ABSTRACT
Antibody formation to human(ized) therapeutic antibodies in humans is highly skewed toward anti-idiotype responses, probably because the idiotype is the only ‘foreign’ part of the antibody molecule. Here, we analyzed antibody responses to F(ab’)2 fragments of a panel of 17 human(ized) therapeutic antibodies in rabbits. Homology between the rabbit germline and the human(ized) antibodies is moderate not only for the variable domains (both the complementarity-determining regions and the framework regions), but also for the constant domains (66% or less). Nevertheless, we observed a highly skewed anti-idiotype response in all cases, with up to >90% of the antibodies directed toward the idiotype. These results indicate that the idiotype may be inherently immunodominant. We used these biased responses to raise monoclonal rabbit anti-idiotype antibodies against secukinumab, ustekinumab, reslizumab, mepolizumab, palivizumab, and dupilumab and demonstrate the potential to develop sensitive pharmacokinetic assays with these antibodies.
Introduction
Antibody formation to biopharmaceutical drugs in animals is highly relevant for several reasons. Animals may be deliberately immunized with these drugs to generate drug-specific antibodies that can serve as positive controls for anti-drug antibody (ADA) assays and reagents for assays assessing drug pharmacokinetics (PK). Furthermore, biopharmaceuticals are often tested in pre-clinical animal models, where ADA formation may interfere with the study of PK and effectiveness. In general, ADA formation in animals is not predictive for ADA formation in humans. Nevertheless, certain aspects of ADA formation may still be evaluated in animals.Citation1, Citation2
Therapeutic monoclonal antibodies (tmAbs) form a special class of biopharmaceuticals that are used in a wide range of diseases. Most of these protein molecules are by design highly homologous to other human IgG antibodies, i.e., humanized or biotechnology-derived human antibodies. They differ in structure largely within a confined region of the molecule called the paratope, or antigen-binding site (). This region largely coincides with the complementarity-determining regions (CDRs) or hypervariable loops within the variable regions of antibodies. The idiotype of an antibody is the set of unique structural determinants of that antibody. From this, it follows that often the idiotype of an antibody largely or completely overlaps with the paratope.Citation3
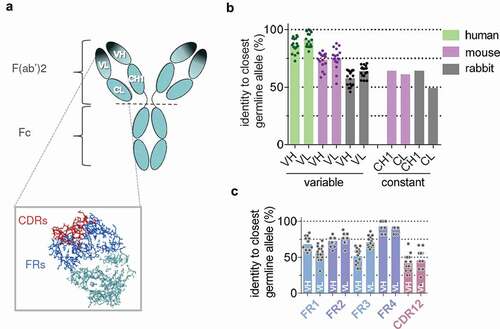
Figure 1. Anatomy of therapeutic monoclonal antibodies (tmAb). (a) Structure of an IgG antibody with variable domains (VL, VH) and constant domains (CL, CH1) in the F(ab’)2 region indicated. Dark shade indicates the antigen binding region or ‘paratope’, which largely coincides with the idiotype. Inset shows complementarity-determining regions (CDRs) in red and framework regions (FRs) in blue. CDRs make up the largest part of the paratope. (b) Homology of human(ized) tmAbs with closest germline allele for rabbit, mouse, and human per domain (n = 17; see materials and methods for details). (c) Homology of tmAb variable domains with rabbit for framework regions (FR1-4) and combined CDR1 and CDR2 regions.
Antibodies that specifically bind to a tmAb are expected to mainly target the idiotype and are therefore called anti-idiotype antibodies. In humans, antibodies to a number of different tmAbs were found to predominantly be anti-idiotype antibodies that inhibit target binding.Citation4–8 However, when a human or humanized therapeutic antibody is injected into an animal, such anti-idiotype antibodies might constitute only a (small) fraction of the total antibody response, since homology with the endogenous animal antibodies would be partial also for other regions of the antibody, including the framework regions (FRs) of the variable domains and the constant domains. Nevertheless, immunizations of mice with adalimumab or rituximab have yielded panels of monoclonal antibodies of which the majority were drug-specific/anti-idiotypic (10/19 and 16/22, respectively).Citation9,Citation10 This might indicate that antibody responses to monoclonal antibodies in general are easily biased toward an anti-idiotype response, although in the case of the chimeric rituximab, the variable domains are of mouse origin, which could explain the observed bias.
In this work, we analyzed antibody responses to a panel of human and humanized tmAbs in rabbits. Our aim was two-fold. First, we wanted to know how dominant the anti-idiotype response is in a setting with substantial ‘mismatch’ across the antibodies used as antigens. Second, we wanted to evaluate the efficiency of anti-idiotype responses in rabbits as a route toward monoclonal high-affinity anti-idiotype antibodies that may be used in PK and ADA assays. Rabbits have been a reliable source of polyclonal antibodies for decades, and have gained interest as a source of monoclonal antibodies in recent years, amongst others because it is suggested that antibody responses in rabbits may yield a more robust, high-affinity repertoire of antibodies in comparison to mice.Citation11,Citation12 Here we demonstrate that rabbits are a convenient source for obtaining monoclonal anti-idiotype antibodies.
