ABSTRACT
While antibody-dependent cellular phagocytosis mediated by activating Fcγ receptor is a key mechanism underlying many antibody drugs, their full therapeutic activities can be restricted by the inhibitory Fcγ receptor IIB (FcγRIIB). Here, we describe a bispecific antibody approach that harnesses phagocytic receptor CLEC5A (C-type Lectin Domain Containing 5A) to drive Fcγ receptor-independent phagocytosis, potentially circumventing the negative impact of FcγRIIB. First, we established the effectiveness of such an approach by constructing bispecific antibodies that simultaneously target CLEC5A and live B cells. Furthermore, we demonstrated its in vivo application for regulatory T cell depletion and subsequent tumor regression.
Introduction
Phagocytosis mediated by Fcγ receptors (FcγRs) is an important mechanism of action in many therapeutic antibodies,Citation1 such as daratumumab,Citation2 trastuzumab,Citation3 and rituximab.Citation4 Macrophages can phagocytose cells and particles through various mechanisms;Citation5 while phagocytosis through FcγRs is the most commonly exploited mechanism in therapeutic antibodies, other mechanisms could provide advantages in specific contexts. For example, we recently described the use of bispecific antibodies targeting MerTK for promoting immunologically silent phagocytosis, which is distinct from FcγR-dependent cellular phagocytosis.Citation6 Thus, the study of alternative phagocytosis mechanisms could reveal opportunities to develop therapeutic antibodies with advantages in some specific indications or patient populations.
CLEC5A (C-type Lectin Domain Containing 5A, also known as myeloid DAP12-associating lectin (MDL-1)) is a type II transmembrane protein that is expressed by monocytes, macrophages and neutrophils.Citation7,Citation8 It is associated with the immunoreceptor tyrosine-based activation motif (ITAM)- or YINM motif-containing adaptor proteins DAP12 and DAP10, respectively.Citation9,Citation10 CLEC5A has not been demonstrated to induce phagocytosis, but other receptors, such as TREM2, that are associated with ITAM-containing proteins like DAP12 have been shown to induce phagocytosis.Citation11 Therefore, we hypothesized that CLEC5A could be harnessed to enhance phagocytosis through a bispecific antibody approach. Here, we show that CLEC5A agonized by bispecific antibodies can mediate phagocytosis in vitro as effectively as FcγRs. Moreover, we show that CLEC5A-directed bispecific antibodies were effective in inhibiting tumor growth in mouse tumor models.
Results
CLEC5A expression in macrophages
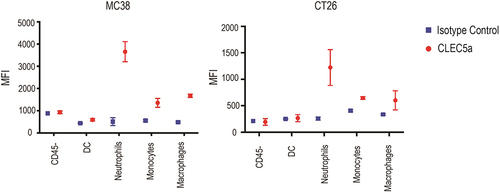
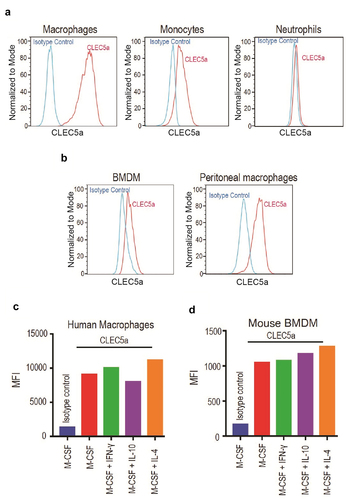
In order to harness the CLEC5A receptor through a bispecific antibody approach, we first characterized its expression. CLEC5A was detected by fluorescence-activated cell sorting (FACS) on human primary macrophages, monocytes, and neutrophils (). Mouse bone marrow-derived macrophages (BMDMs) and peritoneal macrophages also expressed CLEC5A (). Gene expression can be affected by different macrophage polarization states.Citation12 To examine whether CLEC5A expression is modulated by macrophage polarization, we treated both human and mouse macrophages with macrophage colony-stimulating factor (M-CSF), (M-CSF + interferon (IFN)-γ), (M-CSF + interleukin (IL)-10), and (M-CSF + IL-4) to induce them into M0, M1, M2c or M2a phenotypes, respectively.Citation13 Overall, we did not observe a significant difference in CLEC5A expression across different macrophage polarization conditions in vitro, with cells from all polarized states showing a robust signal ( a nd D). To characterize CLEC5A expression in vivo, we examined CLEC5A expression on tumor-infiltrating myeloid cell populations in mouse MC38 colon adenocarcinoma and CT26 colorectal carcinoma tumor models. We confirmed by FACS that CLEC5A was expressed by monocytes, macrophages and neutrophils 12 days after tumor inoculation (, B and S1). Together, these data suggest CLEC5A as a potential target for engineering fit-for-purpose phagocytosis through a bispecific antibody approach.
Figure 1. Characterization of CLEC5A expression on primary myeloid cells (a) FACS plots showing expression of CLEC5A on human primary monocyte-derived macrophages, monocytes, and neutrophils. (b) FACS plots showing expression of CLEC5A on mouse BMDMs and peritoneal macrophages. (c and d) Effect of human and mouse macrophage polarization states on CLEC5A expression. (A) Three panels of FACS histogram of CLEC5A expression on human macrophages (left panel, high signal), monocytes (central panel, moderate signal), and neutrophils (right panel, minimal signal). (B) Two panels of FACS histogram of CLEC5A expression on murine BMDMs (left panel), and murine peritoneal macrophages (right panel). (c). Bar graph with individual bars representing mean fluorescence intensity (MFI) of anti-CLEC5A staining on human macrophages with various treatments. (d). Bar graph with individual bars representing mean fluorescence intensity (MFI) of anti-CLEC5A staining on murine BMDMs with various treatments.
CLEC5A-targeting bispecific agonistic antibodies
We envisioned that a CLEC5A antibody with agonistic potential could be used for further engineering to trigger CLEC5A-dependent phagocytosis. To identify such agonist antibodies, we screened a collection of monoclonal antibodies (mAbs) from a hamster immunization campaign for their ability to stimulate primary human macrophages. Because tumor necrosis factor (TNF) is a known target downstream of CLEC5A signaling,Citation14 its secretion by macrophages incubated with the different anti-CLEC5A antibodies was used as the primary readout for agonist activity. Using this assay, we identified an agonist antibody, 1F7. We found that an effectorless 1F7, produced as a murine IgG2a-carrying mutations D265A and N297A (DANA) in the Fc region to disrupt binding to FcγRs,Citation15,Citation16 could still induce high levels of TNF. In contrast, an effectorless (mIgG2a, LALAPG) agonistic antibody against the macrophage phagocytic receptor MerTK, or a nonmacrophage binding anti-CD20 IgG1, failed to do so (). Having identified an anti-CLEC5A agonistic antibody, we decided to make a bispecific antibody by combining with an antibody against a tumor marker. Rituximab, a CD20-targeting mAb approved for the treatment of B-cell malignancies, relies on multiple mechanisms of actions, including antibody-dependent cellular phagocytosis (ADCP).Citation17 Thus, it provided a good system for comparing phagocytosis mediated by CLEC5A or FcγRs.
