ABSTRACT
An ex situ germplasm collection of the endangered Cycas micronesica was established in a transition zone between biodiverse native forest and mature stands of the invasive species Leucaena leucocephala. Soil chemical properties were determined for the 2 tree cover types to inform management decisions. Total carbon, total nitrogen, calcium, and net ammonification were greater in native forest cover than in L. leucocephala patches. Net nitrification and net mineralization were greater under L. leucocephala cover. Trace metals also differed between the 2 forest cover types, with chromium, cobalt, and nickel accumulating to greater concentration under L. leucocephala cover and zinc accumulating to greater concentration under native forest cover. The results indicated that L. leucocephala cover generated substantial changes in soil chemical properties when compared with native forest tree cover, illuminating one means by which understory vegetation may be affected by changes in invasive tree cover.
Introduction
Cycas micronesica transitioned from the most abundant tree on the island of Guam in 2002Citation1 to Endangered status under IUCNCitation2 and Threatened status under the United States Endangered Species ActCitation3 mainly as a result of exotic phytophagous insects that invaded the island.Citation4,5 Plant mortality following the invasions was epidemic, and population size and structure dramatically changed.Citation6 Ex situ and in situ conservation programs were subsequently initiated, including a living collection of Guam germplasm established on the island of Tinian and funded by the United States Department of the Navy.Citation7 The germplasm collection was situated in a transition zone with a portion of the plants established beneath a diverse native forest canopy, and a portion established beneath adjacent mature stands of non-native Leucaena leucocephala. Growth and health of the C. micronesica plants have been highly variable (see Fig. 2 in ref. Citation8), with larger plants under the L. leucocephala cover than under native tree cover.
Conservation and restoration projects are often initiated in the absence of relevant publications to inform decisions. An adaptive management approach calls for persistent pursuit of new evidence-based outcomes. Therefore, the observations in Tinian reveal a need to identify which factors may be mediating the disparity in C. micronesica plant performance. Invasive plant species often exert profound changes in the habitats they invade.Citation9,10 The literature on this subject reveals a bias toward several well-known invasive plant species, which limits a comprehensive evaluation of how invasive plants fit into global change issues.Citation11 Leucaena leucocephala has been extensively exploited in agroforestry settings as a source of nitrogen-rich green mulch to improve soil quality for cash crops.Citation12 Nitrogen inputs, soil nitrification, and soil ammonification are among the components of the nitrogen cycleCitation13 that may be influenced by L. leucocephala. Therefore, one potential factor that could influence understory C. micronesica plant growth is the difference in nutrient relations of the soils beneath the native forest cover versus the L. leucocephala patches.
Long-standing patches of vegetation contribute to system spatial heterogeneity through chronic influences on biogeochemical cycling and through interactions with microbial communities associated with the vegetation.Citation14 These plant-soil feedbacks are orchestrated by complex integrated relationships among many biotic and abiotic factors.Citation15 Influences of tree genotype on these processes include prolonged extraction and sequestration of soil elements in plant organs, extent of element resorption prior to leaf senescence then abscission, litter quality effects on organic matter lability, and local amplification of root-associated microorganisms and specialist saprophytic microorganisms. These phenomena are one means by which invasive plant species can affect native plants through changes in soil properties.Citation16,17
Our objective was to use paired sampling sites throughout the C. micronesica germplasm to determine how forest tree cover type influenced soil chemical traits and nitrogen mineralization dynamics. The results may improve management decisions in our ex situ conservation program. Moreover, the information will increase knowledge of broader topics of tropical invasive species management, rare plant conservation, and integrative biological influences on soil chemical properties.
Results
Total carbon concentration () and total nitrogen concentration () were less in soils from L. leucocephala sites than soils from native forest sites. The quotient carbon:nitrogen did not differ between the 2 tree cover types. The pH of soils beneath L. leucocephala tree cover was slightly less than that of soils from native tree cover (). From highest to lowest, the concentrations of extractable macronutrients in the soils from both tree cover types were in the following order: calcium>magnesium>potassium>phosphorus (). Calcium concentration was greater under diverse native tree cover than under L. leucocephala cover, but the other nutrients were not influenced by tree cover type ().