Results
Homology of therapeutic human(ized) antibodies with rabbit/mouse antibodies
For a panel of 17 tmAbs, 9 humanized and 8 human (see Materials and Methods), we analyzed the homology of the variable and constant domains of the antigen-binding fragment (Fab) region with the human, mouse, and rabbit germ-line repertoire (). The median homology of the variable domains to the closest human germ-line alleles is 86% and 91% for VH and VL, respectively. Homology to rabbit VH and VL is 59% and 66%, respectively, which is substantially lower not only compared to human, but also to mouse, for which median homology is 77% and 75% for VH and VL, respectively. Furthermore, homology of the human constant domains in the Fab region, CH1 and CL, to both rabbit and mouse counterparts was also fairly low (<65%). For the rabbit variable domains, FR2 and FR4 show higher homology in comparison to the CDR1 and 2 regions and FR1 and FR3 regions ().
Rabbit immunizations
Rabbits were immunized with F(ab’)2 of the 17 different tmAbs according to the scheme outlined in . Serum or plasma obtained 9 d after each booster was tested for the presence of antibodies to the tmAb (). Titers were generally substantially higher after the second or third booster compared to the first booster (). Per tmAb, two rabbits were immunized, and robust responses were invariably observed.
Figure 2. Cross-reactivity of polyclonal rabbit anti-tmAb antibodies to polyclonal human antibodies. (a) Immunization scheme. (b) Assay for evaluating rabbit antibody responses: rabbit antibodies are captured onto protein A Sepharose beads and biotinylated tmAb F(ab’)2 is added to detect anti-tmAb antibodies. If IVIG F(ab’)2 is also added, binding of anti-framework and anti-constant domain antibodies, but not anti-idiotype antibodies will be inhibited. (c) Examples of binding curves for rabbit serum upon immunization with a tmAb F(ab’)2, drawn 9 d after a booster. Left panel: palivizumab; middle panel: cross-reactivity to tocilizumab. Notice that IVIG F(ab’)2 completely inhibits the cross-reactive binding; right panel: adalimumab. (d) Percentages of rabbit antibody response not inhibited by IVIG (Fab’)2 (i.e., response in presence of inhibitor relative to response without) after the 1st or 2nd/3rd booster (n = 17 human(ized) tmAbs, 2 rabbits/mAb). Bars represent mean and SD. (e) Correlation between 1st and 2nd/3rd booster (left panel) and rabbit 1 and rabbit 2 (right panel); Pearson r = 0.73, p < .0001, and r = 0.74, p < .0001, respectively.
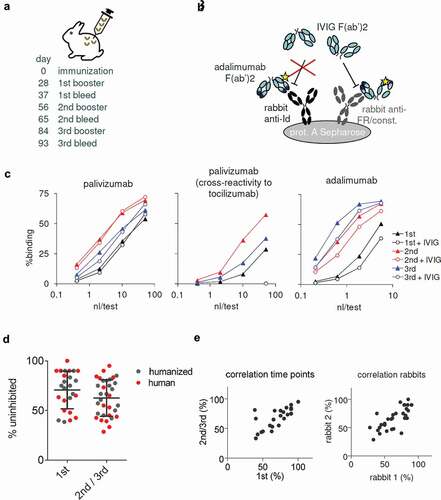
To investigate the contribution of anti-idiotype antibodies to the total anti-tmAb response, samples were tested both in the presence and absence of a 10,000-fold excess of F(ab’)2 prepared from pooled polyclonal human IgG (IVIG; ). Two examples are shown in . For palivizumab, hardly any inhibition was observed, indicating that almost all anti-tmAb antibodies (> 90%) were anti-idiotype. On the other hand, for adalimumab, anti-idiotype antibodies comprise about half the anti-tmAb response. Effectiveness of the IVIG F(ab’)2 inhibition was demonstrated by complete inhibition of cross-reactive binding (example shown for palivizumab). In , the percentage of reactivity not inhibited by IVIG (Fab’)2 is shown for all 17 tmAbs, for both the first booster and 2nd/3rd booster combined, yielding a median of 67 (IQR 48–83)%. There was no significant difference between the 1st and 2nd/3rd booster, and the percentage inhibition correlated well between 1st and 2nd/3rd booster (). Two rabbits were immunized per tmAb, and there was also a good correlation of percent inhibition between rabbits (). To exclude the possibility that these results were affected by the use of biotinylated tmAbs, we also conducted another experiment in which binding of rabbit antibodies to intact, unlabeled tmAb was tested in the presence or absence of IVIG F(ab’)2 (Figure S1). Since immunizations were carried out with F(ab’)2 of the tmAbs, no anti-Fc antibodies will have been raised in the rabbits and the Fc tail conveniently serves as ‘tag’ for detection of bound tmAb using an anti-human Fc antibody. Overall, results were similar to those described above, with a median 68 (IQR 45–80)% non-inhibited reactivity (n = 12).
