ABSTRACT
Immunogold electron microscopy (EM) study of Arabidopsis root apices analyzed using specific IAA antibody and high-pressure freeze fixation technique allowed, for the first time, vizualization of subcellular localization of IAA in cells assembled intactly within plant tissues. Our quantitative analysis reveals that there is considerable portion of IAA gold particles that clusters within vesicles and membraneous compartments in all root apex cells. There are clear tissue-specific and developmental differences of clustered IAA in root apices. These findings have significant consequences for our understanding of this small molecule which is controlling plant growth, development and behavior.
Introduction
Indole-3-acetic acid (IAA or auxin) is considered for a plant hormone, but it has also morphogen and signaling transmitter features. IAA is involved in almost all plant processes during the entire life span of higher plants. After it was predicted by Charles and Francis Darwin in 1880,Citation1 this small signaling molecule was discovered and initially characterized by Went, Kögl, Thimann and others in the first half of the last century.Citation2 Boysen-Jensen confirmed that a mobile signal molecule is involved in phototropism in 19133 and Went coined term auxin (from Greek auxein) in 1926,Citation4 which was then chemically identified as IAA in 1934.Citation5 Finally, IAA was isolated from immature maize seeds in 1946.Citation6
Charles and Francis Darwin, in their experiments with decapped maize roots, followed original experiments accomplished by Theophil Ciesielski in 1872 who discovered that decapped maize roots continue to grow but fail to respond to gravistimulation.Citation7 Julius Sachs heavily criticized these experiments but they were confirmed later by Francis Darwin as well as many other experimental researchers.Citation8-15 Currently, IAA and its transcellular transport are well known to be essential for all kinds of plant tropisms, both in roots and shoots. Especially root apices are unique as almost all PIN proteins are known to be expressed in root apex of Arabidopsis and drive the very complex loops of transcellular auxin fluxes.
IAA is very small molecule (molar mass 175.19 g·mol−1), which can rapidly diffuse through the cytoplasm as well as through plant cell walls, that affects and controls almost every aspect of plant biology.Citation16,Citation17 Surprisingly, although auxin easily diffuse into nuclei trough their nuclear pores to control gene expression, it is not freely transported through larger plasmodesmata.Citation18,Citation19 Either plasmodesmal gating excludes auxin from passing through plasmodesmata or there is some active mechanism behind, which prevents IAA to enter plasmodesmata. Obviously, this is an IAA specific process as synthetic auxin 2,4-dichlorophenoxyacetic acid (2,4-D)Citation20 is passing through via plasmodesmata.Citation21 One possible scenario would be that putative plasma membrane derived recycling vesicles, internalizing IAA via activity of the PIN efflux transporters, patrol plasmodesmata orifices and actively prevent IAA molecules to enter the plasmodesmata channels. 2,4-D molecules, which are not transported via auxin efflux carriers,Citation20,Citation22 are allowed to pass along plasmodesmata. In support of this concept, IAA is known to accumulate within plasma membrane-derived vesicles via an active electrogenic transport of IAA.Citation23 Moreover, immunolocalization of IAA using specific antibodies revealed IAA-enriched vesicles in maize root apex cells, co-localizing with recycling auxin efflux transporter PIN1 and with endosomal recycling pectins.Citation24,Citation25 However, at low resolution of the light immunofluorescence microscopy, it is not possible to reach any conclusive evidence on auxin molecules accumulating within the lumen of recycling vesicles. To localize auxin molecules in cells of plant tissues, the only possible method is immunogold electron microscopy (EM) using specific antibodies. EM immunolocalization of low molecular weight compounds (slightly below of that of IAA's 175,18 Da) have already been reported using anti-GABA and L-glutamate antibodies applied on fixed rat brain sections.Citation26,Citation27 Both GABA and L-glutamate are localized in synaptic vesicles with higher density (clusters) as in the adjacent cytoplasm.Citation26,Citation27
Results
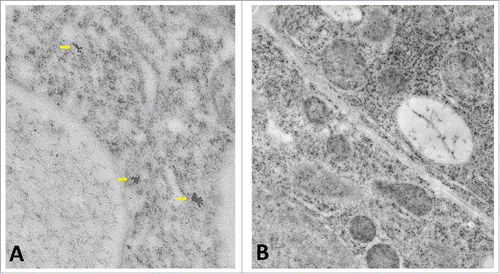
Here, we have used immunogold electron microscopy (EM) analysis of Arabidopsis root apices fixed using high-pressure freeze fixation technique which preserves membranes, organelles and ultrastucture of the fixed cells. Our analysis revealed that all cells of Arabidopsis root apices show auxin-labeled gold particles clustered within vesicular structures (, and ). Most of them were tightly clustered, from 3 up to about 80 gold-particles within one single compartment. Importantly, there are both tissue-specific and developmental differences, with large auxin clusters scored especially in root cap and root epidermis cells (). There were almost no auxin clusters scored within quiescent center cells, and only small clusters in the adjacent initial cells. Size of IAA clusters is increasing along the root apex, reaching a peak in the transition zone, and then decreasing in cells of the early elongation zone (, ). The number of clusters is high in the root cap and the transition zone cells, while no or very few IAA clusters are in the quiescent center and adjacent initial cells (, ). When the first antibody was omitted then no gold particles were prewsent ().