Figure 1. Available nitrate, available ammonium, net nitrification rate, net ammonification rate, net mineralization rate, and total nitrogen of soils as influenced by Leucaena leucocephala tree cover versus biodiverse native tree cover. D = Kolmogorov-Smirnov statistic. Mean + SE, n = 10.
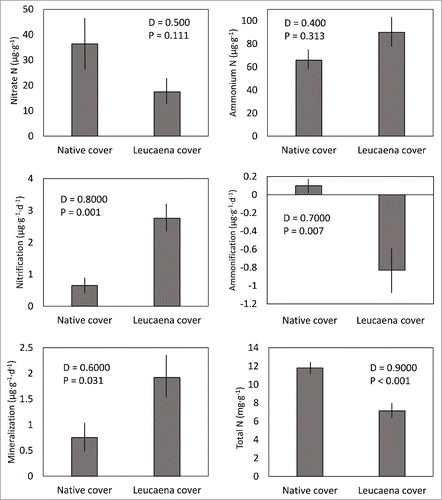
Table 1. Soil properties as influenced by Leucaena leucocephala tree cover vs. native forest cover on Tinian Island. Mean + SE, n = 10. D = Kolmogorov-Smirnov statistic.
The differences in various components of nitrogen cycling were highly contrasting between the 2 forest tree cover types. Net nitrification rate of soils beneath L. leucocephala cover was 4.25-fold greater than soils beneath native tree cover (). Net ammonification of soils was positive beneath native tree cover, but negative beneath L. leucocephala tree cover (). These results caused the soils from L. leucocephala microsites to exhibit net mineralization that was 156% greater than that from the diverse native forest microsites (). Available nitrate, available ammonium, and total available nitrogen did not differ between the 2 forest tree cover types (, ).
From highest to lowest, the concentrations of measured metals in the soils were in the following order: zinc>copper>chromium>cobalt>nickel>cadmium>selenium>lead (). Chromium, cobalt, and nickel were greater in the L. leucocephala soils than in the native tree soils. Selenium and zinc were greater in the native tree soils than in the L. leucocephala soils. Cadmium, copper, and lead were not influenced by tree cover type.
Table 2. Metal content of soils in Tinian Island as influenced by Leucaena leucocephala tree cover versus native forest cover. Mean + SE, n = 10. D = Kolmogorov-Smirnov statistic.
Discussion
Insular habitats including islands may be more susceptible to plant invasions than continental habitats.Citation18 Yet the Pacific islands have been under-represented in publications on invasive plant species.Citation11 We have addressed this bias with a look at a widespread invasive woody tree species in a small oceanic island habitat.
This diagnosis of the integrative changes in soil chemical properties caused by persistent L. leucocephala tree cover is the first empirical look at how this invasive tree affects ecosystems in the Mariana Islands. We have shown that a high proportion of soil nutrients and metals were substantially changed by the L. leucocephala tree cover. Various components of the nitrogen cycle were among the soil properties that were most affected by tree cover type. That total nitrogen was greater under native tree cover may seem counterintuitive, as L. leucocephala is a well-known legume, its Rhizobium endosymbionts are prevalent in the calcareous soils of the Mariana Islands,Citation19 and none of the prevalent native tree species at our site associate with nitrogen-fixing symbionts. Indeed, habitats dominated by legume species tend to exhibit greater soil nitrogen.Citation20 These interesting relationships were reconciled by the acute differences in nitrification and ammonification. Net mineralization rates in the L. leucocephala microsites were 256% of those of native forest microsites. Moreover, net nitrification of the L. leucocephala soils exceeded net mineralization, but net nitrification of the native forest soils was only 87% of net mineralization. Clearly, nitrogen-fixing bacteria genera Nitrosomonas and Nitrobacter were highly active under L. leucocephala cover and less active under native tree cover.