Figure 3. Identification and characterization of CLEC5A agonist antibodies CLEC5A agonist antibody induced TNF production from CLEC5A + human primary macrophages (Mean ± S.D of triplicates from one donor). (b) Schematic depiction of B cell-dependent engagement of CLEC5A via CD20/CLEC5A bispecific antibody. (c) Schematic depiction of different CLEC5A bispecific antibodies. Bispecific antibodies carry LALAPG mutation in their Fc while CD20 hIgG1 has a wild-type Fc. (d) Binding Kd values of CLEC5A bispecific antibodies for human and mouse CLEC5A were determined by Biacore. (e) Activation of CLEC5A signaling (TNF production) in human macrophages by the bispecific antibody in the presence of target Raji cells (Mean ± S.D of triplicates from one donor).
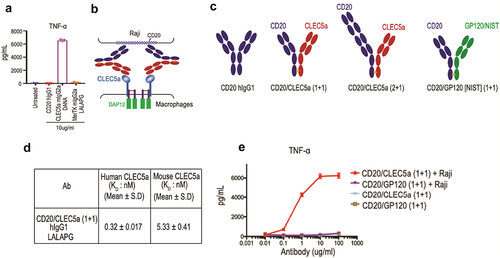
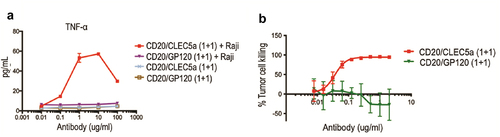
To establish a proof-of-principle of exploiting CLEC5A agonism for targeted phagocytosis, we made bispecific antibodies targeting CLEC5A and CD20 using the knob-hole technologyCitation18,Citation19 ( and 3C). To abrogate phagocytosis mediated by FcγRs, the bispecific antibodies were produced as human IgG1s with the LALAPG mutations (L234A, L235A and P329G) known to minimize binding to FcγRs.Citation20–22 We characterized the specific binding of CD20/CLEC5A bispecific antibodies to CLEC5A using recombinant human and mouse CLEC5A proteins by surface plasmon resonance (SPR), obtaining Kd values of single-digit nM or lower for both species (). While the CD20/CLEC5A (1 + 1) bispecific antibody has a configuration of monovalent binding to CLEC5A, we anticipated that clustering of the bispecific antibodies by CD20-expressing cells could activate CLEC5A expressed by macrophages (). Indeed, when we co-cultured macrophages and Raji cells, we observed robust production of TNF, which was not observed in the absence of Raji cells (). These results suggested that the monovalent CLEC5A arm in the bispecific antibody alone cannot trigger CLEC5A activation, but once clustered by binding to the target cell surface, it became an effective CLEC5A agonist. Similar results were obtained when we measured other cytokines/chemokines in a Luminex panel that includes IL-6, IL-1β, IL-8, RANTES, MIP-1α, MIP-1β and Il-10 (Supplemental Figure 2).
CLEC5A-targeting Bispecific Antibodies Mediate FcγRs-independent Cellular Phagocytosis of B cells by Human Macrophages in vitro
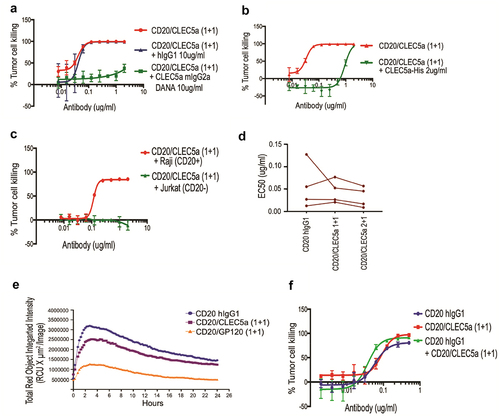
We then assessed the activity of the bispecific antibodies in a phagocytosis assay using human macrophages as phagocytes and CD20-expressing Raji cells as targets in a FACS-based assay with a gating strategy shown in Supplemental Figure 3A. We found that the CD20/CLEC5A bispecific antibody was able to induce macrophage phagocytosis of Raji cells in a dose-dependent manner (). This effect was CLEC5A-dependent since it was blocked by an excessive amount of a monospecific CLEC5A-blocking antibody, but not by an irrelevant IgG1 (). Moreover, soluble recombinant CLEC5A protein was also able to inhibit the pro-phagocytosis effect of the bispecific antibody (). As expected, CD20/CLEC5A bispecific antibodies failed to induce phagocytosis of CD20-negative Jurkat cells by macrophages ( and S3B). Next, we compared the phagocytic activity mediated by CLEC5A with that of FcγRs. For phagocytosis through FcγR, we used anti-CD20 hIgG1 carrying a wild-type Fc. First, we compared the potency of the phagocytic activity mediated by CLEC5A or FcγR. Because the CD20/CLEC5A (1 + 1) bispecific antibody is monovalent for CD20 and differs from anti-CD20 IgG1 not only in the mechanism mediating phagocytosis but also in the valency for CD20, we decided to make a CD20/CLEC5A bispecific antibody bivalent for CD20, CD20/CLEC5A (2 + 1) (). Comparing the EC50 of phagocytosis of Raji cells by primary macrophages derived from monocytes of four different human donors, we found that all three molecules exhibited comparable activity (). The maximum extent of tumor cell phagocytosis was also comparable between the two mechanisms ().
Figure 4. Characterizing activity and potency of CLEC5A-dependent bispecific antibodies (a and b) Raji cell killing by human macrophages in the presence of indicated bispecific antibody and blocking agents (Mean ± S.D of triplicates from one donor, Effector: Target ratio is 5:1). (c) CD20/CLEC5A-dependent human macrophage-mediated cell-killing is specific to Raji (CD20+) but not to Jurkat (CD20−) (Mean ± S.D of triplicates from one donor, Effector: Target ratio is 5:1). (d) EC50 of macrophage-mediated tumor (Raji) cell-killing in the presence of indicated antibodies. Data were derived from 4 independent human donors. Effector: Target ratio is 5:1. (e) IncuCyte phagocytosis assay demonstrates the uptake of pHrodo-labeled Raji cells by the human macrophages in the presence of indicated antibodies. Antibody concentration, 2ug/ml. Effector: Target ratio is 1:5. (f) Testing the dual action of anti-CD20 and CLEC5A bispecific antibody in human macrophage-mediated Raji cell killing (Mean ± S.D of triplicates from one donor, Effector: Target ratio is 5:1).