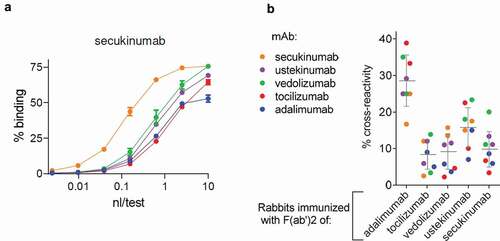
For five tmAbs, cross-reactivity of the rabbit antibody response against any of the respective four other tmAbs was evaluated (). Cross-reactivity was fairly low, at a median of 12 (IQR 6–20)%. These results were confirmed by assessing cross-reactivity in an enzyme-linked immunosorbent assay (ELISA) (Figure S1d), yielding a median of 10 (IQR 8–25%). This implies that on average, less than ca. 12% of the antibodies would be directed against the constant domains of the Fab region. Taken together, the antibody response to tmAbs in rabbits is biased toward an anti-idiotype response.
Figure 3. Cross-reactivity of polyclonal rabbit anti-tmAb antibodies to other tmAbs. (a) Exemplar binding curves for rabbit serum upon immunization with a tmAb F(ab’)2 (secukinumab), drawn 9 d after final booster to secukinumab and four other tmAbs (ustekinumab, vedolizumab, tocillizumab, and adalimumab). (b) Overview of cross-reactivities for rabbits immunized with five different tmAbs (2 rabbits/tmAb), tested against all five tmAbs as in (a). Bars represent mean and SD.
B-cell screening and mAb generation
Given the robust (polyclonal) antibody response to therapeutic antibodies, we wondered if specific, high-affinity monoclonal anti-idiotype antibodies could be obtained following rabbit immunizations. For this purpose, peripheral blood mononuclear cells (PBMCs) were isolated from bleeds 9 d after the first or second booster. Following the strategy originally developed for human single B-cell cloning,Citation5,Citation13 cells were enriched for antigen-specific cells by fluorescence-activated cell sorting (FACS) and collected at one cell per well. An example is shown for secukinumab in . The population of cells positive for antigen was observed mainly as cells somewhat larger – consistent with a plasmablast-like phenotype. However, the paucity of specific antibodies for rabbit lymphocyte cell surface proteins precludes a more elaborate phenotypic analysis. Cells were cultured for 9 d and supernatants screened for the presence of anti-idiotype antibodies. RNA was isolated from positive wells, message-encoding variable domains were amplified by PCR, sequenced, and corresponding antibody was recombinantly expressed.
Figure 4. Enrichment of antigen-specific B cells. Gating strategy for single cell sort. Lymphocytes isolated from a rabbit immunized with secukinumab were defined based on FSC and SSC, subsequent exclusion of doublets and gating on either IgG+ B cells (red) or enriched for secukinumab-specific IgG+ B cells (blue).
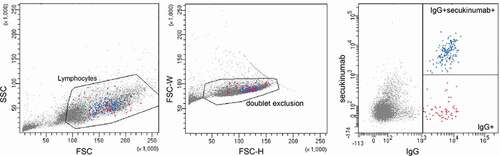
For secukinumab, three clones were sequenced and recombinantly expressed. Of these, one clone (5A9) was expressed in good yields and bound specifically to secukinumab. For five other therapeutic antibodies, a similar procedure was followed, 2–4 clones were sequenced, and the one with the highest yield carried forward for evaluation ().
Table 1. Monoclonal anti-idiotype antibodies obtained from immunized rabbits.
Evaluation of rabbit anti-idiotype monoclonal antibodies for PK assays
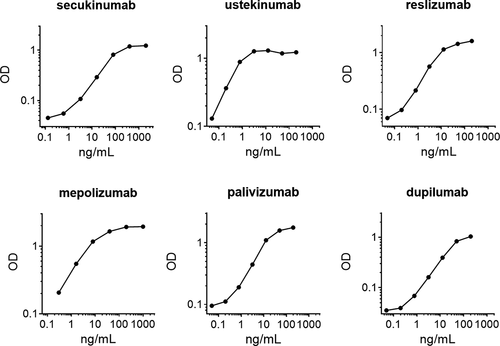
Binding affinities of the anti-idiotype antibodies were measured using biosensor analysis. All the monoclonal antibodies bound with high affinity to their respective target (; Figure S2). This suggests that these antibodies are suitable for the development of sensitive PK assays. To illustrate the applicability of these antibodies for use in PK assays, we tested each antibody in a bridging ELISA format, using the anti-idiotype antibody for both capture and detection. For reslizumab, an IgG4 antibody with an unmodified hinge and therefore capable of Fab arm exchange,Citation14,Citation15 we tested an alternative format, using the anti-idiotype for capture and detection using anti-IgG4. Examples of titration curves for the respective biologics are shown in , demonstrating dose–response relationships in the low ng/mL range. Minimal cross-reactivity was observed against other biologics (Figure S3).
