Abstract
Dithiocarbamates (DTCs) belong to a group of compounds used as fungicides in food production and can be divided into three major groups. Since DTCs easily oxidise and hydrolyse in alkaline and acidic medium respectively, precautions have to be implemented during preparation/homogenisation and extraction of samples. As such, test samples are commonly prepared individually by cutting into small pieces just before the digestion of DTCs with a hot acid to give carbon disulphide (CS2) and the results are expressed as CS2 without any differentiation of individual DTCs. However, individual DTCs have different toxicological potencies whilst their metabolites are more toxic than the parent compound. Apart from the hot digestion method, chromatographic separation of three major groups of DTCs has been developed by a number of different researchers. This review provides a comprehensive examination of sample preparation, extraction, clean-up and chromatographic methods for the determination of individual DTCs and their more toxic metabolites in foodstuffs. Moreover, this review also studies on how dietary exposure of DTCs can be efficiently and effectively estimated using different methods of analysis.
Introduction
Dithiocarbamates (DTCs) have been widely used as fungicides in food production for many years due to their broad spectrum of activity. However, owing to their low stability and poor solubility in water and organic solvents, analysis of DTCs in food is challenging. There are two common analytical approaches: (i) hot acid digestion of DTCs to carbon disulphide (CS2) and then analysing CS2 by gas chromatography, and (ii) alkaline extraction with stabiliser and then analysing by liquid chromatography (LC). However, the first approach cannot distinguish parent DTC(s) and induces uncertainty in dietary exposure assessment as individual DTCs have different toxicological potencies. Moreover, a number of metabolites of DTCs are more toxic than their parent compound. Therefore, there is a need to develop LC methods for simultaneous analysis of individual DTCs and their metabolites.
This review aims to provide an update of current knowledge of analysis of DTCs by LC methods and discuss their advantages and disadvantages, including method performance. The pros- and cons- of analysing dimethyldithiocarbamate (DMDTC), ethylenebisdithiocarbamate (EBDTC), propylenebisdithiocarbamate (PBDTC), etc. for dietary exposure assessment are discussed. Moreover, gaps in the knowledge are identified and recommendations made for further research to develop suitable methods.
Characteristics of DTCs and their metabolites
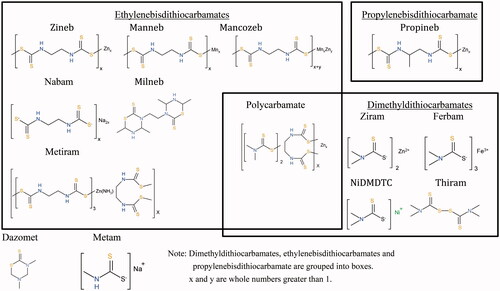
DTCs are a group of commonly used fungicides that include a number of different compounds, including ferbam, thiram, ziram, mancozeb, maneb, nabam, zineb, propineb, dazomet, metam sodium, milneb, metiram and polycarbamate (). Ferbam and ziram are iron and zinc salts of DMDTC respectively while thiram is an oxidised dimer of DMDTC. Maneb, nabam, and zineb are manganese, sodium and zinc salts of EBDTC respectively. Mancozeb is a combination of maneb and zineb while metiram is a common name for zinc ammoniate EBDTC—poly(ethylenebisthiuramdisulphide). Metam sodium and propineb are sodium salts of methyldithiocarbamate (MDTC) and zinc salts of PBDTC respectively. Dazomet and milneb (thiadiazin) are DTC fungicides in the form of heterocyclic six membered ring(s) of methylene DTC and EBDTC respectively. Polycarbamate is a polymeric zinc salt of DMDTC and EBDTC.
Ethylene thiourea (ETU) and propylene thiourea (PTU) are metabolites of EBDTCs and PBDTC, respectively. Both ETU and PTU have been shown to be considerably more toxic than their parent compounds and are DTC metabolites of concern due to their known thyroid toxicity (IPCS Citation1988). Similarly, methyl isothiocyanate (MITC) is the metabolite of metam and dazomet, which is also more toxic than the parent compounds. Although N,N′-dimethylthiourea (DMTU) is an impurity of metam and dazomet, it may be present after their uses. The European Food Safety Authority (EFSA Citation2019) in its Opinion stated that DMTU should be regarded as toxicologically relevant to metam and dazomet.
