a new drug. The records in the database provided detailed information on the collaborative agreements, including the collaboration purposes, project-related technological content, responsibilities of each involved party, and the date of signing agreements. The content of agreements covered the themes on research, clinical development, licensing, cooperative R&D, and commercialization. The various contract contents result in different modes of cooperation underling the linkages between organizations. However, it was impractical to code the information on every partnership within the diverse collaboration modes based on the specific deal description due to the relevant data structure and some limitations in the database. In this study, various collaboration modes are not distinguished in the network and equally shown as network connections. Thus, the relationships related to bilateral or trilateral agreements depended on R&D projects and were extracted to constitute the basis of the inter-organizational interactions.
Evolving network construction
To map the evolving structure of the collaboration network, the continuation of every relationship from the date of signing an agreement was determined based on the agreement declarations and the clinical progress of the projects. Additionally, a yearly time frame was used to measure the duration of the organizational interactions. That is to say, the network in a specific year is shaped by the new/discontinued links and the concomitant entrants/exits on the basis of the one in the previous year. Therefore, the evolution of the network comprises a series of sub-networks existing at particular points in the timeline.
Starting with a connection between 2 organizations in 1985, the collaboration network expanded into a complex system with 537 organizations by 2014 after continual entries and exits over 30 y. Organizations in the network are not always stable due to some significant strategic changes (e.g., failure, departure from the field, and M&A activities) that reflect the network dynamics. First, when an organization changes its name, the new name will always be used throughout its involvement in the network. Thus, situations where 2 different names are referring to the same organization can be avoided. Second, organizations participating in the network should be independently operated. If a company is operated as a subsidiary, the parent company would be used instead of the subsidiary after the year of the control relationship formation. Third, M&A has been taken into consideration throughout the duration of the relevant agreements. In other words, the partnerships built by the acquired company are believed to be taken over by the acquiring company after a merger or an acquisition, consequently resulting in relevant position changes on both sides in the collaboration network.
The collaborative relationships embedded in the mAb R&D projects have involved 853 organizations over the 3 decades from 1985 to 2014. These organizations are sorted into 4 categories according to their main focus: established pharmaceutical companies (and chemical companies), DBFs, PROs (including hospitals, public or private universities, government institutions, and non-profit organizations), and other form organizations. The 4 different types basically represent the conventional organization patterns involved in R&D activities. This classification would be helpful to identify the distinguished organizational changes in the industry structure over the evolution process. Furthermore, considering the distinct roles in terms of organization profiles, the collaboration modes in the network map are established according to the organization types of the 2 parties at both ends of the edge, such as partnerships between 2 DBFs, between pharmaceutical companies and DBFs, and between 2 pharmaceutical companies.
Analysis of the dynamic network
A set of indicators are considered to measure both the specific importance of individual participants and the topologies of network connectivity. In a network, “node degree” is defined as the total number of edges directly connected to a node and is interpreted as the degree of prestige of the node due to the number of connections it has to others. The normalized version is defined as the degree divided by the maximum degree observed in the network, expressed as a value ranging from 0 to 1. In this study, the network is weighted in terms by the number of collaborative R&D projects. The thicker ties mean the larger number of co-development projects that players participate in. Therefore, weighted degree was used, counting not only the number of connections of a node, but also the frequency of those connections, to gauge the intensity of partnering relationships among participants. A component in a network refers to an isolated sub-network that has no connections with nodes in other components of the network. The shortest path between 2 nodes in the network is the minimum number of edges that is required to go from one to the other. This prompts another common actor-level measure, betweenness centrality, which is measured by the frequency of the node positioned on the shortest path between pairs of other nodes. From the network analysis perspective, an actor with high betweenness centrality occupies a high intermediary position in the network among other actors or groups of actors. Triangles in the network denote triads having 3 relationships connecting each node with the other 2 nodes. This is referred to as clustering and is always associated with a node's clustering coefficient, which is defined as the fraction of existing connections to all possible connections between the nearest neighbors of the node. Averaging the clustering coefficients of all nodes generates the clustering coefficient of the network. Moreover, the average path length measures the average distance – the length of the shortest path – between any pair of nodes, while the network diameter is the largest distance between 2 nodes in the network.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental_Materials.zip
Download Zip (566.4 KB)Acknowledgments
The authors gratefully thank Ms. Tianhong Yin and Mr. Yunfeng Lai for their advice on data processing and manuscript preparation.
Funding
This work was supported by University of Macau under the Grant MYRG2015–00145-ICMS-QRCM and the Grant MYRG2015–00172-ICMS-QRCM; and Fundo para o Desenvolvimento das Ciências e da Tecnologia (FDCT) under the Grant 013-2015-A1. The authors declare that they have no conflicts of interest to disclose and no financial conflicts with the subject matter or materials discussed in the manuscript.