Discussion
In this study, we demonstrated a highly biased anti-idiotype antibody response in rabbits following immunization with tmAbs. These robust responses were therefore also explored as a route toward monoclonal rabbit anti-idiotype antibodies. In our hands, this resulted in a convenient and reliable method to generate such antibodies.
Our current observations extend the observations in humans that ADA responses to tmAbs are largely restricted to the idiotype. Despite the overall low homology of variable and constant domains in the human(ized) Fab2 used for immunization of rabbits, the immune responses are largely confined toward drug-specific determinants. This suggests that the idiotype, or antigen-binding site, of an antibody molecule may somehow be ‘immunodominant’, i.e., more easily serve as a B cell epitope, in comparison to other foreign parts of the antibody structure. If these results can be extrapolated to species other than rabbit remains to be investigated, although pilot immunization studies of adalimumab in mice showed a similarly biased antibody response (data not shown). Furthermore, anti-infliximab antibodies in humans appear to be largely restricted to the antigen-binding site even though the framework regions of the variable domains have only moderate homology with human variable domains in this chimeric humanized antibody.Citation6
At a more fundamental level, it has proven hard to define or predict B cell epitopes.Citation16–18 Therefore, by extension, it is difficult to speculate which factors might make certain parts of a protein ‘immunodominant’. One study suggests that immunodominance of one epitope over another may depend on subtle structural features and can substantially shift with just a single amino acid mutation.Citation19 One feature idiotypes have in common is that they largely coincide with the antibodies’ antigen binding site. There is some evidence that protein-protein binding sites in general have certain structural characteristics that make these regions stand out.Citation20 One could speculate that protein binding sites, including antigen binding sites, are in general likely candidates to serve as B cell epitopes precisely because these sites have what it takes to bind another protein. Furthermore, antigen binding sites have a skewed amino acid composition, enriched for aromatic residues, Tyr in particular.Citation16 These are believed to be important for antigen binding, but might also make the antigen binding site an easier target for other B cells. In short, the idiotype might be an immunodominant epitope not only because it will generally be ‘foreign’, but also because of structural features common to binding sites in general.
The strong and highly skewed anti-idiotype response of rabbits toward tmAbs allowed for a comparatively straightforward procedure to obtain rabbit monoclonal anti-idiotype antibodies. Anti-idiotype reagents are useful for the development of PK and ADA assays. Mice are often used as a source of monoclonal antibodies, but rabbits may yield a more robust antibody response in comparison.Citation11,Citation12 The lower homology of tmAbs to rabbit germline sequences in comparison to mice () suggests that rabbits in general might be expected to mount a stronger immune response to tmAbs than mice. The antibodies generated in this study demonstrated high-affinity binding (); and appear suitable for sensitive measurement of the therapeutic antibodies ().
In summary, we demonstrate a strong, highly skewed anti-idiotype response toward tmAbs in rabbits. This makes the rabbit a convenient source for the development of high-affinity monoclonal anti-idiotype antibodies.
Materials and methods
Materials
Monoclonal therapeutic antibodies were: adalimumab (Humira, AbbVie), belimumab (Benlysta, GSK), certolizumab pegol (Cimzia, UCB), dupilumab (Dupixent, Sanofi), golimumab (Simponi, MSD), guselkumab (Tremfya, Janssen-Gilag), mepolizumab (Nucala, GSK), natalizumab (Tysabri, Biogen), nivolumab (Opdivo, BMS), omalizumab (Xolair, Novartis), palivizumab (Synagis, AbbVie), pembrolizumab (Keytruda, MSD), reslizumab (Cinqaero, Teva), secukinumab (Cosentyx, Novartis), tocilizumab (Roactemra, Roche), ustekinumab (Stelara, Janssen-Gilag), vedolizumab (Entyvio, Takeda), and were obtained from the pharmacy.
Immunizations
Immunizations were carried out as described previously.Citation21 Briefly, F(ab’)2 fragments of the therapeutic antibodies were generated by pepsin digestion, except for belimumab, of which F(ab’)2 fragments were prepared via IdeS (Fabricator, Genovis) digestion. Certolizumab pegol is a PEGylated F(ab’) fragment and was used for immunizations without any modifications. Female New Zealand white rabbits were immunized with 100 μg/ml F(ab’)2 fragments/Montanide ISA-50 (Seppic, Paris, France) multiple times at 4-week intervals. Blood was drawn 9 d after the 2nd, 3rd, and 4th immunizations, and either collected as serum, or as EDTA blood. In the latter case, PBMCs were isolated by density centrifugation using Ficoll (GE Healthcare, Chalfont St. Giles, UK). PBMCs were stored in liquid nitrogen, and plasma was stored at −30 °C.