Amongst the above mentioned DTCs, only dazomet, metam and salt of DMDTCs are soluble in organic solvents while other DTCs are polymeric in nature and practically insoluble. As such, DTCs residues on plant-based samples cannot be extracted by organic solvent(s). Moreover, Heise et al. (Citation2000) first showed the decomposition of thiram in aqueous solution, increased by 50%–75% within 20 min, in the presence of cut pieces of apple, cucumber or celeriac. The decomposition rate was demonstrated to be predominantly influenced by the section surface area of the cut fruit and vegetables. Denaturing reaction conditions (exchange of the solvent water by methanol; boiling of sample material) significantly slowed down the decomposition rate. Furthermore, the influences of light, acid and organic solvent could be excluded as a cause of the reductions of thiram concentrations in the presence of the sample material. They concluded that thiram decomposition was caused by enzymes on the cut surface of the fruit and vegetable samples and contact of the surface (peel) with the fleshy tissue or juice should be strictly avoided. For the determination of thiram, a simple rinsing of the intact fruit and vegetable sample was appropriate as a method. DTCs have low stability in the presence of plant matrix, especially in the presence of acidic plant juices, since DTCs rapidly decompose into CS2 and the respective amine. Thus, plant-based samples should only be cut into small pieces before extraction instead of homogenisation.
Toxicity of DTCs and their metabolites
The Joint FAO/WHO Meeting on Pesticide Residues (JMPR) has established Acceptable Daily Intake (ADI) values for different DTCs based on their thyroid toxicity and these are summarised in . Some DTCs are considered as a group but some are considered individually, for example, a group ADI for ferbam and ziram but a separate ADI for thiram. Besides, ADIs have also been allocated to the thyroid toxicity of their common metabolites, ETU and PTU. EFSA re-assessed ADIs of individual DTCs after considering new toxicological data acquired since 2010. Most of the ADIs established by EFSA () are different from those of the JMPR.
Table 1. Established ADI of DTCs and their metabolites.
Issues concerning the hot acid digestion (CS2) method
The hot acid digestion method has been adopted by many to become the most commonly used method for quantification of DTCs in plant-based foods. Crnogorac and Schwack (Citation2009) have provided a comprehensive review in this area. They made reference to an article of Perz et al. (Citation2000), which showed that hot acid digestion methods generally face a big problem when crops of phytogenic CS2 sources are analysed, especially members of the Brassica or Carica family (e.g. broccoli, cabbage, cauliflower or papaya) as they are known to produce glucosinolates and mustard oils, which are responsible for a false-positive CS2 formation during hot acid hydrolysis. Arslan et al. (Citation2019) reported that the sulphurisation process, which is allowed to be used for different apricot products, can also lead to false positive detection of CS2 owing to the effect of residual sulphur or sulphur compounds. However, most reported dietary exposure studies on DTCs have employed the hot acid digestion method for the analysis of DTCs.
Food Standards Australia and New Zealand (FSANZ, Citation2001) reported in its total diet study (TDS) for Australia that the hot acid digestion method was used to analyse DTC in crops, such as Brassica and onions, which produce CS2 naturally under the acid digestion conditions. Even so, it was assumed that all CS2 generated by acid digestion was from DTCs and can therefore lead to an overestimation of the dietary exposure for DTCs. Similarly, Ministry for Primary Industries (MPI, Citation2018) also reported for New Zealand that some commodities, such as brassica vegetables (cabbage, broccoli, cauliflower), contain natural compounds, which can produce CS2 under the conditions used for DTC analysis and can lead to an overestimation of the DTC content of these products. DTCs detected on cabbage accounted for ∼28%–40% of the estimated exposure to DTC fungicides in the 2016 New Zealand TDS among adult populations.