Figure 1. Quantification of gold particle distributions in root apex zones using the polyclonal IAA antibody.
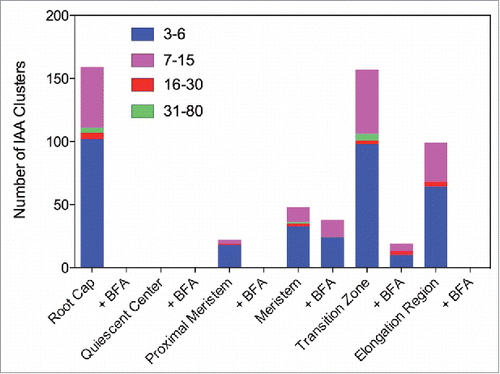
Figure 2. Quantification of gold particle distributions in root apex zones using the monoclonal IAA antibody.
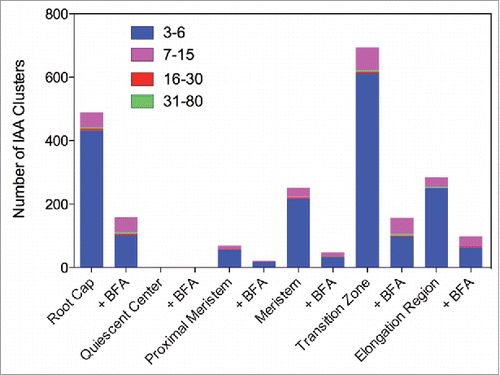
Figure 3. (A) Three IAA clusters in a transition zone epidermis cell labeled with the polyclonal IAA antibody. (B) Negative control of similar cell using only the secondary antibody results in no gold particles.
Interestingly, this pattern of clustered IAA, shown here for both IAA antibodies, closely mimicks rates of endoctic vesicle recycling in the root apex zone, being the highest in the transition zone cells, both in maize and Arabidopsis.Citation24,Citation28 Clusters of IAA were scored from 3 up to 80 gold particles with both IAA antibodies (, ). Interestingly, exposure of Arabidopsis roots to inhibitor of exocytosis and endocytic vesicle recycling Brefeldin A (BFA) resulted in loss of IAA clusters in all root cap and elongation region cells, whereas the size of clusters decreased in meristem and transition zone cells (, )
Discussion
Current models of transcellular auxin transport are based on membrane transporters of PIN and ABC families which are considered to be active as transporters only when inserted in the plasma membrane. However, these auxin transporters are actively recycling between the plasma membrane and endosomes via recycling vesicles.Citation29-31 Importantly, there is no evidence available, whatsoever, that these transporters stop to transport IAA when localized within the vesicle membrane. In fact, some authors insert PIN arrows (indicating their auxin transport activities) also into the vesicle and endosomal membranes in their schemes.Citation32-35 Our present data strongly support this scenario and PINs represent the best candidate for vesicular auxin transporters.
There are several features of the polar auxin transport (PAT) implicating exocytic secretion of auxin.Citation28,Citation36,Citation37 First of all, there is tight correlation between exo/endocytic vesicle recycling and rate of the PAT. Second, inhibitors of PAT inhibit endocytic vesicle recycling whereas exocytosis inhibitor brefeldin A (BFA) inhibits endocytic vesicle recycling and PAT.Citation38-41 Third, amounts of PINs inserted within plasma membrane do not correlate with rates of PAT.Citation36 Importantly, BFA mimicking PAT transport inhibitors also similarly affects gravistimulation-induced calcium spikes in Arabidopsis seedlings.Citation42
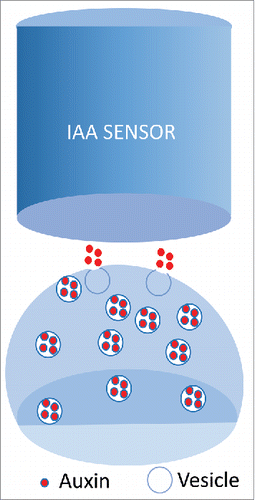
Recently, vesicular secretion of IAA via single exocytic vesicle fusion was even experimentally confirmed with in situ core-shell titanium-carbide carbon (TiC&C) quasi-aligned nanofiber arrays (QANFAs). Quantal secretion of IAA using this amperometric monitoring system of IAA release allows real time quantification of IAA release.Citation43 Importantly, the quantal size of IAA secretion events was increased by pre-loading of plant cells with IAA molecules.Citation43 The same system was used also for quatification of GABA and L-glutamate release at brain synapses.Citation44-46 This depolarization-induced IAA secretion release from plant protoplasts ()Citation43 closely resembles neurotransmitter vesicular release of GABA and L-glutamate at the neuronal synapses. As IAA is well-known to induce electrical responses, and even plant action potentials, all this strongly supports the neurotransmitter-like concept of auxin actions in plants.Citation24,Citation28,Citation36,Citation37
Figure 4. Plasma membrane depolarization-induces quantal IAA exocytosis from plant protoplasts probed with auxin-specific micro-electrochemical sensor.Citation43
Interestingly, there is a clear negative corelation between vesicular auxin and auxin-mediated gene expressionCitation47 as well as auxin response maximum mediated via synthetic auxin-response reporter DR5,Citation48 and PLETHORA transcription factor gradient in Arabidopsis root apex.Citation49,Citation50 Our data suggest that auxin-accumulating vesicles control how much IAA molecules are free, available to enter nuclei for the auxin induced gene expression. IAA enriched vesicles and/or endosomes may correspond to the mysterious compartment X proposed by Markus GrebeCitation51 to explain role of intracellular proton pump AVP1 in control of PAT and cell wall acidification.Citation52,Citation53 AVP1 belongs to pyrophosphatases which localize to trans-Golgi networks and multivesicular bodies,Citation54,Citation55 both being part of the plant endosomal system. Importantly, AVPs are localized within the BFA-induced compartmentsCitation55 in which also IAA and PINs are enriched.Citation24,Citation25 More recently, the possible input of AVP1 into PAT was toned down due to a second T-DNA insertion close to the ARF-GEF GNOM gene in the avp1–1 mutant line.Citation56,Citation57 It is relevant, however, that GNOM is plant-specific ARF-GEF which is mediating BFA-mediated endosomal recycling-based PAT.Citation58-60 Both GNOM and AVP1 localize to BFA-sensitive endosomal compartments accumulating within the BFA-induced compartments.