Radioimmunoassays for ADA testing
Rabbit antibodies to the therapeutic antibodies were measured with radioimmunoassay (RIA) essentially as described previously.Citation22 Serial dilutions of serum in phosphate-buffered saline (PBS)/0.3% bovine serum albumin (BSA) and 0.02% polysorbate 20 was incubated with 1 mg protein A Sepharose (GE healthcare, Chalfont St. Giles, UK) or CaptureSelect IgG-Fc (ms) Affinity Matrix (ThermoFisher) and 1 ng of biotinylated F(ab’)2 fragments of the respective monoclonal antibody, with or without 10 μg of IVIG F(ab’)2 (prepared from Nanogam, Sanquin) in 800 μl of total volume. The F(ab’)2 fragments were biotinylated with Sulfo-NHS LC-biotin (Pierce/Thermo Fisher Scientific, Rockford, IL, USA). After overnight incubation, samples were washed and125I radioactive labeled streptavidin was added. After overnight incubation, unbound radiolabel was washed out and Sepharose-bound radioactivity was measured.
ELISAs for ADA testing
Maxisorp microtiter plates (NUNC, Rochester, NY) were coated with 1 μg/mL goat anti-rabbit IgG H & L (Abcam; ab6702) overnight in PBS. After washing 5 times with PBS/0.02% Tween (PBS-T), plates were incubated for 1 hour with serially diluted rabbit sera, starting from 1:1000. After washing 5 times with PBS-T, therapeutic antibody was added at a final concentration of 0.25 μg/mL with or without 50 μg of IVIG F(ab’)2 in PBS-T supplemented with 0.3% gelatin (PTG) and incubated for 1 hour. Plates were washed 5 times, and incubated with 0.5 μg/mL mouse anti-human IgG-horseradish peroxidase (HRP) (clone MH16-1, Sanquin) for 30 minutes. Alternatively, plates were coated with 1 μg/mL mouse anti-human IgG (MH16-1), followed by capture of therapeutic antibody at 0.25 μg/mL, incubation with serially diluted rabbit sera, and detection of bound rabbit IgG using mouse monoclonal anti-rabbit IgG-HRP (clone 2A9, Abcam). Subsequently, after washing, plates were developed with 100 μg/ml tetramethylbenzidine in 0.11 M sodium acetate (pH 5.5) containing 0.003% (v/v) H2O2. The reaction was stopped with 0.2 M H2SO4. Absorption was measured as OD = 450–540 nm.
B-cell isolation, sorting, and culture
B cells were isolated from thawed PBMC samples by index FACS sorting. Cells were stained with FITC-labeled mouse anti-rabbit IgG antibody clone 2A9 (Abcam, Cambridge, UK) and biotinylated F(ab’)2 fragments of a tmAb in combination with Streptavidin-APC (BD Biosciences, San Jose, CA). Cells were seeded at 1 cell/well in 96-well flat-bottom microtiter plates and cultured for 9 d essentially as described before,Citation5,Citation24 but with mouse IL-1β and mouse tumor necrosis factor (BioLegend, San Diego, CA; 575106, 575206), and recombinant IL-21 (R&D systems, Minneapolis, MN; 7274-RB-025).
Screening ELISA
Supernatants were screened for the presence of specific anti-idiotype antibodies by bridging ELISA. Maxisorp microtiter plates (NUNC, Rochester, NY) were coated with 0.25–1 ug/mL therapeutic antibody in PBS overnight at room temperature. After washing 5 times with PBS-T, plates were incubated for 1 hour with culture supernatant diluted 5x in high-performance ELISA buffer (HPE, Sanquin, The Netherlands), supplemented with 1 mg/mL IVIG (Nanogam, Sanquin) (HPE-I). After washing 5 times with PBS-T, plates were incubated with 50 − 100 ng/mL biotinylated F(ab’)2 of therapeutic antibody in HPE-I for 1 h. Plates were washed 5 times, and incubated with streptavidin-HRP (Sanquin) for 30 minutes. After washing, plates were developed with 100 μg/ml tetramethylbenzidine in 0.11 M sodium acetate (pH 5.5) containing 0.003% (v/v) H2O2. The reaction was stopped with 0.2 M H2SO4. Absorption was measured as OD = 450–540 nm.
RNA isolation, sequencing
Total RNA was isolated with TriFast (Erlangen, Germany) according to the manufacturer’s instructions. Briefly, 150 µl TriFast was added to lyse single cells. To visualize the pellet during RNA isolation, 45 µg GlycoBlue (ThermoFisher) was added. The aqueous phase was isolated after chloroform addition. Subsequently, RNA was precipitated with 180 µl 2-propanol and washed with 75% ethanol. The RNA pellet was resuspended in RNase-free water (ThermoFisher). For cDNA synthesis, 400 ng random primers (Invitrogen) were added to up to 2.5 µg mRNA and incubated for 10 minutes at 70°C. After cooling the mixture, 1x First strand buffer (Invitrogen), 0.01 M dithiothreitol (DTT; Invitrogen), 2 mM dNTPs (Invitrogen), 20 Units RNase OUT and 100 Units Superscript II (Invitrogen) were added. Reverse transcription was performed by initial incubation at room temperature for 10 minutes followed by an incubation of 50 minutes at 42°C. Incubation at 70°C for 10 minutes terminated the reaction and cDNA was stored at −20°C.