In contrast, Jensen et al. (Citation2008) reported an exposure assessment study for the Danish populations to DTC residues based on the data obtained from Danish pesticide monitoring programme with exclusion of brassica vegetables, as these are known to give false-positives by the hot acid digestion method. Having considered that most commodities with detectable DTC residues can be eaten raw (without processing), except for melon, which is peeled, no processing factors for cooking and peeling were applied in the exposure assessment.
Analysis of individual DTCs by chromatographic methods
As the hot acid digestion method cannot differentiate residues of individual group of DTCs, LC methods were established for the analysis of DMDTC, EBDTC, PBDTC, etc. Gustafsson and Fahlgren (Citation1983) first reported the determination of DTCs in vegetables by LC-ultraviolet (UV) detection. Crnogorac and Schwack (Citation2009) have reviewed those LC methods published previously. On the other hand, Nakamura et al. (Citation2010) first showed DMDTC, EBDTC, PBDTC and milneb can be analysed separately by gas chromatography-mass spectrometry after extraction with alkaline ethylenediaminetetraacetic acid (EDTA)/cysteine and then derivatisation of the DTCs to their methyl esters with iodomethane.
Sample preparation
Owing to rapid decomposition of DTCs, samples are normally cut into small pieces just before extraction. In 2011, France reported their results on dietary exposure of DTCs in the second French TDS. They claimed that the absence of detection in composite samples of fruit and vegetables as consumed did not appear to be involved in any loss of DTC residues, but could be due to a ‘dilution’ of potential residues related to the TDS sample (where a number of samples are pooled in a form of composite samples) and the particularly high analytical limits for fruit and vegetables (limit of detections (LODs) ranging from 0.025 to 0.2 mg/kg). First, the sub-samples were ground in the presence of liquid nitrogen to avoid a loss of DTCs by enzymatic degradation before the analysis. Second, the samples were not thawed before the analysis. However, before cryogenic milling, each sub-sample underwent single cutting and rapid mixing. Although this single cutting may contribute to DTC loss at ambient temperature, it generally reflects the culinary practice of cutting foods before consumption, which is consistent with the very objective of the TDS.
Roussev et al. (Citation2019) reported that analysis of DTCs as CS2 has shown a significant increase of thiram recoveries (up to 95%) when using liquid nitrogen during sample comminution. They also mentioned that the practical use of liquid nitrogen among working laboratories not only improved sample processing in the routine monitoring of pesticide residues in foods, but also gave promise for feasible test sample size reduction in high-throughput miniaturised methods.
No matter whether an individual or composite sample approach is adopted, sample comminution using liquid nitrogen has become the preferred practice for analysing DTCs with reference to above-mentioned studies.
Extraction of DTCs from foodstuffs
Pflugmacher and Ebing (Citation1980) first used aqueous EDTA solution for sample-surface extraction of EBDTCs (maneb, zineb, mancozeb) and PBDTC (propineb) from fruit and vegetables and then separated the analytes by a gel-permeation chromatography method (Sephadex LH-20 column) with 0.1 M aqueous EDTA as eluent. The main function of the EDTA is chelating the metal ion(s) of EBDTCs and PBDTC so that both EBDTC and PBDTC exist as doubly charged anions. This method was subsequently adopted as an official method by the German Research Foundation for the analysis of EBDTC and PBDTC fungicides (DFG Citation1987). For different crops, a limit of quantification (LOQ) of 0.05 mg/kg was reported except for samples of lettuce with a higher LOQ of 0.5 mg/kg. The mean recoveries were 78%–106% after spiking of samples in the range 0.25–2.0 mg/kg.