While phagocytosis mediated by FcγR and CLEC5A seemed to have comparable potencies and efficiencies, it was possible that one mechanism had faster kinetics than the other. To investigate this possibility, we performed a time-course IncuCyte phagocytosis assay over a 24-hour period. Raji cells prelabeled with a pH-sensitive dye (pHrodo) were incubated with human macrophages in the presence of anti-CD20/CLEC5A (1 + 1), anti-CD20/GP120 (1 + 1) as a control antibody or anti-CD20 IgG1, and images were taken at different time points (). Image analysis showed very few intracellular pHrodo-positive macrophages in the absence of anti-CD20/CLEC5A (Figure S4A). In contrast, in the presence of anti-CD20/CLEC5A, a high proportion of macrophages were intracellular pHrodo-positive (Figure S4B-E). The time course analysis indicated that CD20/CLEC5A bispecific antibody and FcγR-dependent CD20 monospecific antibody exhibited similar kinetics ().
Cellular phagocytosis driven by dual action of CLEC5A and FcγRs
To explore whether phagocytosis is mediated by FcγR and CLEC5A with two independent mechanisms that can be combined for an additive effect, we compared the phagocytic activity of CLEC5A/CD20 (1 + 1), anti-CD20 hIgG1, and a combination of both. We observed a moderate increase in activity in the combination treatment (). It is important to note that the bispecific antibody and the anti-CD20 IgG1 competed for binding to overlapping epitopes on CD20,Citation23 and a more pronounced effect could be observed for noncompeting antibodies.
CLEC5A-targeting bispecific antibody for cellular phagocytosis of epithelial tumor cells
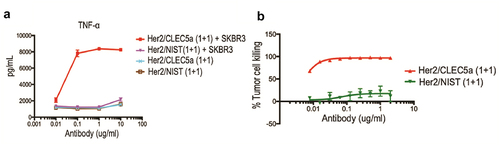
To determine whether our approach is applicable to epithelial cancer cells, we developed CLEC5A bispecific antibodies against HER2 receptor, a well-characterized protein overexpressed in breast and gastric tumors. Indeed, the HER2/CLEC5A (1 + 1) bispecific antibody robustly induced target-dependent TNF secretion () and phagocytosis of HER2+ SK-BR-3 cells () by human macrophages. Overall, these findings support that CLEC5A could be broadly harnessed for targeted phagocytosis.
Figure 5. Demonstration of CLEC5A-dependent phagocytosis of solid tumor cells Activation of CLEC5A (TNF production) by the bispecific antibody in the presence of target SK-BR-3 cells (Mean ± S.D of triplicates from one donor). (b) Macrophage-mediated tumor (SK-BR-3) cell killing in the presence of indicated bispecific antibody (Mean ± S.D of triplicates from one donor). Effector: Target ratio for phagocytosis and cytokine assay is 5:1.
Treg depletion and tumor growth inhibition by CLEC5A-targeting bispecific antibody
Our CLEC5A bispecific antibodies bind both human and mouse CLEC5A () although with slightly lower affinity for the murine CLEC5A. To determine if they also exhibit cross-species agonistic activity, we used mouse BMDMs and tested the ability of CD20/CLEC5A (1 + 1) bispecific antibody to induce TNF release when co-incubated with Raji cells. Indeed, CD20/CLEC5A (1 + 1) antibody was able to trigger TNF production in a dose-dependent manner () although at a lower level than those previously observed with human macrophages. The TNF production was both CLEC5A- and target cell-dependent (). Similar to what was observed with human macrophages, the CLEC5A/CD20 bispecific antibody led to robust phagocytosis of target cells in a dose-dependent manner (). These studies paved the way for further in vivo evaluation in mouse models.
Figure 6. Characterization of cross-species agonistic activity of CLEC5A bispecific antibody Activation of CLEC5A (TNF production) in mouse BMDMs by the bispecific antibody in the presence of target Raji cells (Mean ± S.D of triplicates). (b) Mouse BMDM-mediated tumor (Raji) cell-killing in the presence of indicated bispecific antibodies (Mean ± S.D of triplicates). Effector: Target ratio for phagocytosis and cytokine assays is 5:1.
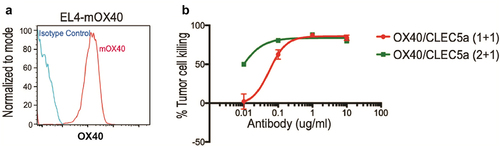
Having characterized the efficiency of CLEC5A-engaging bispecific antibodies in vitro, we next explored their activity in vivo. Targeting regulatory T cells (Tregs) in the tumor microenvironment (TME) has been explored to enhance antitumor immunity. For instance, depletion of Treg cells through activation of FcγRs leads to robust antitumor efficacy in preclinical models.Citation24,Citation25 We decided to explore Treg depletion using the CLEC5A bispecific antibody approach. OX40, a trimeric co-stimulatory receptor belonging to the TNFR superfamily, exhibits higher expression in FoxP3+ Tregs compared to FoxP3− CD4+ and CD8+ T cells.Citation25 We generated OX40 (clone OX86)/CLEC5A (1 + 1) and (2 + 1) bispecific mouse IgG2a antibodies with LALAPG mutations to abolish binding to FcγRs. Monospecific bivalent anti-OX40 (clone OX86) antibody (mIgG2a) was used as a positive control of ADCP in this study. First, we examined the activity of both OX40/CLEC5A (1 + 1) and (2 + 1) bispecific antibodies in an in vitro phagocytosis assay using mOX40-overexpressing EL4 murine lymphoma cells as the target cells (). Both antibodies showed dose-dependent activity, with the (2 + 1) format being more potent ().
Figure 7. Characterization of the OX40/CLEC5A bispecific antibodies FACS plots depicting stable expression of mOX40 on EL4 cells (left panel). (b) Human macrophage-mediated EL4-mOX40 cell-killing in the presence of indicated bispecific antibody (Mean ± S.D of triplicates from one donor, Effector: Target ratio is 5:1).
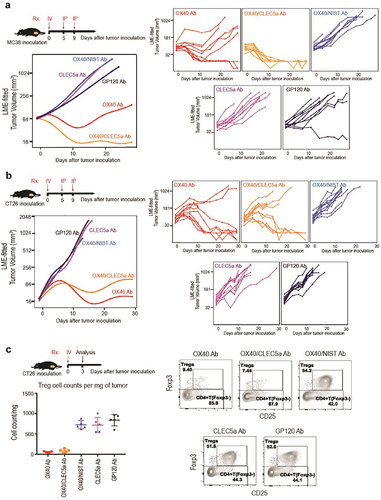
We next decided to investigate OX40/CLEC5A in vivo using syngeneic mouse tumor models. MC38 and CT26 are two syngeneic mouse tumor models widely used for preclinical mechanistic studies and have been previously used to study the effect of anti-OX40 mAb clone OX86.Citation25,Citation26 We found that in both models, the OX40/CLEC5A bispecific antibody exhibited robust antitumor activity ( a nd B). To determine that the antitumor efficacy by the OX40/CLEC5A bispecific was due to depletion of Tregs, we quantified CD4+ FoxP3+ CD25+ Treg cells in CT26 tumors 3 days after a single intravenous dosing. Indeed, treatment with the OX40/CLEC5A bispecific antibody resulted in significant depletion of Treg cells, comparable to that caused by the Ox86 mAb ( and S5). Together, these data showed that the bispecific OX40/CLEC5A exhibited the antitumor activity in vivo by the expected mechanism of action, making CLEC5A an attractive target for further exploring therapeutic applications.