References
- Staropoli C. Cooperation in R&D in the pharmaceutical industry – The network as an organizational innovation governing technological innovation. Technovation. 1998; 18(1):13-23. doi:10.1016/S0166-4972(97)00107-7
- Tushman ML, Anderson P. Technological discontinuities and organizational environments. Admin Sci Quart. 1986; 31(3):439-65. doi:10.2307/2392832
- Malerba F, Orsenigo L. The evolution of the pharmaceutical industry. Bus Hist. 2015; 57(5):664-87. doi:10.1080/00076791.2014.975119
- Teece DJ. Competition, cooperation, and innovation. J Econ Behav Organ. 1992; 18(1):1-25. doi:10.1016/0167-2681(92)90050-L
- Hunter J, Stephens S. Is open innovation the way forward for big pharma? Nat Rev Drug Discov. 2010; 9:87-8. doi:10.1038/nrd3099
- Ni J, Wan J, Kong X, Cai Y, Yang F, Wang Y, Hu Y. Pharmaceutical technology licensing: An analysis in the field of cardiovascular disease. J Pharm Innov. 2016; 11(1):34-45. doi:10.1007/s12247-015-9234-5
- Powell WW. Learning from collaboration: Knowledge and networks in the biotechnology and pharmaceutical industries. Calif Manage Rev. 1998; 40(3):228-40. doi:10.2307/41165952
- Belderbos R, Carree M, Lokshin B, Sastre JF. Inter-temporal patterns of R&D collaboration and innovative performance. J Technol Transf. 2015; 40:123-37. doi:10.1007/s10961-014-9332-4
- Powell WW, Koput KW, Smith-Doerr L. Interorganizational collaboration and the locus of innovation: Networks of learning in biotechnology. Admin Sci Quart. 1996; 41(1):116-45. doi:10.2307/2393988
- Hoang H, Antoncic B. Network-based research in entrepreneurship: A critical review. J Bus Venturing. 2003; 18(2):165-87. doi:10.1016/S0883-9026(02)00081-2
- Powell WW, White DR, Koput KW, Owen-Smith J. Network dynamics and field evolution: The growth of interorganizational collaboration in the life sciences. Am J Sociol. 2005; 110(4):1132-205. doi:10.1086/421508
- Zaheer A, Gözübüyük R, Milanov H. It's the connections: The network perspective in interorganizational research. Acad Manage Perspect. 2010; 24(1):62-77. doi:10.5465/AMP.2010.50304417
- Brass DJ, Galaskiewicz J, Greve HR, Tsai W. Taking stock of networks and organizations: a multilevel perspective. Acad Manage J. 2004; 47(6):795-817; doi:10.2307/20159624
- Borgatti SP, Foster PC. The network paradigm in organizational research: A review and typology. J Manage. 2003; 29(6):991-1013. doi:10.1016/S0149-2063(03)00087-4
- Bergenholtz C, Waldstrøm C. Inter-organizational network studies—A literature review. Ind Innov. 2011; 18(6):539-62. doi:10.1080/13662716.2011.591966
- Provan KG, Fish A, Sydow J. Interorganizational networks at the network level: A review of the empirical literature on whole networks. J Manage. 2007; 33(3):479-516. doi:10.1177/0149206307302554
- McKelvey M, Rake B. Product innovation success based on cancer research in the pharmaceutical industry: co-publication networks and the effects of partners. Ind Innov. 2016; 23(5):383-406. doi:10.1080/13662716.2016.1150157
- Knoben J, Oerlemans LAG, Rutten RPJH. Radical changes in inter-organizational network structures: The longitudinal gap. Technol Forecast Soc. 2006; 73(4):390-404. doi:10.1016/j.techfore.2005.05.010
- Powell WW, Koput KW, Smith-Doerr L, Owen-Smith J. Network position and firm performance: organizational returns to collaboration in the biotechnology industry. Res Sociol Organizations. Greenwich, CT: JAI Press 1999; 16:129-59
- Koka BR, Prescott JE. Designing alliance networks: the influence of network position, environmental change, and strategy on firm performance. Strategic Manage J. 2008; 29(6):639-61. doi:10.1002/smj.679
- Roijakkers N, Hagedoorn J. Inter-firm R&D partnering in pharmaceutical biotechnology since 1975: Trends, patterns, and networks. Res Policy. 2006; 35(3):431-46. doi:10.1016/j.respol.2006.01.006
- Li J, Zhu Z. Research and development of next generation of antibody-based therapeutics. Acta Pharmacol Sin. 2010; 31:1198-207. doi:10.1038/aps.2010.120. PMID:20694021
- Munro TP, Mahler SM, Huang EP, Chin DY, Gray PP. Bridging the gap: facilities and technologies for development of early stage therapeutic mAb candidates. mAbs. 2011; 3(5):440-52. doi:10.4161/mabs.3.5.16968. PMID:21822050
- Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov. 2010; 9(10):767-74. doi:10.1038/nrd3229. PMID:20811384
- Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. mAbs. 2015; 7(1):9-14. doi:10.4161/19420862.2015.989042. PMID:25529996
- Gay B, Dousset B. Innovation and network structural dynamics: Study of the alliance network of a major sector of the biotechnology industry. Res Policy. 2005; 34(10):1457-75. doi:10.1016/j.respol.2005.07.001
- Geng X, Kong X, Hu H, Chen J, Yang F, Liang H, Chen X, Hu Y. Research and development of therapeutic mAbs: An analysis based on pipeline projects. Hum Vaccin Immunother. 2015; 11(12):2769-76. doi:10.1080/21645515.2015.1074362. PMID:26211701
- Petering J, McManamny P, Honeyman J. Antibody therapeutics - the evolving patent landscape. N Biotechnol. 2011; 28(5):538-44. doi:10.1016/j.nbt.2011.03.023. PMID:21515427
- Gulati R. Social structure and alliance formation patterns: a longitudinal analysis. Admin Sci Quart. 1995; 40(4):619-52. doi:10.2307/2393756.