Perz and Schwack (Citation2003) first added sulphite and cysteine to stabilise DTCs in the alkaline (sodium hydrogen carbonate) extraction buffer. Subsequently, different stabilising agents such as dl-penicillamine, l-cysteine, dithiothreitol, etc. were added to prevent degradation of EBDTC and PBDTC in alkaline extraction buffers. They are summarised in . Nakazawa et al. (Citation2004) examined the performance of different antioxidant stabilisers for DTCs including l-cysteine, dithiothreitol, mercaptoethanol, sodium bisulphite and tri (2-carboxyethyl)phosphine (TCEP). l-Cysteine, dithiothreitol and mercaptoethanol react with luminol to produce a broad tailing peak with intense chemiluminescence that interferes in the detection of DTCs on the LC-chemiluminescence detector system. Sodium bisulphite and TCEP did not affect the detection of DTCs on the chromatogram while TCEP was better than sodium bisulphite in reducing the decomposition of DTCs. Furthermore, Nakazawa et al. (Citation2004) mentioned using TCEP as the antioxidant can prevent the decomposition of DTCs.
Table 2. Summary of method performance of liquid chromatographic methods for analysing DTCs published from 2000 to 2020.
After alkaline buffer extraction with EDTA and l-cysteine, Gustafsson and Fahlgren (Citation1983) converted DTC anions into ion pairs of tetrabutylammonium and S-alkylated with iodomethane in one step at room temperature. The methylated DMDTC and EBDTC were analysed by LC with UV detection at 272 nm. In 2008, both Brewin et al. (Citation2008) and Hayama and Takada (Citation2008) reported similar derivatisation of EBDTCs but using dimethyl sulphate instead of iodomethane. Their differences are mainly relied on the clean-up of methyl ester of EBDTC.
Clean-up of extract
Amongst the reported LC-UV studies, none of them included a clean-up step. It is expected that the matrix effect was not significant and there was no closely eluted peak around the analyte(s). On the other hand, assessment of the matrix effect is a must for all LC-MS studies. Hence, some of the reported studies introduced a clean-up step with solid-phase extraction (SPE) or dispersive SPE (dSPE). Different sorbents have been used, including, C18, primary and secondary amine (PSA) and graphitised carbon black, and the selection depends on the food matrix under study. Of course, clean-up with an SPE cartridge provides better clean-up efficiency but is relatively time-consuming. If the matrix effect can be reduced to an acceptable level, dSPE would be the choice. de Silva et al. (Citation2021) recently evaluated the dSPE clean-up efficiencies of PSA, non-end-capped octadecyl (C18-OH), C18, silica, aluminium oxide, and Florisil, for extraction of soybean by LC coupled to diode-array detector and an evaporative light-scattering detector. They concluded that silica was an effective and cheap sorbent to remove co-extracted matrix components. However, soybean does not necessarily represent other food matrices and thus researchers should evaluate sorbents with respect to their scope of matrices.
Liquid chromatographic separation of DTCs
Bardarov et al. (Citation1989) first applied a strong anion exchange column to analyse EBDTC and PBDTC. Even though pH and strength of perchlorate were studied, EBDTC and PBDTC could not be baseline separated owing to similar charge, mass and configuration.van Lishaut and Schwack (Citation2000) first demonstrated that all three DTC sub-classes [i.e. DMDTCs (ziram, ferbam), EBDTCs (maneb, zineb, mancozeb), and PBDTC (propineb)] can be baseline separated by an ion-pair LC system with UV and electrochemical (EC) detection. On the other hand, Blasco et al. (Citation2004) showed two neutral DTCs, that is, dazomet and thiram, and two metabolites, that is, ETU and PTU, can be analysed by a reversed phase LC system. The targeted analytes did not require derivatisation by the above-mentioned systems.
Kim et al. (Citation2010) first reported an LC system that can analyse DMDTC, EBDTC, PBDTC, ETU and PTU simultaneously in fruit, vegetables and cereals. Kakitani et al. (Citation2017) reported a reversed phase LC system that can analyse DMDTC, EBDTC, PBDTC, milneb, metiram and thiram. Around the same time, Al-Alam (Citation2017) reported another reverse phase LC system that could analyse DMDTC, EBDTC, PBDTC, dazomet and metam. Both of these systems required DTCs be methylated before LC analysis.