Figure 8. Depletion of Tumor-infiltrating regulatory T cells by the OX40/CLEC5A bispecific antibody results in antitumor efficacy (a and b) Growth of MC38 and CT26 tumors in mice treated with the indicated antibodies (n = 10 mice/group). Treatment started 9 days after tumor cell inoculation. (c) Quantification of CD4+ FoXP3+ CD25+ tumor Treg cells after treatment with indicated antibodies (Left panel). Representative FACS plots showing the percentage of Treg cells within total CD4+ T cells in tumors with different treatments (Right panel). Mice were treated with indicated antibodies (n = 5 mice/group) on day 9 after CT26 tumor inoculation, and tumors were harvested on day 3 after antibody treatment. Antibodies used: OX40 mIgG2a (OX40 Ab), OX40/CLEC5A mIgG2a LALAPG (OX40/CLEC5A Ab), OX40/NIST mIgG2a LALAPG (OX40/NIST Ab), CLEC5A mIgG2a DANA (CLEC5A Ab), and GP120 mIgG2a LALAPG (GP120 Ab).
Discussion
While CLEC5A shows high homology to TREM2, another lectin expressed on myeloid cells with a well-known role in phagocytosis, to our knowledge, a direct observation of a similar function for CLE5A, was not previously made. Here, we describe, for the first time, the use of agonistic antibodies to exploit a CLEC5A-mediated phagocytosis.
A key requirement for the potential therapeutic use of CLEC5A-mediated phagocytosis is that tumor-associated macrophages (TAMs) express CLEC5A and respond to CLEC5A-engaging antibodies. As it has been widely reported that macrophages in different states of polarization express different sets of genes,Citation27 we first characterized whether CLEC5A expression was specific for a specific type of polarization. We observed that, although with minor variations in mean fluorescence intensity, CLEC5A was expressed in macrophages polarized with different stimuli. This observation opened the possibility that the mechanism could be functional across different sets of macrophage subpopulations. Macrophage polarization is a continuum between fully M1 and M2 sites, with multiple factors present in the TME being capable of influencing the polarization state. As a result, macrophages present in the TME are usually a mixed population in different polarization states.Citation28 In order to analyze macrophages in the context of TME, TAMs from two different tumor models, MC38 and CT26, were characterized and confirmed for their expression of CLEC5A, consistent with data from patients with ovarian cancer,Citation29 osteosarcoma,Citation30 and melanoma.Citation31
Human macrophages express three activating Fcγ receptors (FcγR), FcγRI, FcγRIIA and FcγRIIIA, which contain ITAM, and one inhibitory FcγRIIB with an immunoreceptor tyrosine-based inhibitory motif (ITIM).Citation15,Citation32 Antibody-opsonized targets, such as cancer and virus-infected cells, cross-link activating FcγRs, which results in phosphorylation of tyrosine residues in ITAMs and recruitment of spleen tyrosine kinase (SYK) to activate downstream signaling events that result in phagocytosis and pro-inflammatory cytokine release. The overactivation of phagocytosis by ITAM-containing receptors is prevented by the function of inhibitory FcγRIIB. Concurrent activation of FcγRIIB results in ITIM phosphorylation and recruitment of SHIP (SH2- domain-containing inositol polyphosphate 5′ phosphatase) and SHP1 (SH2-domain-containing protein tyrosine phosphatase 1), which dampens activating signaling pathways.Citation32 One potential application of CLEC5A-targeting bispecific antibodies is promoting the phagocytosis in a way less dependent on the expression of inhibitory FcγRs. To this end, we compared antibodies with the same target arm and valency, but relying on either CLEC5A or FcγR for triggering phagocytosis. Our results showed comparable potency and kinetics. Thus, the potential advantage of using CLEC5A-mediated phagocytosis to avoid the negative effect of FcγRIIB expression can be achieved without the expense of potency.
A role of CLEC5A in proinflammatory responses has been observed in a variety of settings, during infections with CLEC5A-binding virus such as influenza,Citation14 dengueCitation7 and Japanese encephalitis virus,Citation33 in chronic obstructive pulmonary diseaseCitation8 and in inflammatory colon diseases.Citation31 Interestingly, an activating mutation in CLEC5A was associated with Crohn’s disease.Citation34 In one study, the proinflammatory responses mediated by CLEC5A led to an increase of immune cell infiltration in the lungs of mice infected with influenza virus.Citation14 Similarly, a CLEC5A-blocking antibody prevented the infiltration of immune cells in the brains of mice infected with Japanese encephalitis virus.Citation33 In this study, we show that agonism of CLEC5A using a bispecific antibody also resulted in the secretion of proinflammatory cytokines. In addition to the direct cancer cell elimination by phagocytosis, the concurrent release of proinflammatory chemokines and cytokines could have additive antitumor effects by recruiting other immune cells. It is known that FcγRIIB, in addition to negatively regulating phagocytosis, also dampens secretion of proinflammatory cytokines.Citation35 On the other hand, activation mediated by DAP10/DAP12 is not as sensitive to ITIM-inhibition as those initiated by ITAM-containing receptors, at least in the context of NKG2D.Citation36,Citation37 Thus, a CLEC5A-based bispecific antibody could lead to a more robust antitumor response.
The CLEC5A-dependent and FcγRs-dependent mechanisms need not be mutually exclusive for targeted phagocytosis. We investigated whether a monospecific anti-CD20 mAb with a wild-type Fc could be combined with an anti-CD20/CLEC5A bispecific antibody to get an additive effect. Although we used anti-CD20 mAbs with competing epitopes,Citation23 we did observe a modest additive effect, opening the possibility that a more robust effect could be obtained for noncompeting antibodies. Alternatively, the two mechanisms could be integrated into a single molecule in an anti-CD20/CLEC5A antibody with a wild-type Fc. However, a concern for this application is the potential bridging of two different immune cells. While in a scenario of a balanced expression of CLEC5A and FcγRs on macrophages, the molecule would likely bind to both receptors in cis, an imbalanced expression of both receptors could open the possibility of the binding of both CLEC5A and FcγRs in trans with the potential unwanted redirection of immune cells against each other.