Interestingly in this respect, our data show that the BFA exposure induces loss of almost all vesicular IAA in root cap cells as well as elongating root cells, which are rather inactive in the endosomal vesicle recycling and PAT; but only decrease in cells of meristem and transition zone which are active in the endosomal vesicle recycling and PAT.Citation24,Citation28,Citation36,Citation37,Citation61-63 Several aspects of the aluminum (Al) toxicity of the root apex transition zone support the emerging concept that the recycling auxin-accumulating vesicles control how much auxin is available for nuclear transport to control gene expression. Similarly as BFA, Al toxicity inhibits endocytic vesicle recycling and PAT in the transition zone of both maize and Arabidopsis root apices.Citation64-67 Moreover, Al induces IAA accumulation within nuclei of the Arabidopsis root apex transition zone, as visualized with the DNA transcription-based IAA reporter DR5.Citation68,Citation69
Conclusions
In conclusion, the EM immunogold visualization of IAA in Arabidopsis root apex cells is incompatible with the currently popular models of PAT considering auxin to be diffusely distributed within the cytoplasm and ignoring endosomes / vesicles in the PAT. Together with the recent discovery of vesicular secretion of IAA from protoplasts, our data implicate vesicular secretion of IAA in the PAT. This feature has far-reaching consequences not only for auxin biology but also, as IAA affects all aspects of plant biology,Citation16,Citation17 for our understanding of plants in their sensory and behavioral complexity. PAT is essential for all kinds of plant movements and tropisms,Citation15,Citation28-37 as well as for integrating plant bodies via long-range IAA fluxes via vascular systemsCitation70 and short-range IAA fluxes.Citation71 Ability to actively enrich IAA within vesicles and larger compartments is important for plant growth, polarity and development as it allows plant cells to control precisely when and how much IAA molecules are reaching their signaling targets and receptors, controling plant signaling at the plasma membrane and gene expresion levels witin the nucleus. Quantal release of IAA from cells is also critical for IAA acting as transmitter molecule controlling cell-cell communication via neuronal-like processes.Citation72,Citation73 Last but not least, the secretion of auxin via recycling vesicles can solve the mystery of the elusive flux sensor which is required for the PAT models based on IAA canalization.Citation35,Citation74 All published models of PAT ignore IAA enriched vesicles and endosomes. This is a serious drawback of these models, limiting their reliability and predictability. If the neurotransmitter-like recycling of the IAA-enriched vesiclesCitation24,Citation72,Citation73 will be confirmed in future, this will represent significant breakthrough not only in auxin biology and physiology, but also in plant sensory and behavioral biology. Relevantly in this respect, recent support for roles of vesicles in PAT emerges from studies of large algal cells of Chara,Citation75 plant-specific myosins,Citation76,Citation77,Citation78,Citation79 and ER-dependent endosome streaming via RHD3 proteinCitation80 expressed highly in the root apex transition zone.Citation81
Materials and methods
Root apices of 7 d old control and BFA-treated (50 μM BFA for 2 hours) Arabidopsis seedlings were used. Immunogold labeling was performed as previously reported.Citation82-84 Three root apices were placed in gold platelet carriers prefilled with 1-hexadecene and immediately frozen in a high-pressure freezing apparatus (HPM100; Bal-Tec). Subsequently, samples were chemically fixed with 0,1% uranyl acetate and 0, 25% glutaraldehyde in dry acetone. Then, samples were cryosubstituted at −80°C and embedded in Lowicryl HM20 (Polysciences, Warrington PA). Ultrathin sections were blocked and incubated in a moist chamber with primary antibodies (1:200) diluted overnight at 4°C. Ultrathin sections were blocked against binding to unspecific proteins, post-fixed with 2% glutaraldehyde, and post-stained with 2% uranyl acetate. Ultrathin sections were incubated primary IAA antibodies diluted 1:200 (affinity purified anti-N1-IAA; polyclonal)Citation24,Citation85 or 1:200 (anti-IAA monoclonal 1E11-C11, Sigma-Aldrich) overnight at 4°C. This step was followed by rinsing and incubation with secondary antibodies conjugated to 10 nm gold particles. Secondary antibodies were diluted 1:50 with PBS and incubated for 2 h at room temperature. Controls were performed using only the secondary antibodies conjugated to 10 nm gold particles. Labeled sections were examined with an Leo 912ab transmission electron microscope (Zeiss) operated at 80 keV. For BFA treatments, the roots were incubated with 35.6 µM BFA at room temperature for 60 min. Clusters of auxin-labeled gold particles were quantified across the examined root apex tissues/zones and were not calculated at the cellular level.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Gerd Hause for providing us with the high-pressure freeze fixation samples of Arasbidopsis root apices.