Oellig and Schwack (Citation2017) compared the use of different brands of hydrophilic interaction chromatography (HILIC) column to separate DTCs. They reported that silica-based columns were not suitable for the sensitive analysis of DTCs owing to ion suppression by the buffer and the limited alkaline pHs. The polymer-based iHILIC-Fusion column was an alternative that offered high mass spectrometric sensitivity when a buffer containing 15 mM aqueous ammonium hydroxide and 7.5 mM ammonium hydrogen carbonate (pH 9.8) was used. However, the separation of DMDTC, EBDTC and PBDTC was poor. They concluded that the originally proposed polymer-based ZIC-pHILIC column out-performed all the tested newly available alternative HILIC columns in terms of selectivity and sensitivity.
Amongst DTCs, DMDTC, EBDTC, PBDTC, metam, thiram and dazomet have established ADIs with different values and should be quantified separately to assess the health risk from dietary exposure. As ETU and/or PTU are likely to be present if the produce was sprayed with EBDTCs and/or PBDTC, they should also be analysed to provide a better picture on the dietary exposure of DTCs. However, there is still no established method, which can perform the task. Based on established ADIs, the method developed by Al-Alam et al. (Citation2017) seems to be the best as only thiram, ETU and PTU are missing in its scope of analysis while the method of Kim et al. (Citation2010) did not include metam, thiram and dazomet.
Detection of DTCs
Amongst the reported studies, mass spectrometer (MS) and UV detection were the most commonly used detection systems. Although UV detection is a convenient and low-cost approach, it is subject to interferences and does not provide sufficient selectivity. Apart from UV, chemiluminescence, EC and amperometric detection were also reported. Nakazawa et al. (Citation2004) showed their complex post-column LC system with chemiluminescence detection can detect EBDTCs and PBDTC down to 1 mg/kg in a sample. However, selectivity was not studied. van Lishaut and Schwack (Citation2000) showed both UV and EC detection gave comparable sensitivity. However, they concluded that MS can further confirm identity of analytes. On the other hand, Charoenkitamorn et al. (Citation2015) noted that sensitivity of the amperometric system was comparable to the UV system but more selective. Hence, MS, especially tandem MS (MS/MS), offers the best selectivity amongst the reported detection techniques.
For analysing DTCs by LC-MS, both atmospheric pressure chemical ionisation (APCI) and electrospray ionisation (ESI) have been used. Blasco et al. (Citation2004) reported that owing to the differences in sensitivity of APCI and ESI for the studied DTCs (dazomet and thiram) and their metabolites, a compromise was necessary. APCI was selected because it achieves better sensitivity for ETU and PTU whereas the sensitivity for thiram was considered sufficient. In addition, APCI is more robust and gives more reproducible spectra of DTCs without adduct formation. Similarly, Vaclavik et al. (Citation2018) reported that positive APCI mode yielded approximately six-fold higher response than optimised positive ESI mode for protonated molecules [M + H]+ of PTU. On the other hand, Hayama and Takada (Citation2008) mentioned that dimethyl-EBDTC was found to have better sensitivity for ESI than APCI. Hence, selection of APCI or ESI depends on target analytes and instrument used.
During the last two decades, the sensitivity of MS/MS increased in orders of magnitude while UV detection achieved almost no improvement. Even though UV and single quadrupole LC-MS may have similar sensitivity some decades ago, LC-MS/MS is the best choice in terms of sensitivity and selectivity nowadays. Hence, LC-MS/MS is the best instrument for the detection of DTCs if the instrumentation is affordable.
Method performance of selected LC methods for analysis of DTCs
Since there is no established standard method employing LC to analyse DTCs in foodstuffs, all published methods are considered as single-laboratory validated methods. Their performance should fulfil the requirements as stipulated in the SANTE guidance document (SANTE/11312/2021) on quality control procedures for pesticide residue analysis. However, some of the articles published a decade ago did not contain sufficient method performance characteristics as required. For illustration and ease of reference, summarises the method performance characteristics of those methods in which the scope of analysis contained at least one group of DTCs (DMDTC, EBDTCs and/or PBDTCs) and their metabolites from 2000 to 2020.