IGHV and IGLV sequences were amplified by PCR.Citation12 The following primers were used: VH forward primer (5ʹ AAGCTTGCCACCATGGAGACTGGGCTGCGCTGGCTTC), VH reverse primer (5ʹ CCATTGGTGAGGGTGCCCGAG), V kappa forward primer (5ʹ AAGCTTGCCACCATGGACAYGAGGGCCCCCACTC) and V kappa reverse primer (5ʹ CAGAGTRCTGCTGAGGTTGTAGGTAC (R = G or A)). For separate PCR of IGHV and IGLV genes, 2.5 µl cDNA was added to 22.5 µl AccuPrime Pfx SuperMix (Invitrogen) and 10 µM of forward and reverse primer. PCR product was purified by gel electrophoresis and DNA isolation with NucleoSpin Gel PCR Cleanup kit (Macherey-Nagel, Düren, Germany). Subsequently, 20 ng DNA was used for Sanger sequencing reaction. Reaction mixture consisted of 4 µl BDT (Applied Biosystems, Waltham, MA), 0.5x BDT buffer and 10 µM forward and reverse primer. Sequencing was performed with an AB Analyzer. Sequences were assessed using FinchTV and aligned to reference sequences from the IMGT database.
Recombinant antibody expression
Recombinant anti-idiotype antibodies were prepared by cloning constructs coding for VH and VL of the rabbit anti-idiotype antibody and the constant domains of the human IgG1 or mouse IgG2b and human, mouse, or rabbit kappa genes (obtained from GeneArt, Invitrogen) into a pcDNA3.1 expression vector (Invitrogen) essentially as described before.Citation5,Citation25 Expression vectors were used for transient transfection of HEK293F cells with polyethylenimine (PEI) and OptiMEM (Invitrogen), using the Freestyle HEK293F expression system (Invitrogen) according to the instructions supplied by the manufacturer. Cell culture supernatants were centrifuged for 15 min at 1700 G, followed by loading on a Protein G column (Protein G 4 fast flow, GE Healthcare) and elution of IgG with 0.1 M glycine, pH 2.5. The eluate was neutralized immediately with 2 M Tris–HCl, pH 9, and dialyzed overnight to PBS. After dialysis, samples were stored at −20°C.
Affinity measurements
Affinity of anti-idiotype antibodies was determined by surface plasmon resonance (SPR) technology using a Biacore T200. A protein G chip, or a CM5 chip to which either a rat anti-mouse IgG2b or mouse anti-human IgG monoclonal antibody was coupled, was used to capture anti-idiotype antibodies (9 µg/mL; 15 µL injected at 15 µL/min), yielding approximately 100 response units (RU) of bound antibody. Subsequently, binding of Fabs of the corresponding biologics was measured (60, 20, and 6 nM) at a flow rate of 15 µl/min for 600 s followed by monitoring dissociation for another 600 s. Alternatively, a single-cycle experiment was carried out at 30 µl/min and 16, 8, 4, 2, and 1 nM of Fab, and with a final dissociation time of 2000 s. To produce Fabs, F(ab’)2 fragments of tmAbs were reduced with 3 mM DTT at 37°C for 2 hours and subsequently alkylated with 6 mM iodoacetamide. All Biacore experiments were performed at 25°C. PBS containing 0.05% polysorbate-20 was used as running buffer. After each run, immobilized ligand was regenerated by removing bound analyte with 5 μL of 0.1 M phosphoric acid. Adsorptions obtained in the reference channel without bound anti-idiotype antibody was subtracted from the adsorptions in the other cells. Association and dissociation kinetics were fitted using the models provided with the Biacore analysis software. There was good agreement between the runs carried out with either protocol (15 vs 30 µl/min), and final results () were reported as the average of all data combined.
PK assays
PK assays were carried out as bridging ELISAs using anti-idiotype monoclonal antibodies as capture and biotinylated monoclonal antibodies for detection except for reslizumab, where detection was carried out using biotinylated anti-human IgG4 (clone MH164.4, Sanquin). Maxisorp enzyme-linked immunosorbent assay (ELISA) plates were coated overnight at room temperature with 0.25 or 0.5 μg/mL rabbit anti-idiotype antibodies in PBS. Plates were washed five times with PBS/0.02% Tween (PBS-T), then washed and incubated for 1 h with serially diluted biological in 5% human serum in high-performance ELISA (HPE) buffer. After washing five times with PBS-T, plates were incubated for 1 h with biotinylated anti-idiotype antibody (250 ng/mL in HPE buffer) or anti-IgG4 (500 ng/mL in HPE buffer). After washing, streptavidin–HRP (Sanquin, Amsterdam, The Netherlands) (1:8000, in HPE buffer) was added for 1 h. After washing, the ELISA was developed with 100 μg/mL tetramethylbenzidinein 0.11 M sodium acetate (pH 5.5) containing 0.003% (v/v) H2O2. The reaction was stopped with 0.2 M H2SO4. Absorption was measured as OD = 450–540 nm.