Funding
This work was funded by the Ministry of Education, Youth and Sports of the Czech Republic (the National Program for Sustainability I Nr. LO1204).
References
- Darwin C (with Darwin F). The Power of Movement in Plants. John Murray, London; 1880.
- Went F, Thimann KV. Phytohormones. MacMillan Company, New York; 1937.
- Boysen-Jensen P. Über die Leitung des phototropischen Reizes in der Avenakoleoptile. Ber Deut Bot Ges 1913; 31:559-66.
- Went FW. On growth-accelerating substances in the coleoptile of Avena sativa. Proc Kon Ned Akad Wet 1926; 30:1.
- Kögl F, Haagen-Smit AJ, Erxleben H. Über den Einfluss der Auxine auf das Wurzelwachstum und über die chemische Natur des Auxins der Graskoleoptilen. Zeit Physiol Chem 1934; 228:104-12; https://doi.org/10.1515/bchm2.1934.228.1-2.104 10.1515/bchm2.1934.228.1-2.90 10.1515/bchm2.1934.228.3-6.113
- Haagen-Smit AJ, Dandliker WB, Wittwer SH, Murneek AE. Isolation of 3-indoleacetic acid from immature corn kernels. Am J Bot 1946; 33:118-20; https://doi.org/10.2307/2437327
- Ciesielski T. 1872. Untersuchungen über die Abwärtskrümmung der Wurzel. Beitr Biol Pflanzen 1872; 1:1-30.
- Heslop Harrison J. Darwin and the movement of plants: a retrospect. In: Skoog F (ed), Plant Growth Substances 1979, Springer Verlag 1980; 3-14.
- Juniper BE, Groves S, Landau-Schachar B, Audus LJ. Root cap and the perception of gravity. Nature 1966; 209:93-4; https://doi.org/10.1038/209093a0
- Konings H. The significance of the root cap for geotropism. Acta Bet Neerl 1968; 17:203-21; https://doi.org/10.1111/j.1438-8677.1968.tb00074.x
- Pilet PE. Root cap and georeaction. Nat New Biol 1971; 233:115-6; PMID:5315327; https://doi.org/10.1038/233115a0 10.1038/newbio233115b0 10.1038/newbio233115a0
- Barlow PW. Recovery of geotropism after removal of the root cap. J Exp Bot 1974; 25:1137-46; https://doi.org/10.1093/jxb/25.6.1137
- Wilkins H, Wain RL. The role of the root cap in the response of the primary roots of Zea mays L. seedlings to white light and to gravity. Planta 1975; 123:217-22; PMID:24435121; https://doi.org/10.1007/BF00390700
- Mancuso S, Barlow PW, Volkmann D, Baluška F. Actin turnover-mediated gravity response in maize root apices: gravitropism of decapped roots implicates gravisensing outside of the root cap. Plant Signal Behav 2009; 1:52-8; https://doi.org/10.4161/psb.1.2.2432
- Baluška F, Mancuso S, Volkmann D, Barlow PW. The 'root-brain' hypothesis of Charles and Francis Darwin: Revival after more than 125 years. Plant Signal Behav 2009; 4:1121-7; PMID:20514226; https://doi.org/10.4161/psb.4.12.10574 10.4161/psb.4.6.8870
- Paque S, Weijers D. Auxin: the plant molecule that influences almost anything. BMC Biol 2016; 14:67; PMID:27510039; https://doi.org/10.1186/s12915-016-0291-0
- Woodward AW, Bartel B. Auxin: regulation, action, and interaction. Ann Bot 2005; 95:707-35; PMID:15749753; https://doi.org/10.1093/aob/mci083
- Cande WZ, Ray PM. Nature of cell-to-cell transfer of auxin in polar transport. Planta 1976; 129:43-52; PMID:24430814; https://doi.org/10.1007/BF00390912
- Drake G, Carr DJ. Plasmodesmata, tropisms, and auxin transport. J Exp Bot 1978; 28:1309-18; https://doi.org/10.1093/jxb/29.6.1309
- Simon S, Petrášek J. Why plants need more than one type of auxin. Plant Sci 2011; 180:454-60; PMID:21421392; https://doi.org/10.1016/j.plantsci.2010.12.007
- Han X, Hyun TK, Zhang M, Kumar R, Koh EJ, Kang BH, Lucas WJ, Kim JY. Auxin-callose-mediated plasmodesmal gating is essential for tropic auxin gradient formation and signaling. Dev Cell 2014; 28:132-46; PMID:24480642; https://doi.org/10.1016/j.devcel.2013.12.008
- Enders TA, Strader LC. Auxin activity: Past, present, and future. Am J Bot 2015; 102:180-96; PMID:25667071; https://doi.org/10.3732/ajb.1400285
- Lomax TL, Mehlhorn RJ, Briggs WR. Active auxin uptake by zucchini membrane vesicles: quantitation using ESR volume and delta pH determinations. Proc Natl Acad Sci USA 1985; 82:6541-5; PMID:2995970; https://doi.org/10.1073/pnas.82.19.6541