We propose that the prodigious nitrification in soils beneath L. leucocephala patches causes excessive nitrogen losses from the system through leaching since nitrate is highly mobile.Citation21 In contrast, the limited nitrification of the native forest microsites protects those soils from similar losses. Annual precipitation in Tinian is 204 cm (www.wunderground.com), evincing considerable leaching potential. As a result, the soil nitrogen pool is labile in the L. leucocephala patches and recalcitrant in the native forest patches.
Biodiversity is critical for sustaining many components of ecosystem services and maintaining forest productivity.Citation22 Indeed, species mixtures and high plant biodiversity may increase nitrogen retention, reduce nitrogen losses, and decrease the potential for groundwater contamination due to nitrogen leaching.Citation23,24 Therefore, our native forest may have exhibited greater retention of nitrogen simply because it had greater biodiversity than the L. leucocephala microsites, which were monospecific.
Two issues from our study are relevant for climate change research. First, climate change is predicted to increase nitrate leaching as extreme events increase in frequency.Citation25 Therefore, in a climate change scenario nitrate leaching in L. leucocephala sites could be aggravated. Second, soil organic matter is the largest terrestrial carbon pool,Citation26 and soil carbon and nitrogen mineralization are closely coupled.Citation27,28 Our results indicate that monospecific L. leucocephala sites act as less effective carbon sinks than native forest sites.
Ecosystem changes caused by invasive species are some of the complex consequences of anthropogenic changes to the global environment. A full understanding of how to manage invasive species cannot develop in the absence of an all-inclusive viewpoint founded in empirical studies.Citation29 Meta-analyses have shown that net primary production, litter decomposition, and altered nitrogen cycle are some of the most common ways that successful invasive plants modify nutrient cycling.Citation30 Bardon et al.Citation31 recently reported that root exudates of a successful invasive plant reduced metabolic activity of 2 denitrifying bacteria species, adding a previously unknown means by which invasive plants may directly influence the nitrogen cycle.
The differences in soil chemical components we have reported from L. leucocephala vs. diverse native tree forest cover may influence understory vegetation growth and health. In our experimental site, the main understory species of interest was the Guam-sourced C. micronesica germplasm that we introduced and managed. Cycas micronesica is one of more than 350 species of extant cycads.Citation32 This plant group is among the most threatened groups of plants worldwide.Citation33,34 Developing successful management strategies for cycad conservation is an urgent agenda. An ongoing refinement of our C. micronesica conservation program based on national threatened and international endangered listingsCitation2,3 fits into that international agenda. Although we have shown the soils in L. leucocephala microsites exhibited substantial differences from soils in biodiverse native forest microsites, the magnitude and direction of differences in macronutrients () and metals () were not likely to explain why C. micronesica plants have grown better as understory plants in the L. leucocephala microsites. Furthermore, the increased net nitrification and net mineralization of soils in L. leucocephala microsites are not likely to substantially benefit the understory C. micronesica plants, as available nitrogen did not differ between the soils from the 2 forest cover types. Additionally, all cycad plants associate with nitrogen-fixing cyanobacteria endosymbionts, therefore cycads may not be affected by ecologically relevant differences in nitrogen among various soils.
Several critical research needs are illuminated by this study. A greater understanding of litterfall quantity, seasonality, and quality may reveal some of the factors that mediate the differences in soil traits between the native forest cover and L. leucocephala cover. Reciprocal litter incubation studies would tease apart the influences of litter quality and soil microbes that influence decomposition speed. Which soil microbes are influential players in the changes that L. leucocephala imposes on soils may be identified by DNA sequencing of bulk soils or rhizosphere, and would greatly improve mechanistic insight. Cycas micronesica plant growth has been greater under L. leucocephala cover than under native tree cover in our ex situ germplasm, yet soil nutrition does not appear to mediate this response. Site differences in incident light, relative humidity, and temperature may be more important factors than the soil properties for explaining the disparity in growth and health of the C. micronesica germplasm.