Data processing, visualization & statistics
Sequence information of the tmAbs was retrieved from either http://www.imgt.org/mAb-DB or from patents. Sequence similarity to human, mouse, and rabbit germline variable domain alleles was carried out using the DomainGapAlign tool from IMGT(Citation23), and further processed using Microsoft Excel. Other data processing was carried out in Microsoft Excel; Graphpad Prism was used for statistics and production of graphs.
Abbreviations
| Anti-drug antibodies | = | (ADA) |
| Pharmacokinetics | = | (PK) |
| Therapeutic monoclonal antibody | = | (tmAb) |
| Complementarity determining region | = | (CDR) |
| Framework region | = | (FR) |
Supplemental Material
Download Zip (1.4 MB)Acknowledgments
We gratefully acknowledge the contributions of Karien Bloem, Els de Groot, Margreet Hart, Irma Rensink, Ellen Vermeulen, Henk te Velthuis, and Annick de Vries, and would furthermore like to acknowledge the Netherlands Cancer Institute Protein Facility for using the Biacore.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- Boysen M, Schlicksupp L, Dreher I, Loebbert R, Richter M. SEC based method for size determination of immune complexes of therapeutic antibodies in animal matrix. J Immunol Res. 2016;2016:9096059. doi:https://doi.org/10.1155/2016/9096059.
- Jiskoot W, Kijanka G, Randolph TW, Carpenter JF, Koulov AV, Mahler H-C, Joubert MK, Jawa V, Narhi LO. Mouse models for assessing protein immunogenicity: lessons and challenges. J Pharm Sci. 2016;105(5):1567–9. doi:https://doi.org/10.1016/j.xphs.2016.02.031.
- van Schie KA, Wolbink GJ, Rispens T. Cross-reactive and pre-existing antibodies to therapeutic antibodies–Effects on treatment and immunogenicity. mAbs. 2015;7(4):662–71. doi:https://doi.org/10.1080/19420862.2015.1048411.
- Chatenoud L, Baudrihaye MF, Chkoff N, Kreis H, Goldstein G, Bach JF. Restriction of the human in vivo immune response against the mouse monoclonal antibody OKT3. J Immunol. 1986;137:830–38.
- van Schouwenburg PA, Kruithof S, Votsmeier C, van Schie K, Hart MH, de Jong RN, van Buren EEL, van Ham M, Aarden L, Wolbink G, et al. Functional analysis of the anti-adalimumab response using patient-derived monoclonal antibodies. J Biol Chem. 2014;289(50):34482–88. doi:https://doi.org/10.1074/jbc.M114.615500.
- van Schie KA, Hart MH, de Groot ER, Kruithof S, Aarden LA, Wolbink GJ, Rispens T. The antibody response against human and chimeric anti-TNF therapeutic antibodies primarily targets the TNF binding region. Ann Rheum Dis. 2015;74(1):311–14. doi:https://doi.org/10.1136/annrheumdis-2014-206237.
- van Schie KA, Kruithof S, van Schouwenburg PA, Vennegoor A, Killestein J, Wolbink G, Rispens T. Neutralizing capacity of monoclonal and polyclonal anti-natalizumab antibodies: the immune response to antibody therapeutics preferentially targets the antigen-binding site. J Allergy Clin Immunol. 2017;139(3):1035–7 e6. doi:https://doi.org/10.1016/j.jaci.2016.09.014.
- van Schie KA, Kruithof S, Ooijevaar-de Heer P, Derksen NIL, van de Bovenkamp FS, Saris A, Vidarsson G, Bentlage AEH, Jiskoot W, Romeijn S, et al. Restricted immune activation and internalisation of anti-idiotype complexes between drug and antidrug antibodies. Ann Rheum Dis. 2018;77(10):1471–79. doi:https://doi.org/10.1136/annrheumdis-2018-213299.
- Bian S, Stappen TV, Baert F, Compernolle G, Brouwers E, Tops S, Vries AD, Rispens T, Lammertyn J, Vermeire S, et al. Generation and characterization of a unique panel of anti-adalimumab specific antibodies and their application in therapeutic drug monitoring assays. J Pharm Biomed Anal. 2016;125:62–67. doi:https://doi.org/10.1016/j.jpba.2016.03.029.
- Tada M, Suzuki T, Ishii-Watabe A. Development and characterization of an anti-rituximab monoclonal antibody panel. mAbs. 2018;10(3):370–79. doi:https://doi.org/10.1080/19420862.2018.1424610.