- McCafferty J, Griffiths AD, Winter G, Chiswell DJ. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990; 348(6301):552-4. doi:10.1038/348552a0. PMID:2247164
- Vaughan TJ, Williams AJ, Pritchard K, Osbourn JK, Pope AR, Earnshaw JC, McCafferty J, Hodits RA, Wilton J, Johnson KS. Human antibodies with sub-nanomolar affinities isolated from a large non-immunized phage display library. Nat Biotechnol. 1996; 14(3):309-14. doi:10.1038/nbt0396-309. PMID:9630891
- Christensen CM, Rosenbloom RS. Explaining the attacker's advantage: technological paradigms, organizational dynamics, and the value network. Res Policy. 1995; 24(2):233-57. doi:10.1016/0048-7333(93)00764-K
- Berchicci L. Towards an open R& D system: internal R&D investment, external knowledge acquisition and innovative performance. Res Policy. 2013; 42(1):117-27. doi:10.1016/j.respol.2012.04.017
- Rigby D, Zook C. Open-market innovation. Harv Bus Rev. 2002; 80(10):80-9. PMID:12389463
- Albert R, Jeong H, Barabási AL. Internet: diameter of the World-Wide Web. Nature. 1999; 401:130-1; doi:10.1038/43601
- Jeong H, Tombor B, Albert R, Oltvai ZN, Barabási AL. The large-scale organization of metabolic networks. Nature. 2000; 407:651-4; doi:10.1038/35036627. PMID:11034217
- Barabási AL, Jeong H, Néda Z, Ravasz E, Schubert A, Vicsek T. Evolution of the social network of scientific collaborations. Physica A. 2002; 311(3–4):590-614. doi:10.1016/S0378-4371(02)00736-7
- Barabási AL, Albert R. Emergence of scaling in random networks. Science. 1999; 286(5439):509-12. doi:10.1126/science.286.5439.509. PMID:10521342
- Bianconi G, Barabási AL. Competition and multiscaling in evolving networks. Europhys Lett. 2001; 54(4):436-42. doi:10.1209/epl/i2001-00260-6.
- Freeman C. The economics of technical change. Cambridge J Econ. 1994; 18(5):463-514. doi:10.1093/oxfordjournals.cje.a035286
- Hagedoorn J. Strategic technology partnering during the 1980s: Trends, networks and corporate patterns in non-core technologies. Res Policy. 1995; 24(2):207-31. doi:10.1016/0048-7333(94)00763-W
- Tripsas M. Unraveling the process of creative destruction: complementary assets and incumbent survival in the typesetter industry. Strategic Manage J. 1997; 18(1):119-42. doi:10.1002/(SICI)1097-0266(199707)18:1+3.3.CO;2-S
- Schumpeter J. The theory of economic development: An inquiry into profits, capital, credit, interest, and the business cycle. London: Transactions Publishers; 1934
- Cohen WM, Levinthal DA. Absorptive capacity: A new perspective on learning and innovation. Admin Sci Quart. 1990; 35(1):128-52. doi:10.2307/2393553
- West J, Bogers M. Leveraging external sources of innovation: A review of research on open innovation. J Prod Innovat Manag. 2014; 31(4):814-31. doi:10.1111/jpim.12125
- Gautam A, Pan X. The changing model of big pharma: Impact of key trends. Drug Discov Today. 2016; 21(3):379-84. doi:10.1016/j.drudis.2015.10.002. PMID:26477304
- Newman MEJ. The structure of scientific collaboration networks. Proc Natl Acad Sci U S A. 2001; 98(2):404-9. doi:10.1073/pnas.98.2.404. PMID:11149952
- Liu X, Bollen J, Nelson ML, Van de Sompel H. Co-authorship networks in the digital library research community. Inform Process Manag. 2005; 41(6):1462-80. doi:10.1016/j.ipm.2005.03.012
- Khan GF, Park HW. The e-government research domain: A triple helix network analysis of collaboration at the regional, country, and institutional levels. Gov Inform Q. 2013; 30(2):182-93. doi:10.1016/j.giq.2012.09.003
- You H, Ni J, Barber M, Scherngell T, Hu Y. China's landscape in oncology drug research: perspectives from research collaboration networks. Chin J Cancer Res. 2015; 27(2):138-47. doi:10.3978/j.issn.1000-9604.2015.04.05. PMID:25937775