- Schlicht M, Strnad M, Scanlon MJ, Mancuso S, Hochholdinger F, Palme K, Volkmann D, Menzel D, Baluška F. Auxin immunolocalization implicates vesicular neurotransmitter-like mode of polar auxin transport in root apices. Plant Signal Behav 2006; 1:122-33; PMID:19521492; https://doi.org/10.4161/psb.1.3.2759
- Šamaj J, Baluška F, Voigt B, Schlicht M, Volkmann D, Menzel M. Endocytosis, actin cytoskeleton, and signaling. Plant Physiol 2004; 135:1150-61; PMID:15266049; https://doi.org/10.1104/pp.104.040683
- Bergersen LH, Storm-Mathisen J, Gundersen V. Immunogold quantification of amino acids and proteins in complex subcellular compartments. Nat Protoc 2008; 3:144-52; PMID:18193031; https://doi.org/10.1038/nprot.2007.525
- Bergersen LH, Morland C, Ormel L, Rinholm JE, Larsson M, Wold JF, Røe AT, Stranna A, Santello M, Bouvier D, Ottersen OP, Volterra A, Gundersen V. Immunogold detection of L-glutamate and D-serine in small synaptic-like microvesicles in adult hippocampal astrocytes. Cereb Cortex 2012; 22:1690-7; PMID:21914633; https://doi.org/10.1093/cercor/bhr254
- Mancuso S, Marras AM, Mugnai S, Schlicht M, Zársky V, Li G, Song L, Xue HW, Baluška F. Phospholipase dzeta2 drives vesicular secretion of auxin for its polar cell-cell transport in the transition zone of the root apex. Plant Signal Behav 2007; 2:240-4; PMID:19516994; https://doi.org/10.4161/psb.2.4.4566
- Krecek P, Skupa P, Libus J, Naramoto S, Tejos R, Friml J, Zazímalová E. The PIN-FORMED (PIN) protein family of auxin transporters. Genome Biol 2009; 10:249; PMID:20053306; https://doi.org/10.1186/gb-2009-10-12-249
- Rakusová H, Fendrych M, Friml J. Intracellular trafficking and PIN-mediated cell polarity during tropic responses in plants. Curr Opin Plant Biol 2015; 23:116-23; PMID:25553419; https://doi.org/10.1016/j.pbi.2014.12.002
- Adamowski M, Friml J. PIN-dependent auxin transport: action, regulation, and evolution. Plant Cell 2016; 27:20-32; https://doi.org/10.1105/tpc.114.134874
- Vanneste S, Friml J. Auxin: a trigger for change in plant development. Cell 2009; 136:1005-16; PMID:19303845; https://doi.org/10.1016/j.cell.2009.03.001
- Petrášek J, Friml J. Auxin transport routes in plant development. Development 2009: 136:2675-88; PMID:19633168; https://doi.org/10.1242/dev.030353
- Paciorek T, Friml J. Auxin signaling, J Cell Sci 2006; 119, 1199-202.
- Merks RM, Van de Peer Y, Inzé D, Beemster GT. Canalization without flux sensors: a traveling-wave hypothesis. Trends Plant Sci 2007; 12:384-90; PMID:17765595; https://doi.org/10.1016/j.tplants.2007.08.004
- Baluška F, Schlicht M, Volkmann D, Mancuso S. Vesicular secretion of auxin: Evidences and implications. Plant Signal Behav 2008; 3:254-6; PMID:19704646; https://doi.org/10.4161/psb.3.4.5183
- Baluška F, Mancuso S. Root apex transition zone as oscillatory zone. Front Plant Sci 2013; 4:354; PMID:24106493; https://doi.org/10.3389/fpls.2013.00354
- Geldner N, Friml J, Stierhof YD, Jurgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 2001;413:425-8; PMID:11574889; https://doi.org/10.1038/35096571
- Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 2002;415:806-9; PMID:11845211; https://doi.org/10.1038/415806a
- Dhonukshe P, Grigoriev I, Fischer R, Tominaga M, Robinson DG, Hasek J, Paciorek T, Petrasek J, Seifertova D, Tejos R, Meisel LA, Zazimalova E, Gadella TW, Jr, Stierhof YD, Ueda T, Oiwa K, Akhmanova A, Brock R, Spang A, Friml J. Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proc Natl Acad Sci USA 2008;105:4489-94; PMID:18337510; https://doi.org/10.1073/pnas.0711414105
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jürgens G. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 2003; 112:219-30; PMID:12553910; https://doi.org/10.1016/S0092-8674(03)00003-5
- Toyota M, Furuichi T, Tatsumi H, Sokabe M. Critical consideration on the relationship between auxin transport and calcium transients in gravity perception of Arabidopsis seedlings. Plant Signal Behav 2008; 3:521-4; PMID:19513245; https://doi.org/10.4161/psb.3.8.6339