Many Guam and Tinian habitats that have experienced past disturbance are characterized by L. leucocephala cover. Once established, this species effectively monopolizes emergent canopy cover. Future large-scale restoration plans for Guam and Tinian include landscape-scale efforts to remove invasive, non-native plant species.Citation35 Our results indicate that forest restoration goals may require many years to achieve following the invasive tree removal, considering the transformed soil properties that need to be restored.
Materials and methods
The experimental site was located on the island of Tinian in karsty outcrop soils (Loamy-skeletal, carbonatic, isohyperthermic Lithic Haplustolls).Citation36 A robust C. micronesica planting is being maintained along a north-south oriented ecotone between diverse native tree cover and an adjacent belt of L. leucocephala cover. The soil samples were obtained within this ex situ germplasm. The L. leucocephala sites were primarily mono-specific for the emergent canopy cover, but contained a diverse understory vegetation palette. The native forest sites were highly diverse, and dominant tree species were Pisonia grandis, Psychotria mariana, Aglaia mariannensis, Cynometra ramiflora, and Eugenia palumbis.
We collected paired soil samples from 10 locations along the ecotone on 10 Sept 2014. Each sample from the L. leucocephala cover was located 25-35 m east of its paired sample from the native forest cover. The soil cores were collected from the A horizon from 0-10 cm. Rainfall for the 2 weeks prior to the soil harvests averaged 9.54 mm·d-1 (www.wunderground.com).
Analyses
A portion of each sample was dried at 50°C then total carbon and nitrogen were determined by dry combustion (Nelson and Sommers);Citation37 extractable P, K, Mg, and Ca were determined by Mehlich-3 digestion (Mehlich 1984);Citation38 and total metals were determined by nitric acid digestion.Citation39 Nitrate and ammonium were determined colorimetrically from fresh moist soil samples following 2M KCl extraction. Soil was incubated using the buried bag methodCitation40 in a homogeneous site at 28°C (range 25°C – 31°C) soil temperature for 32 d. Nitrate and ammonium were determined at the end of the incubation period. Net nitrification rate was calculated by subtracting initial from final nitrate concentration and dividing by the incubation period. Net ammonification rate was calculated by subtracting initial from final ammonium concentration and dividing by the incubation period. Net mineralization was calculated as the sum of nitrification and ammonification.
The data did not meet requirements for parametric analysis, primarily because of unequal variances. We used the non-parametric and distribution-free Kolmogorov-Smirnov 2-sample testCitation41 to test the null hypothesis that the 2 groups of soil samples were not different in each of the chemical properties that were quantified. This test does not require any assumption about the distribution of data. Levels of significance of at least P < 0.05 were considered significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Support provided by the United States Department of the Navy through N40192-12-2-8000 and N40192-13-2-8003, administered by Naval Facilities Engineering Command Marianas, Guam.
References
- Donnegan JA, Butler SL, Grabowiecki W, Hiserote BA, Limtiaco D. Guam's forest resources, 2002. Resource Bulletin PNW-RB-243. Portland: US. Department of Agriculture, Forest Service, Pacific Northwest Research Station; 2004.
- Marler T, Haynes J, Lindström A. Cycas micronesica. The IUCN Red List of Threatened Species 2010; e.T61316A12462113; http://dx.doi.org/10.2305/IUCN.UK.2010-3.RLTS.T61316A12462113.en
- USFWS [US. Fish and Wildlife Service]. Endangered and threatened wildlife and plants; endangered status for 16 species and threatened status for 7 species in Micronesia. Federal Register 2015; 80(190):59424-97.
- Marler TE. Cycad aulacaspis scale invades the Mariana Islands. Memoirs New York Botanical Garden 2012; 106:20-35.
- Marler TE. Temporal variations in leaf miner, butterfly, and stem borer infestations of Cycas micronesica in relation to Aulacaspis yasumatsui incidence. HortScience 2013; 48:1334-8.