- Weber J, Peng H, Rader C. From rabbit antibody repertoires to rabbit monoclonal antibodies. Exp Mol Med. 2017;49:e305.
- Seeber S, Ros F, Thorey I, Tiefenthaler G, Kaluza K, Lifke V, Fischer JAA, Klostermann S, Endl J, Kopetzki E, et al. A robust high throughput platform to generate functional recombinant monoclonal antibodies using rabbit B cells from peripheral blood. PLoS One. 2014;9(2):e86184. doi:https://doi.org/10.1371/journal.pone.0086184.
- Dohmen SE, Mulder A, Verhagen OJ, Eijsink C, Franke-van Dijk ME, van der Schoot CE. Production of recombinant Ig molecules from antigen-selected single B cells and restricted usage of Ig-gene segments by anti-D antibodies. J Immunol Methods. 2005;298(1–2):9–20. doi:https://doi.org/10.1016/j.jim.2004.12.013.
- Labrijn AF, Buijsse AO, van den Bremer ET, Verwilligen AY, Bleeker WK, Thorpe SJ, Killestein J, Polman CH, Aalberse RC, Schuurman J, et al. Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo. Nat Biotechnol. 2009;27(8):767–71. doi:https://doi.org/10.1038/nbt.1553.
- Rispens T, Davies AM, Ooijevaar-de Heer P, Absalah S, Bende O, Sutton BJ, Vidarsson G, Aalberse RC. Dynamics of inter-heavy chain interactions in human immunoglobulin G (IgG) subclasses studied by kinetic Fab arm exchange. J Biol Chem. 2014;289(9):6098–109. doi:https://doi.org/10.1074/jbc.M113.541813.
- Peng HP, Lee KH, Jian JW, Yang AS. Origins of specificity and affinity in antibody-protein interactions. Proc Natl Acad Sci U S A. 2014;111(26):E2656–65. doi:https://doi.org/10.1073/pnas.1401131111.
- Kringelum JV, Lundegaard C, Lund O, Nielsen M. Reliable B cell epitope predictions: impacts of method development and improved benchmarking. PLoS Comput Biol. 2012;8(12):e1002829. doi:https://doi.org/10.1371/journal.pcbi.1002829.
- Jespersen MC, Mahajan S, Peters B, Nielsen M, Marcatili P. Antibody specific B-cell epitope predictions: leveraging information from antibody-antigen protein complexes. Front Immunol. 2019;10:298. doi:https://doi.org/10.3389/fimmu.2019.00298.
- Chiesa MD, Martensen PM, Simmons C, Porakishvili N, Justesen J, Dougan G, Roitt IM, Delves PJ, Lund T. Refocusing of B-cell responses following a single amino acid substitution in an antigen. Immunology. 2001;103(2):172–78. doi:https://doi.org/10.1046/j.1365-2567.2001.01242.x.
- Ma B, Elkayam T, Wolfson H, Nussinov R. Protein-protein interactions: structurally conserved residues distinguish between binding sites and exposed protein surfaces. Proc Natl Acad Sci U S A. 2003;100(10):5772–77. doi:https://doi.org/10.1073/pnas.1030237100.
- Rispens T, Leeuwen A, Vennegoor A, Killestein J, Aalberse RC, Wolbink GJ, Aarden LA. Measurement of serum levels of natalizumab, an immunoglobulin G4 therapeutic monoclonal antibody. Anal Biochem. 2011;411(2):271–76. doi:https://doi.org/10.1016/j.ab.2011.01.001.
- Rispens T, de Vrieze H, de Groot E, Wouters D, Stapel S, Wolbink GJ, Aarden LA. Antibodies to constant domains of therapeutic monoclonal antibodies: anti-hinge antibodies in immunogenicity testing. J Immunol Methods. 2012;375(1–2):93–99. doi:https://doi.org/10.1016/j.jim.2011.09.011.
- Ehrenmann F, Kaas Q, Lefranc MP. IMGT/3Dstructure-DB and IMGT/DomainGapAlign: a database and a tool for immunoglobulins or antibodies, T cell receptors, MHC, IgSF and MhcSF. Nucleic Acids Res. 2010;38(Databaseissue):D301–7. doi:https://doi.org/10.1093/nar/gkp946.
- Lighaam LC, Vermeulen E, Bleker T, Meijlink KJ, Aalberse RC, Barnes E, Culver EL, van Ham SM, Rispens T. Phenotypic differences between IgG4+ and IgG1+ B cells point to distinct regulation of the IgG4 response. J Allergy Clin Immunol. 2014;133(1):267–70e1–6. doi:https://doi.org/10.1016/j.jaci.2013.07.044.
- Vink T, Oudshoorn-Dickmann M, Roza M, Reitsma JJ, de Jong RN. A simple, robust and highly efficient transient expression system for producing antibodies. Methods. 2014;65(1):5–10. doi:https://doi.org/10.1016/j.ymeth.2013.07.018.