- Liu JT, Hu LS, Liu YL, Chen RS, Cheng Z, Chen SJ, Amatore C, Huang WH, Huo KF. Real-time monitoring of auxin vesicular exocytotic efflux from single plant protoplasts by amperometry at microelectrodes decorated with nanowires. Angew Chem Int Ed Engl 2014; 53:2643-7; PMID:24482020; https://doi.org/10.1002/anie.201408226 10.1002/anie.201408153 10.1002/anie.201406258 10.1002/anie.201406764 10.1002/anie.201308972 10.1002/anie.201408298 10.1002/anie.201403463 10.1002/anie.201403998 10.1002/anie.201404197 10.1002/anie.201404643 10.1002/anie.201407799 10.1002/anie.201408795 10.1002/anie.201408493 10.1002/anie.201408269 10.1002/anie.201408927 10.1002/anie.201408896
- Wang W, Zhang SH, Li LM, Wang ZL, Cheng JK, Huang WH. Monitoring of vesicular exocytosis from single cells using micrometer and nanometer-sized electrochemical sensors. Anal Bioanal Chem 2009; 394:17-32; PMID:19274456; https://doi.org/10.1007/s00216-009-2703-2 10.1007/s00216-009-2832-7 10.1007/s00216-009-2759-z
- Li YT, Zhang SH, Wang L, Xiao RR, Liu W, Zhang XW, Zhou Z, Amatore C, Huang WH. Nanoelectrode for amperometric monitoring of individual vesicular exocytosis inside single synapses. Angew Chem Int Ed Engl 2014; 53:12456-60; PMID:25060546; https://doi.org/10.1002/anie.201409314 10.1002/anie.201403817 10.1002/anie.201404320 10.1002/anie.201407225 10.1002/anie.201407000 10.1002/anie.201407211 10.1002/anie.201405779 10.1002/anie.201406554 10.1002/anie.201408757 10.1002/anie.201406180 10.1002/anie.201408265 10.1002/anie.201405058 10.1002/anie.201407935 10.1002/anie.201405178 10.1002/anie.201408754
- Li YT, Zhang SH, Wang XY, Zhang XW, Oleinick AI, Svir I, Amatore C, Huang WH. Real-time monitoring of discrete synaptic release events and excitatory potentials within self-reconstructed neuromuscular junctions. Angew Chem Int Ed Engl 2015; 54:9313-8; PMID:26079517; https://doi.org/10.1002/anie.201507157 10.1002/anie.201505242 10.1002/anie.201584261 10.1002/anie.201507272 10.1002/anie.201506972 10.1002/anie.201505192 10.1002/anie.201505232 10.1002/anie.201507608 10.1002/anie.201584461 10.1002/anie.201507176 10.1002/anie.201505278 10.1002/anie.201505329 10.1002/anie.201506458 10.1002/anie.201505064 10.1002/anie.201503801 10.1002/anie.201505025
- Lewis DR, Olex AL, Lundy SR, Turkett WH, Fetrow JS, Muday GK. A kinetic analysis of the auxin transcriptome reveals cell wall remodeling proteins that modulate lateral root development in Arabidopsis. Plant Cell 2013; 25:3329-46; PMID:24045021; https://doi.org/10.1105/tpc.113.114868
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, Scheres B. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 1999; 99:463-72; PMID:10589675; https://doi.org/10.1016/S0092-8674(00)81535-4
- Mähönen AP, ten Tusscher K, Siligato R, Smetana O, Díaz-Triviño S, Salojärvi J, Wachsman G, Prasad K, Heidstra R, Scheres B. PLETHORA gradient formation mechanism separates auxin responses. Nature 2014; 515:125-9; PMID:25156253; https://doi.org/10.1038/nature13663
- Santuari L, Sanchez-Perez GF, Luijten M, Rutjens B, Terpstra I, Berke L, Gorte M, Prasad K, Bao D, et al. The PLETHORA gene regulatory network guides growth and cell differentiation in Arabidopsis roots. Plant Cell 2016: In press; PMID:27920338; https://doi.org/10.1105/tpc.16.00656
- Grebe M. Growth by auxin: when a weed needs acid. Science 2005; 310:60-1; PMID:16210521; https://doi.org/10.1126/science.1119735
- Li J, Yang H, Peer WA, Richter G, Blakeslee J, Bandyopadhyay A, Titapiwantakun B, Undurraga S, Khodakovskaya M, Richards EL, Krizek B, Murphy AS, Gilroy S, Gaxiola R. Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science 2005; 310:121-5; PMID:16210544; https://doi.org/10.1126/science.1115711 10.1126/science.1118391
- Yang H, Zhang X, Gaxiola RA, Xu G, Peer WA, Murphy AS. Over-expression of the Arabidopsis proton-pyrophosphatase AVP1 enhances transplant survival, root mass, and fruit development under limiting phosphorus conditions. J Exp Bot 2014; 65:3045-53; PMID:24723407; https://doi.org/10.1093/jxb/eru139 10.1093/jxb/eru149 10.1093/jxb/eru259
- Ratajczak R, Hinz G, Robinson DG. Localization of pyrophosphatase in membranes of cauliflower inflorescence cells. Planta 1999; 208: 205-11; PMID:10333584; https://doi.org/10.1007/s004250050551