To assess CLEC5A-engaging bispecific antibody in vivo, we treated mice carrying either MC38 or CT26 syngeneic tumors with anti-OX40/CLEC5A. As a comparator, we used an anti-OX40 monospecific antibody with a wild-type Fc. While the treatment with both monospecific and bispecific antibodies in the CT26 model led to robust tumor growth inhibition and response curves with comparable trends, treatment of MC38 tumors with the bispecific mAb led to a more pronounced antitumor effect than with the anti-OX40 monospecific antibody. While OX86 relies on engaging activating Fcγ receptorsCitation25 present on macrophages and natural killer cells (NK), CLEC5A bispecific antibodies engage macrophages and potentially neutrophils. The content of NK cells, for example, is less than 1% of CD45+ cells in MC38 tumors, but 15–20% of CD45+ cells in CT26 tumors.Citation38 It is possible that the lower NK-cell content in MC38, among other factors, could lead to the less robust antitumor effect of OX86 IgG.
TAMs are among the most abundant immune cells in the TME, making them an attractive therapeutic target for cancer therapy. Our current study demonstrated that targeting CLEC5A on TAMs could be a new weapon in our arsenal against cancer.
Materials and methods
CLEC5A antibodies
Hamsters were immunized with murine CLEC5A, and hybridoma clones were obtained from the immunized animals by standard procedures. Cell clones that produced IgGs reactive with murine and human CLEC5A by enzyme-linked immunosorbent assay (ELISA) and FACS were selected for subsequent screening for agonistic properties using TNF secretion from primary human macrophages as a readout. Clone 1F7 showed the strongest agonist activity and was selected for molecular cloning and reformatting into a half antibody.
Bispecific antibodies were expressed in either hIgG1 or mIgG2a framework carrying LALAPG mutations to abrogate effector functions.Citation20–22 Bispecific antibodies were produced in knobs-into-holes format as previously described.Citation19 Briefly, half antibodies were expressed in separate Chinese hamster ovary cell cultures, purified by standard ProteinA affinity capture and annealed in vitro using redox conditions that allowed the formation of hinge disulfides.Citation39,Citation40 After in vitro annealing, undesired side products were removed by ion exchange chromatography using a pH gradient. Characterization of the purified antibodies by analytical size exclusion chromatography, mass spectrometry and LAL assays indicated the absence (<0.5%) of aggregates, half antibodies (<1%), homodimers (<2%) or endotoxins (< 0,5EU/mg). The CD20 arm is clone 2H7,Citation18 HER2 arm is clone 4D5,Citation41 whereas the OX40 arm is clone OX86.Citation25 In the in vivo experiments, an anti-GP120 mAb was used as a control for the OX86 mAb, whereas and anti-OX40/NIST antibody was used as a control for the bispecific mAb. NIST is a humanized IgG1ĸ specific to the respiratory syncytial virus protein F (RSVF)Citation42 and often used as a nonbinding control arm.
Affinity determination
To measure the binding affinity of CLEC5A agonistic antibody, a SPR BIAcore™-T200 instrument was used. Series S sensor chip Protein A (GE Healthcare) was applied to capture each antibody on different flow cell (FC) to achieve approximately 200 response units (RU), followed by injection of five-fold serial dilutions of human (R&D systems, Catalog 8544-CL) or mouse (R&D systems, Catalog 8438-CL) CLEC5A (0.8 nM to 500 nM) in HBS-EP buffer (100 mM HEPES pH7.4, 150 mM NaCl, 3 mM EDTA, 0.05% (v/v) Surfactant P20) with a flow rate of 100 ul/min at 25°C. Association rates (kon) and dissociation rates (koff) were calculated using a simple one-to-one Langmuir binding model (BIAcore T200 evaluation software version 2.0). The equilibrium dissociation constant (KD) was calculated as the ratio koff/kon.
Preparation of human primary macrophages
Human peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated from buffy coat using a Ficoll gradient centrifugation. CD14+ monocytes were purified from PBMCs by negative selection using a CD14 isolation kit without depletion of CD16+ cells (StemCell Technologies, Catalog 19058). About 20X10^6 cells were differentiated in 15 cm2 plates to M0 for 7 days with 50 ng/ml macrophage colony stimulating factor (M-CSF, StemCell Technologies, Catalog 78057) in RPMI media containing penicillin/streptomycin, 10% heat inactivated fetal bovine serum (FBS), 2 mM glutamine (RPMI complete media). On day 6, M0 macrophages were either left unpolarized or polarized with 25 ng/mL of IL-10 (StemCell Technologies, Catalog 78024), or 25 ng/mL of IL-4 (StemCell Technologies, Catalog 78045), or 50 ng/mL of IFN-γ (StemCell Technologies, Catalog 78020) for another 24 hours (day 7).
Preparation of human primary neutrophils
Human primary neutrophils were isolated using a human neutrophil isolation kit according to the manufacturer’s instructions (StemCell Technologies, Catalog 17957). Cell purity was determined by neutrophil marker CD66b-FITC (Biolegend, Catalog 305103).
Isolation of mouse primary macrophages
For BMDM differentiations, femurs from C57BL/6 J mice were dissected and sterilized. Bone marrow was flushed with the syringe filled with RPMI complete media. The resulting marrow suspension was filtered through 100 μM nylon filter and treated with ACK lysis buffer to lyse red blood cells. Cells were washed twice with RPMI media and differentiated with 50 ng/ml M-CSF (StemCell Technologies, Catalog 78059) for 7 days in RPMI complete media. On day 6, M0 macrophages were either left unpolarized or polarized with 25 ng/mL of IL-10 (StemCell Technologies, Catalog 78079.1), or 25 ng/mL of IL-4 (StemCell Technologies, Catalog 78047), or 50 ng/mL of IFN-γ (StemCell Technologies, Catalog 78021) for another 24 hours (day 7).
For peritoneal macrophages, cells were harvested from the mouse peritoneal cavity in 10 ml of phosphate-buffered saline (PBS) with 4% HI-FBS and plated on culture dishes in RP10 medium (RPMI supplemented with 10% heat-inactivated fetal bovine serum, 10 mM HEPES, 2 mM L-Glutamine, 10 mM MEM nonessential amino acid solution, 55 μM β-mercaptoethanol, 100 units/ml penicillin and 100 μg/ml streptomycin). After incubation at 37°C for 2 hours, nonadherent cells were removed, and the adherent macrophages were cultured overnight prior to assays.
All mice were either generated at Genentech or obtained from Charles River Laboratories or Jackson Laboratory and maintained in accordance with American Association of Laboratory Animal Care (AALAC) guidelines. The experiments were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Genentech Institutional Animal Care and Use Committee.
Twenty-four hours phagocytosis assay with human/mouse macrophages
Human primary macrophages differentiated with M-CSF (M0, Day7) were harvested with cell dissociation buffer (Gibco, Catalog 13151–014). Dead/apoptotic cells from Raji cell culture were depleted using a dead cell removal kit (Miltenyi Biotec, Catalog 130–090-101). Cells with viability more than 95% (as determined by Vi-Cell) were labeled with 0.5 μM carboxyfluorescein succinimidyl ester (CFSE), using a CFSE Cell Proliferation Kit (Life Technologies, Catalog C34554). About 20,000 labeled target cells and 100,000 human macrophages (1:5) were added into a 96-well round bottom (ultra-low attachment plate, corning catalog 4520) in complete RPMI media in the presence of serially diluted indicated antibodies for 24 hours (final volume 200 ul). After incubation, 50 ul of counting beads were added (ThermoFisher, Catalog C36950). The absolute number of Raji cells in each well was quantified by counting a fixed number of counting beads.