- Marler TE, Lawrence JH. Demography of Cycas micronesica on Guam following introduction of the armoured scale Aulacaspis yasumatsui. J Trop Ecol 2012; 28:233-42; http://dx.doi.org/10.1017/S0266467412000119
- Anonymous. Conserving our nation's only native cycad species. Currents 2014; Fall, p. 28-31. Available at: http://greenfleet.dodlive.mil/files/2014/10/Fall14_Conserving_Cycad_Species.pdf
- Marler TE, Dongol N, Cruz GN. Plastic responses mediated by identity recognition in below-ground competition in Cycas micronesica K.D. Hill. Trop Cons Science 2016; 9:648-57.
- Pyšek P, Jarošík V, Hulme PE, Pergl J, Hejda M, Schaffner U, Vilà M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species' traits and environment. Glob Change Biol 2012; 18:1725-37; http://dx.doi.org/10.1111/j.1365-2486.2011.02636.x
- Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošík V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P. Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Letters 2011; 14:702-8; http://dx.doi.org/10.1111/j.1461-0248.2011.01628.x
- Hulme PE, Pyšek P, Jarošík V, Pergl J, Schaffner U, Vilà M. Bias and error in understanding plant invasion impacts. Trends Ecol & Evol 2013; 28:212-8; http://dx.doi.org/10.1016/j.tree.2012.10.010
- Read MD, Kang BT, Wilson GF. Use of Leucaena leucocephala (Lain.de Wit) leaves as nitrogen source for crop production. Fert Res 1985; 8:107-16; http://dx.doi.org/10.1007/BF01048894
- Smil V. Cycles of Life. Scientific American Library. New York: 2000.
- Van Der Heijden MG, Bardgett RD, Van Straalen NM. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 2008; 11:296-310; PMID:18047587; http://dx.doi.org/10.1111/j.1461-0248.2007.01139.x
- Van der Putten WH, Bardgett RD, Bever JD, et al. Plant-soil feedback: the past, present and future challenges. J Ecol 2013; 101:265-76; http://dx.doi.org/10.1111/1365-2745.12054
- Haubensak KA, Parker IM. Soil changes accompanying invasion of the exotic shrub Cytisus scoparius in glacial outwash prairies of western Washington [USA]. Plant Ecology 2004; 175:71-9. http://dx.doi.org/10.1023/B:VEGE.0000048088.32708.58; http://dx.doi.org/10.1023/B:VEGE.0000048088.32708.58
- Niu H, Liu W, Wan F, Liu B. An invasive aster (Ageratina adenophora) invades and dominates forest understories in China: altered soil microbial communities facilitate the invader and inhibit natives. Plant Soil 2007; 294:73-85; http://dx.doi.org/10.1007/s11104-007-9230-8
- Gimeno I, Vilà M, Hulme PE. Are islands more susceptible to plant invasion than continents? A test using Oxalis pes-caprae L. in the western Mediterranean. J Biogeography 2006; 33:1559-65; http://dx.doi.org/10.1111/j.1365-2699.2006.01525.x
- Marutani M, Manalastas E. Evaluation of two methods to estimate the populations of indigenous N-fixers associated with tropical legumes. Micronesica 2000; 33:153-60.
- Chimphango SBM, Potgieter G, Cramer MD. Differentiation of the biogeochemical niches of legumes and non-legumes in the Cape Floristic Region of South Africa. Plant Ecology 2015; 216:1583-95; http://dx.doi.org/10.1007/s11258-015-0542-0
- Cameron KC, Di HJ, Moir JL. Nitrogen losses from the soil/plant system: a review. Ann Appl Biol 2013; 162:145-73; http://dx.doi.org/10.1111/aab.12014
- Scherer-Lorenzen M. The functional role of biodiversity in the context of global change. In Forests and global change, Eds Burslem D, Coomes D, Simonson W, Cambridge Univ Press, Cambridge, MA: 2014; 195-238.