- Mitsuda N, Enami K, Nakata M, Takeyasu K, Sato MH. Novel type Arabidopsis thaliana H+-PPase is localized to the Golgi apparatus. FEBS Letts 2001; 488:29-33; https://doi.org/10.1016/S0014-5793(00)02400-5
- Kriegel A, Andrés Z, Medzihradszky A, Krüger F, Scholl S, Delang S, Patir-Nebioglu MG, Gute G, Yang H, Murphy AS, Peer WA, Pfeiffer A, Krebs M, Lohmann JU, Schumacher K. Job sharing in the endomembrane system: vacuolar acidification requires the combined activity of V-ATPase and V-PPase. Plant Cell 2015; 27:3383-96; PMID:26589552; https://doi.org/10.1105/tpc.15.00733
- Schilling RK, Tester M, Marschner P, Plett DC, Roy SJ. AVP1: one protein, many roles. Trends Plant Sci 2016; In press; PMID:27989652
- Steinmann T, Geldner N, Grebe M, Mangold S, Jackson CL, Paris S, Gälweiler L, Palme K, Jürgens G. Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 1999; 286:316-8; PMID:10514379; https://doi.org/10.1126/science.286.5438.316
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jürgens G. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 2003; 112:219-30; PMID:12553910; https://doi.org/10.1016/S0092-8674(03)00003-5
- Geldner N, Richter S, Vieten A, Marquardt S, Torres-Ruiz RA, Mayer U, Jürgens G. Partial loss-of-function alleles reveal a role for GNOM in auxin transport-related, post-embryonic development of Arabidopsis. Development 2004; 131:389-400; PMID:14681187; https://doi.org/10.1242/dev.00926
- Baluška F, Hlavacka A, Šamaj J, Palme K, Robinson DG, Matoh T, McCurdy DW, Menzel D, Volkmann D. F-actin-dependent endocytosis of cell wall pectins in meristematic root cells. Insights from brefeldin A-induced compartments. Plant Physiol 2002; 130:422-31.
- Baluška F, Liners A, Hlavacka A, Schlicht M, van Custem P, McCurdy DW, Menzel D. Cell wall pectins and xyloglucans are internalized into dividing root cells and accumulate within cell plates during cytokinesis. Protoplasma 2005; 225:141-55; PMID:16228896; https://doi.org/10.1007/s00709-005-0095-5
- Mancuso S, Marras AM, Magnus V, Baluska F. Noninvasive and continuous recordings of auxin fluxes in intact root apex with a carbon nanotube-modified and self-referencing microelectrode. Anal Biochem 2005; 341:344-51; PMID:15907881; https://doi.org/10.1016/j.ab.2005.03.054
- Kollmeier M, Felle HH, Horst WJ. Genotypical differences in aluminum resistance of maize are expressed in the distal part of the transition zone. Is reduced basipetal auxin flow involved in inhibition of root elongation by aluminum? Plant Physiol 2000; 122:945-56; PMID:10712559
- Shen H, Hou NY, Schlicht M, Wan YL, Mancuso S, Baluška F. 2008. Aluminium toxicity targets PIN2 in Arabidopsis root apices: effects on PIN2 endocytosis, vesicular recycling, and polar auxin transport. Chin Sci Bull 2008; 53:2480-7; https://doi.org/10.1007/s11434-008-0143-6 10.1007/s11434-008-0107-x
- Amenós M, Corrales I, Poschenrieder C, Illés P, Baluska F, Barceló J. Different effects of aluminum on the actin cytoskeleton and brefeldin A-sensitive vesicle recycling in root apex cells of two maize varieties differing in root elongation rate and aluminum tolerance. Plant Cell Physiol 2009; 50:528-40. J Exp Bot 2006; 57:4201-13; PMID:19176573; https://doi.org/10.1093/pcp/pcp013
- Illés P, Schlicht M, Pavlovkin J, Lichtscheidl I, Baluška F, Ovecka M. Aluminium toxicity in plants: internalization of aluminium into cells of the transition zone in Arabidopsis root apices related to changes in plasma membrane potential, endosomal behaviour, and nitric oxide production. J Exp Bot 2006; 57:4201-13; PMID:17085753; https://doi.org/10.1093/jxb/erl197
- Yang ZB, Geng X, He C, Zhang F, Wang R, Horst WJ, Ding Z. TAA1-regulated local auxin biosynthesis in the root-apex transition zone mediates the aluminum-induced inhibition of root growth in Arabidopsis. Plant Cell 2014; 26:2889-904; PMID:25052716; https://doi.org/10.1105/tpc.114.127993 10.1105/tpc.114.124867 10.1105/tpc.114.133769 10.1105/tpc.114.129965
- Liu G, Gao S, Tian H, Wu W, Robert HS, Ding Z. Local transcriptional control of YUCCA regulates auxin promoted root-growth inhibition in response to aluminium stress in Arabidopsis. PLoS Genet 2016; 12:e1006360; PMID:27716807; https://doi.org/10.1371/journal.pgen.1006085 10.1371/journal.pgen.1006373 10.1371/journal.pgen.1005742 10.1371/journal.pgen.1006460 10.1371/journal.pgen.1005818 10.1371/journal.pgen.1005767 10.1371/journal.pgen.1006360 10.1371/journal.pgen.1006026 10.1371/journal.pgen.1006083