Percentage of tumor cells killed is calculated as follows:
{(Number of CFSE-positive target cells in no mAb control) – (Number of CFSE-positive target cells in the presence of mAb)/(Number of CFSE-positive target cells in no mAb control)} *100.
Live-cell imaging phagocytosis assay with human macrophages
Human primary macrophages differentiated with M-CSF (M0, Day7) were harvested with cell dissociation buffer (Gibco, Catalog 13151–014). About 15,000 macrophages were seeded into a 96-well flat-bottom plate 24 hours prior to the assay in the presence of M-CSF. Dead/apoptotic cells from Raji cell culture were depleted using a dead cell removal kit (Miltenyi Biotec, Catalog 130–090-101). Cells with viability more than 95% (as determined by Vi-Cell) were labeled with pHrodo (ThermoFisher, Catalog P36600). For preparing pHrodo stock solution, pHrodo was reconstituted in 154 ul of dimethyl sulfoxide (DMSO). Furthermore, it was diluted at 1:25 in DMSO to prepare a working solution. Raji cells at a 1 106/mL concentration in PBS were prepared and were treated with 1:1000 of pHrodo working solution. Cells were mixed gently and kept on rocker in the dark for 30 mins. To quench the excess dye, cells were pelleted and resuspended in RPMI complete media for 5 mins. Finally, labeled cells were resuspended at a 1.5 × 106/ml concentration and 50 ul (75 K cells) was used per reaction with a final volume of 200 ul.
Incucyte acquisition settings:
10× objective. Standard Scan, 1 image/well. Every 15 mins for 24-hours. “RED” and “PHASE” images were acquired (800 ms acquisition time).
Cytokine release assays
For activation of human primary macrophages with soluble anti-CLEC5A, indicated antibodies at 10 ug/ml in 300 ul of RPMI complete media were added to 2 × 105 human primary macrophages in a 24-well plate (differentiated in M-CSF for 7 days and polarized with 50 ng/ml of IFN-γ). After 24 hours, supernatant was collected to quantify TNF.
For activation of human primary macrophages/mouse BMDM with bispecific antibodies and target cells, about 20,000 target cells and 100,000 human macrophages/BMDM (differentiated in M-CSF for 7 days and polarized with 50 ng/ml of IFN-γ) (1:5) were combined into a 96-well round bottom plate (Corning, Catalog 4520) in complete RPMI media in the presence of serially diluted antibodies for 24 hours (final volume 200ul). After incubation, cytokines were analyzed by Luminex analysis (Millipore). Additionally, TNF levels were measured with human (Abcam, Catalog ab181421) and mouse (R&D systems, Catalog MTA00B) ELISA kits.
Tumor studies
Female C57BL/6 J and BALB/C mice were inoculated subcutaneously on the lower right flank with 0.1 million MC38 and CT26 cells in PBS/Matrigel (1:1, v/v). Animals were grouped based on weight and tumor volume to ensure similar weight and starting tumor volume distribution before treatment. Antibodies were administered via intravenous (IV) tail injection for the first dose and subsequently two more doses of intraperitoneal (IP) injection at a dose of 1 mg/kg. Isotype-matched anti-GP120 antibody was used as control antibody. Tumors were monitored twice a week. Mice were humanely euthanized if ulceration occurred or tumor volume reached 2,000 mm3. Tumor volume was calculated as (length x widthCitation2)/2. A linear mixed effects (LME) modeling was performed to analyze repeated measurements of tumor volume over time from the same animals to properly account for both latitudinal variability (differences in tumor volumes at each study day) and longitudinal variability (differences in how individual tumors change over the course of the study).Citation17 LME modeling also accounts for missing values from study dropouts due to ulceration, or tumor volume exceeds 2,000 mm3. Tumors tend to exhibit exponential growth for the most part, and therefore, tumor volumes were subjected to natural log transformation before being analyzed. Changes in ln(tumor volumes) over time for all groups are described by fits (regression splines with auto-generated spline bases). Both individual tumor growth curves and LME-fitted tumor growth curves of each group are presented for each tumor growth efficacy study.
Tumor digestion and flow cytometry
Tumors were dissected and dissociated using a mouse Tumor Dissociation Kit (Miltenyi Biotec, Catalog 130–096-730) to obtain single cell suspension following the manufacturer’s protocol. After lysis of red blood cells using ACK lysing buffer (Lonza), cells were filtered through a 70-μm cell strainer. Cells were blocked with mouse Fc Block (Miltenyi Biotec, Catalog 130–092-575). For characterizing expression of CLEC5A, cells were then stained with fluorochrome-conjugated antibodies: anti-CLEC5A antibody (R&D systems, Catalog FAB1639P), isotype antibody (R&D systems, Catalog IC00P), anti-CD11b antibody (eBioscience, Catalog 45–0112), anti-CD45 antibody (BD Bioscience, Catalog 564279), anti-CD11c antibody (BD Bioscience, Catalog 558079), anti-Ly6G antibody (BD Bioscience, Catalog 565707), anti-MHC Class II antibody (BD Bioscience, Catalog 562366), anti-CD24 antibody (BD Bioscience, Catalog 565308), anti-Ly6C antibody (BioLegend, Catalog 128036), anti-CD64 antibody (BioLegend, Catalog 139309), anti-F4/80 antibody (BioLegend, Catalog 123108), and live/dead dye (eBioscience, Catalog 65–0865-14).
To examine Treg depletion, cells were surface stained with live/dead dye (ThermoFisher scientific, catalog L10119), anti-CD25 antibody (ThermoFisher scientific, catalog 53–0251-82), anti-CD4 antibody (BioLegend, catalog 100528), anti-CD44 antibody (ThermoFisher scientific, catalog 17–0441-83), anti-CD8b antibody (BD Biosciences, catalog 740387), anti-CD90.2 antibody (BioLegend, catalog 105331), and anti-CD45 antibody (BD Biosciences, catalog 564279). Cells were fixed and intracellularly stained with anti-Foxp3 antibody (ThermoFisher scientific, catalog 45–5773-82) using the Foxp3 transcription factor staining buffer set (ThermoFisher scientific, catalog 00–5523-00) following manufacturer’s protocol.