- Lang AC, von Oheimb G, Scherer-Lorenzen M, et al. Mixed afforestation of young subtropical trees promotes nitrogen acquisition and retention. J Applied Ecol 2014; 51:224-33; http://dx.doi.org/10.1111/1365-2664.12157
- Scherer-Lorenzen M, Palmborg C, Prinz A, Schulze E-D. The role of plant diversity and composition for nitrate leaching in grasslands. Ecology 2003; 84:1539-52; http://dx.doi.org/10.1890/0012-9658(2003)084%5b1539:TROPDA%5d2.0.CO;2
- Dirnböck T, Kobler J, Kraus D, Grote R, Kiese R. Impacts of management and climate change on nitrate leaching in a forested karst area. J Envir Management 2016; 165:243-52; http://dx.doi.org/10.1016/j.jenvman.2015.09.039
- Schlesinger WH, Bernhardt ES. Biogeochemistry: An analysis of global change. 3rd ed. Academic Press; 2013.
- Gutiérrez-Girón A, Díaz-Pinés E, Agustín Rubio A, Gavilán RG. Both altitude and vegetation affect temperature sensitivity of soil organic matter decomposition in Mediterranean high mountain soils. Geoderma 2015; 237:1-8; http://dx.doi.org/10.1016/j.geoderma.2014.08.005
- Quan Q, Wang CH, He NP, et al. Forest type affects the coupled relationships of soil C and N mineralization in the temperate forests of northern China. Sci Rep 2014; 4:1-8.
- Barney JN. Invasive plant management must be driven by a holistic understanding of invader impacts. Applied Vegetation Science 2016; 19:183-4; http://dx.doi.org/10.1111/avsc.12239
- Liao C, Peng R, Luo Y et al. Altered ecosystem carbon and nitrogen cycles by plant invasion: a meta-analysis. New Phytol 2008; 177:706-14; PMID:18042198; http://dx.doi.org/10.1111/j.1469-8137.2007.02290.x
- Bardon C, Piola F, Bellvert F, Zhou X, Wu X, Fang C, Chen J, Li B. Evidence for biological denitrification inhibition (BDI) by plant secondary metabolites. New Phytol 2014; 204:621-31; http://dx.doi.org/10.1111/nph.12944
- Osborne R, Calonje M, Hill K, Stanberg L, Stevenson DW. The world list of cycads. Memoirs New York Botanical Garden 2012; 106:480-508.
- Brummitt NA, Bachman SP, Griffiths-Lee J, Lutz M, Moat JF, Farjon A, Donaldson JS, Hilton-Taylor C, Meagher TR, Albuquerque S, et al. Green plants in the red: A baseline global assessment for the IUCN sampled Red List Index for plants. PloS one 2015; 10:e0135152; PMID:26252495; http://dx.doi.org/10.1371/journal.pone.0135152
- Fragniere Y, Bétrisey S, Cardinaux L, Stoffel M, Kozlowski G. Fighting their last stand? A global analysis of the distribution and conservation status of gymnosperms. J Biogeography 2015; 42:809-20; http://dx.doi.org/10.1111/jbi.12480
- USFWS [US. Fish and Wildlife Service]. Biological opinion for the joint Guam program office relocation of the US. Marine Corps from Okinawa to Guam and associated activities on Guam and Tinian. 2010; United States: Department of the Interior.
- Young FJ. Soil survey of the islands of Aguijan, Rota, Saipan, and Tinian, Commonwealth of the Northern Mariana Islands. United States Department of Agriculture, Soil Conservation Service; 1989.
- Nelson DW, Sommers LE. Total carbon, organic carbon, and organic matter. p. 961-1010. In: Sparks DL (ed.) Methods of soil analysis. Part 3. Chemical methods. SSSA Book Series No. Five. SSSA and ASA, Madison, WI: 1996.
- Mehlich A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun Soil Sci Plant Anal 1984; 15:1409-16; http://dx.doi.org/10.1080/00103628409367568
- E.P.A. [United States Environmental Protection Agency]. EPA Method 3005. Cincinnati, Ohio: USEPA; 1992.
- Eno CF. Nitrate production in the field by incubating the soil in polyethylene bags. Proc Soil Sci Soc Am 1960; 24:277-9; http://dx.doi.org/10.2136/sssaj1960.03615995002400040019x
- Sokal RR, Rohlf FJ. Biometry: The principles and practice of statistics in biological research. 4th Edition. New York: WH Freeman; 2011.