- Leyser O. Auxin, self-organisation, and the colonial nature of pants. Curr Biol 2011; 21:R331-7; PMID:21549955; https://doi.org/10.1016/j.cub.2011.02.031
- Bennett T, Hines G, van Rongen M, Waldie T, Sawchuk MG, Scarpella E, Ljung K, Leyser O. Connective auxin transport in the shoot facilitates communication between shoot apices. PLoS Biol 2016; 14:e1002446; PMID:27119525; https://doi.org/10.1371/journal.pbio.1002446
- Baluška F, Šamaj J, Menzel D. Polar transport of auxin: carrier-mediated flux across the plasma membrane or neurotransmitter-like secretion? Trends Cell Biol 2003: 13:282-5; PMID:12791291; https://doi.org/10.1016/S0962-8924(03)00084-9
- Baluška F, Volkmann D, Menzel D. Plant synapses: actin-based domains for cell-to-cell communication. Trends Plant Sci 2005; 10:106-11; PMID:15749467; https://doi.org/10.1016/j.tplants.2005.07.004 10.1016/j.tplants.2005.01.002
- Bennett T, Hines G, Leyser O. Canalization: what the flux? Trends Genet 2014; 30:41-8; PMID:24296041; https://doi.org/10.1016/j.tig.2013.11.001
- Boot KJM, Libbenga KR, Hille SC, Offringa R, van Duijn B. Polar auxin transport: an early invention. J Exp Bot 2012; 63:4213-8; PMID:22473986; https://doi.org/10.1093/jxb/ers106
- Peremyslov VV, Morgun EA, Kurth EG, Makarova KS, Koonin EV, Dolja VV. Identification of myosin XI receptors in Arabidopsis defines a distinct class of transport vesicles. Plant Cell 2013; 25: 3022-3038; PMID:23995081; https://doi.org/10.1105/tpc.113.113704
- Kurtha EG, Peremyslova VV, Turnera HL, Makarova KS, Iranzo J, Mekhedov SL, Koonin EV, Dolja VV. Myosin-driven transport network in plants. Proc Natl Acad Sci USA 2017; 114:E1385-E1394; PMID:28096376; https://doi.org/10.1073/pnas.1620577114
- Drdová EJ, Synek L, Pečenková T, Hála M, Kulich I, Fowler JE, Murphy AS, Zárský V. The exocyst complex contributes to PIN auxin efflux carrier recycling and polar auxin transport in Arabidopsis. Plant J 2013; 73:709-19.
- Tan X, Feng Y, Liu Y, Bao Y. Mutations in exocyst complex subunit SEC6 gene impaired polar auxin transport and PIN protein recycling in Arabidopsis primary root. Plant Sci 2016; 250:97-104.
- Stefano G, Renna L, Lai Y, Slabaugh E, Mannino N, Buono RA, Otegui MS, Brandizzi F. ER network homeostasis is critical for plant endosome streaming and endocytosis. Cell Discov 2015; 1:15033; PMID:27462431; https://doi.org/10.1038/celldisc.2015.33 10.1038/cddiscovery.2015.33
- Wang HY, Lee MM, Schiefelbein JW. Regulation of the cell expansion gene RHD3 during Arabidopsis development. Plant Physiol 2002; 129: 638-649; PMID:12068108; https://doi.org/10.1104/pp.002675
- Dhonukshe P, Baluska F, Schlicht M, Hlavacka A, Samaj J, Friml J, Gadella TW Jr.. Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Dev Cell 2006; 10:137-150; PMID:16399085; https://doi.org/10.1016/j.devcel.2005.11.015
- Müller J, Beck M, Mettbach U, Komis G, Hause G, Menzel D, Šamaj J. Arabidopsis MPK6 is involved in cell division plane control during early root development, and localizes to the pre-prophase band, phragmoplast, trans-Golgi network and plasma membrane. Plant J 2010; 61:234-48; PMID:19832943; https://doi.org/10.1111/j.1365-313X.2009.04046.x
- Li R, Liu P, Wan Y, Chen T, Wang Q, Mettbach U, Baluška F, Šamaj J, Fang X, Lucas WJ, Lin J. A membrane microdomain-associated protein, Arabidopsis Flot1, is involved in a clathrin-independent endocytic pathway and is required for seedling development. Plant Cell 2012; 24:2105-22; PMID:22589463; https://doi.org/10.1105/tpc.112.104232 10.1105/tpc.112.105106 10.1105/tpc.112.103945 10.1105/tpc.111.094748 10.1105/tpc.112.095695
- Nishimura T, Toyooka K, Sato M, Matsumoto S, Lucas MM, Strnad M, Baluska F, Koshiba T. Immunohistochemical observation of indole-3-acetic acid at the IAA synthetic maize coleoptile tips. Plant Signal Behav 2011; 6:2013-22; PMID:22112455; https://doi.org/10.4161/psb.6.12.18080