Multicolor analysis was performed on a BD FACSymphony analyzer, and data were analyzed using Flowjo software (FlowJo LLC). Gating strategies for analysis of immune cell populations have been described previously.Citation18,Citation19
Abbreviations
ADCP Antibody-Dependent Cellular Phagocytosis
BMDM Bone Marrow-Derived Macrophages
CFSE Carboxyfluorescein Succinimidyl Ester
CLEC5A C-type Lectin Domain Containing 5A
DAP DNAX-Activating Protein
DMSO Dimethyl Sulfoxide
ELISA Enzyme-Linked Immunosorbent Assay
FACS Fluorescence-Activated Cell Sorting
FBS Fetal Bovine Serum
FcγR Fcγ receptor
FITC Fluorescein isothiocyanate
IgG Immunoglobulin G
IP Intraperitoneal
IV Intravenous
ITAM Immunoreceptor Tyrosine-based Activation Motif
ITIM Immunoreceptor Tyrosine-based Inhibitory Motif
LME Linear Mixed Effects
mAb monoclonal antibody
M-CSF: Macrophage Colony Stimulating Factor
MERTK MER proto-oncogene Tyrosine Kinase
MFI: Mean Fluorescence Intensity
NK Natural Killer
PBMC Peripheral Blood Mononuclear Cell
PBS Phosphate-buffered Saline
SHIP SH2-domain-containing inositol polyphosphate 5′ phosphatase
SHP1 SH2-domain-containing protein tyrosine phosphatase 1
SPR surface Plasmon Resonance
SYK Spleen Tyrosine Kinase
TAM Tumor Associated Macrophage
TME: Tumor Microenvironment
TNF Tumor Necrosis Factor
TREM2 Triggering Receptor Expressed on Myeloid Cells 2
Treg Regulatory T cell
YINM Tyrosine-containing Motif
Authors Contributions
VK and MY designed the research and wrote the paper, with input from all authors. VK performed all in vitro experiments and tumor studies and analyzed the data. DE designed and purified bispecific antibodies and wrote the paper. MF designed and performed Treg depletion assay. GZ assisted in tumor studies and Treg depletion assay. WCL performed BIAcore experiments. EC and JZ carried out IV antibody dosing. HH and WL supervised IV antibody dosing. YC designed bispecific antibody constructs. YW supervised antibody generation.
Supplemental Material
Download MS Word (12.5 MB)Acknowledgments
We thank Weilan Ye for comments and ushering the publication of this manuscript, Andres Martinez for measuring cytokine levels by Luminex and Genentech FACS core for technical assistance an Stacey Yeung for assistance with Incucyte data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Weiskopf K, and Weissman IL. Macrophages are critical effectors of antibody therapies for cancer. mAbs. 2015;7;(2):303–10.
- Overdijk MB, Verploegen S, Bögels M, van Egmond M, Lammerts Van Bueren JJ, Mutis T, Groen RWJ, Breij E, Martens ACM, Bleeker WK, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. mAbs. 2015;7(2):311–12. doi:10.1080/19420862.2015.1007813.
- Shi Y, Fan X, Deng H, Brezski RJ, Rycyzyn M, Jordan RE, Strohl WR, Zou Q, Zhang N, An Z. Trastuzumab triggers phagocytic killing of high her2 cancer cells in vitro and in vivo by interaction with Fcγ receptors on macrophages. J Immunol. 2015;194(9):4379–86. doi:10.4049/jimmunol.1402891.
- Grandjean CL, Garcia Z, Lemaître F, Bréart B, Bousso P. Imaging the mechanisms of anti-CD20 therapy in vivo uncovers spatiotemporal bottlenecks in antibody-dependent phagocytosis. Science Advances. 2021;7(8). doi:10.1126/sciadv.abd6167.
- Uribe-Querol E, Rosales C. Phagocytosis: our current understanding of a universal biological process. Front Immunol. 2020;11. doi:10.3389/fimmu.2020.01066.
- Kedage V, Ellerman D, Chen Y, Liang WC, Borneo J, Wu Y, and Yan M. Harnessing MerTK agonism for targeted therapeutics. mAbs. Jan-Dec 2020;12(1):1685832.
- Chen ST, Lin YL, Huang MT, Wu MF, Cheng SC, Lei HY, Lee CK, Chiou TW, Wong CH, and Hsieh SL. CLEC5A is critical for dengue-virus-induced lethal disease. Nature. 2008 May 29;453(7195):672–6.
- Wortham BW, Eppert BL, Flury JL, Garcia SM, Donica WR, Osterburg A, Joyce-Shaikh B, Cua DJ, Borchers MT. Cutting edge: CLEC5A mediates macrophage function and chronic obstructive pulmonary disease pathologies. J Immunol. 2016;196(8):3227–31. doi:10.4049/jimmunol.1500978.
- Bakker ABH, Baker E, Sutherland GR, Phillips JH, and Lanier LL. Myeloid DAP12-associating lectin (MDL)-1 is a cell surface receptor involved in the activation of myeloid cells. Proc Natl Acad Sci U S A 1999 Aug 17; 96(17):9792–6.
- Inui M, Kikuchi Y, Aoki N, Endo S, Maeda T, Sugahara-Tobinai A, Fujimura S, Nakamura A, Kumanogoh A, and Colonna M, et al. Signal adaptor DAP10 associates with MDL-1 and triggers osteoclastogenesis in cooperation with DAP12. Proc Natl Acad Sci U S A 2009 Mar 24; 106(12):4816–21.
- Takahashi K, Rochford CDP, and Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005 Feb 21;201(4): 647–657.
- Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177(10):7303–11. doi:10.4049/jimmunol.177.10.7303.
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, and Lawrence T, et al. Macrophage activation and polarization. Nomenclature and Experimental Guidelines Immunity. 2014 Jul 17; 41(1):14–20.
- Teng O, Chen S-T, Hsu T-L, Sia SF, Cole S, Valkenburg SA, Hsu T-Y, Zheng JT, Tu W, and Bruzzone R, et al. CLEC5A-mediated enhancement of the inflammatory response in myeloid cells contributes to influenza virus pathogenicity in vivo. J Virol. 2017Jan 1; 91(1): e01813–16.
- Clynes RA, Towers TL, Presta LG, V RJ, Towers TL, Presta LG, V RJ, Presta LG, V RJ, V RJ, et al. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(1):6. doi:10.1038/71545.
- Chao DT, Ma X, Li O, Park H, and Law D. Functional characterization of N297A, a murine surrogate for low-Fc binding anti-human CD3 antibodies. Immunol Invest. 2009 Jan;38(1):76–92.
- Pierpont TM, Limper CB, Richards KL. Past, present, and future of Rituximab-The world’s first oncology monoclonal antibody therapy. Front Oncol. 2018;8. doi:10.3389/fonc.2018.00163.
- Sun LL, Ellerman D, Mathieu M, Hristopoulos M, Chen X, Li Y, Yan X, Clark R, Reyes A, Stefanich E, et al. Anti-CD20/CD3 T cell-dependent bispecific antibody for the treatment of B cell malignancies. Sci Transl Med [Internet]. 2015;7:287ra70. Available from https://www.ncbi.nlm.nih.gov/pubmed/25972002.
- Atwell S, Ridgway JBB, Wells JA, Carter P. Stable heterodimers from remodeling the domain interface of a homodimer using a phage display library. J Mol Biol. 1997;269(2):270. doi:10.1006/jmbi.1997.1023.
- Schlothauer T, Herter S, Koller CF, Grau-Richards S, Steinhart V, Spick C, Kubbies M, Klein C, Umaña P, Mössner E. Novel human IgG1 and IgG4 Fc-engineered antibodies with completely abolished immune effector functions. Protein Engineering Design and Selection. 2016;29(10):457–66. doi:10.1093/protein/gzw040.
- Hezareh M, Hessell AJ, Jensen RC, van de Winkel JGJ, Pwhi P. Effector Function activities of a panel of mutants of a broadly neutralizing antibody against human immunodeficiency virus type 1. J Virol. 2001;75(24):12161–68. doi:10.1128/JVI.75.24.12161-12168.2001.
- Lo M, Kim HS, Tong RK, Bainbridge TW, Vernes JM, Zhang Y, Lin YL, Chung S, Dennis MS, and Zuchero YJY, et al. Effector-attenuating substitutions that maintain antibody stability and reduce toxicity in mice. J Biol Chem. 2017 Mar 3; 292(9):3900–3908.
- Klein C, Lammens A, Schäfer W, Georges G, Schwaiger M, Mössner E, Hopfner KP, Umaña P, and Niederfellner G. Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. MAbs. 2013 Jan-Feb;5(1):22–33.
- Arce Vargas F, Furness AJS, Solomon I, Joshi K, Mekkaoui L, Lesko MH, Miranda Rota E, Dahan R, Georgiou A, and Sledzinska A, et al. Fc-optimized anti-cd25 depletes tumor-infiltrating regulatory T cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity. 2017 Apr 18;46(4):577–586.
- Bulliard Y, Jolicoeur R, Zhang J, Dranoff G, Wilson NS, Brogdon JL. OX40 engagement depletes intratumoral Tregs via activating FcγRs, leading to antitumor efficacy. Immunol Cell Biol. 2014;92(6):475–80. doi:10.1038/icb.2014.26.
- Mo S, Gu L, Xu W, Liu J, Ding D, Wang Z, Yang J, Kong L, and Zhao Y. Bifunctional macromolecule activating both OX40 and interferon-α signaling displays potent therapeutic effects in mouse HBV and tumor models. Int Immunopharmacol. 2020 Dec;89(Pt B):107099.
- Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage polarization: different gene signatures in M1(Lps+) vs. Classically and M2(LPS-) vs. Alternatively activated macrophages. Front Immunol. 2019;10. doi:10.3389/fimmu.2019.01084.
- Chávez-Galán L, Olleros ML, Vesin D, Garcia I. Much more than M1 and M2 macrophages, there are also CD169+ and TCR+ macrophages. Front Immunol. 2015;6(6). 10.3389/fimmu.2015.00263.
- Allavena P, Chieppa M, Bianchi G, Solinas G, Fabbri M, Laskarin G, Mantovani A. Engagement of the Mannose receptor by tumoral mucins activates an immune suppressive phenotype in human tumor-associated macrophages. Clin Dev Immunol. 2010;2010:1–10. doi:10.1155/2010/547179.
- Buddingh EP, Kuijjer ML, Duim RAJ, Bürger H, Agelopoulos K, Myklebost O, Serra M, Mertens F, Hogendoorn PCW, Lankester AC, et al. Tumor-infiltrating macrophages are associated with metastasis suppression in high-grade osteosarcoma: a rationale for treatment with macrophage activating agents. Clin Cancer Res. 2011;17(8):2110–19. doi:10.1158/1078-0432.CCR-10-2047.
- Gonzalez-Dominguez E, Samaniego R, Flores-Sevilla JL, Campos-Campos SF, Gomez-Campos G, Salas A, Campos-Pena V, Corbi AL, Sanchez-Mateos P, and Sanchez-Torres C. CD163L1 and CLEC5A discriminate subsets of human resident and inflammatory macrophages in vivo. J Leukoc Biol. 2015 Oct;98(4):453–66.
- Nimmerjahn F, and V RJ. Fcγ receptors as regulators of immune responses. Nat Rev Immunol. 2008 Jan;8(1):34–47.
- Chen ST, Liu RS, Wu MF, Lin YL, Chen SY, Tan DTW, Chou TY, Tsai IS, Li L, and Hsieh SL. CLEC5A regulates Japanese encephalitis virus-induced neuroinflammation and lethality. PLoS Pathog. 2012;8(4):e1002655.
- Elleisy N, Rohde S, Huth A, Gittel N, Ä G, Möller S, Lamprecht G, Schäffler H, and Jaster R. Genetic association analysis of CLEC5A and CLEC7A gene single nucleotide polymorphisms and Crohn’s disease. World J Gastroenterol. 2020 May 14;26(18): 2194–2202.
- Vogelpoel LTC, Baeten DLP, de Jong EC, den Dunnen J. Control of cytokine production by human Fc gamma receptors: implications for pathogen defense and autoimmunity. Front Immunol. 2015;6(6). 10.3389/fimmu.2015.00079.
- Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, and Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress- inducible MICA. Science. 1999 Jul 30;285(5428):727–9.
- Cerwenka A, Baron JL, and Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc Natl Acad Sci U S A. 2001 Sep 25; 98(20):11521–6.
- Taylor MA, Hughes AM, Walton J, Coenen-Stass AML, Magiera L, Mooney L, Bell S, Staniszewska AD, Sandin LC, Barry ST, et al. Longitudinal immune characterization of syngeneic tumor models to enable model selection for immune oncology drug discovery. J ImmunoTher Cancer. 2019;7(1). doi:10.1186/s40425-019-0794-7.
- Williams AJ, Giese G, Persson J. Improved assembly of bispecific antibodies from knob and hole half-antibodies. Biotechnol Prog [Internet]. 2015;31(5):1315–22. Available from https://www.ncbi.nlm.nih.gov/pubmed/26097217.
- Giese G, Williams A, Rodriguez M, Persson J. Bispecific antibody process development: assembly and purification of knob and hole bispecific antibodies. Biotechnol Prog [Internet]. 2017;34(2):397–404. doi:10.1002/btpr.2590. Available from
- Lewis GD, Figari I, Fendly B, Wong WL, Carter P, Gorman C, Shepard HM, and Lewis GD. Differential responses of human tumor cell lines to anti-p185 nEu2 monoclonal antibodies Cancer Immunol Immunother. 1993 Sep;37(4):255–63.
- Formolo T, Ly M, Levy M, Kilpatrick L, Lute S, Phinney K, Marzilli L, Brorson K, Boyne M, and Davis D, et al. Determination of the NISTmAb primary structure. In: ACS symposium series Vol. 1201, p. 1–62. Washington, DC: ACS publication. 2015.