Among the method performance characteristics, spike recovery at known level with a blank matrix and its precision were mostly provided. Besides, the LOQ and/or LOD were also provided. The acceptance criteria for mean recoveries and precision as stipulated in the SANTE/11312/2021 are within the range of 70%–120% and ±20%, respectively, while LOQ is the lowest spiked level of the validation meeting those method performance acceptability criteria. As the maximum residue limits established by Codex Alimentarius for DTCs in food varied from 0.05 (eggs, milk, meat) to 30 mg/kg (dry hops) (expressed as CS2), the established LOQ for monitor of DTCs in foods should be <0.05 mg/kg. As such, some of the published method cannot fulfil the above-mentioned specified requirements for analysing DTCs in eggs, milk and meat. If the results were used for risk assessment, the guidance document SANTE/2020/12830 should also be fulfilled. By doing so, the LOQ of residue methods should be as low as required to meet the study needs.
To ensure the results of different laboratories can be compared with one another, participating in proficiency tests, such as Food Analysis Performance Assessment Scheme provided by the UK FERA Science Ltd is considered necessary.
Total diet studies on DTCs or its metabolites
Even though DTCs (analysed by the hot acid digestion method) were found for 13 foods in the 25th Australian TDS, seven of them (apple, capsicum, grapes, nectarine, strawberries, sultanas and tomatoes) were unprocessed fruit/fruiting vegetables. For the three microwave cooked foods, bok choi (a type of Chinese cabbage), broccoli and cauliflower belong to brassica vegetables and are well known to produce false positive results with the hot acid digestion method owing to the breakdown of naturally occurring substances in the plant (Perz et al. Citation2000). The remaining three foods are hamburger, grilled lamb chops and cooked prawns. It is not known why CS2 was detected in these heat-treated animal origin foods. Similarly, CS2 residues were detected in 36 food samples from 16 different food types in the 2016 New Zealand TDS. The foods with detected CS2 residues included all eight samples of cabbage and three broccoli/cauliflower samples. The other foods with detected CS2 were mostly unprocessed fruit, where the use of DTCs was the likely origin. These unprocessed fruit included strawberries (four samples), avocados and grapes (three samples each). On the other hand, the second French TDS did not report any residues of DTCs in 562 foods of plant and animal origin, except for vegetables consumed raw, that were cooked. Its report also mentioned that DTCs are among the most frequently detected active substances in unprocessed vegetables and particularly leafy vegetables in 2008 French surveillance programme. In summary, DTCs are almost likely detected in fruit if brassica vegetables are excluded from TDS food list.
For the above-mentioned TDSs, the hot acid digestion method was adopted for the analysis of DTCs, where only residues of CS2 were determined. By doing so, the risk from estimated dietary exposure was commonly characterised by applying a selected ADI amongst various ADIs of DTCs. It should be noted that the ADIs for the same DTCs may vary in different countries too. Although DTCs are authorised for use in France on fruit and vegetable crops (mancozeb, maneb, thiram) and in vineyards (propineb), the second French TDS used the lowest ADI of propineb (0.007 mg/kg body weight (bw)/day) as reference point for risk characterisation. The Australian TDS selected the lowest ADIs of thiram (0.004 mg/kg bw/day as adopted in Australia) amongst that of mancozeb, thiram, zineb and ziram. The New Zealand TDS also applied the lowest ADI for ferbam, thiram and ziram (0.003 mg/kg bw/day as adopted in New Zealand) among three different groups of ADIs. It was considered conservative in the risk characterisation by assuming any detected residues of CS2 were derived from the most toxic DTC.
Since DMDTCs can be easily degraded to CS2 and lost during food processing and no other toxic metabolites are formed, the health risk can be assessed correctly by analysing DTCs with the hot acid digestion method. On the other hand, processing of food containing EBDTCs, PBDTC and metam/dazomet can lead to formation of more toxic degradation products ETU, PTU and MITC, respectively. Hence, quantification of these DTCs by hot acid digestion method underestimates the risk from dietary exposure to DTCs, particularly to their metabolites.
In contrast to above-mentioned TDSs, Wong et al. (Citation2014) reported ETU and PTU were detected in 80 composite TDS samples (13%) while most of them had undergone heat treatment. The foods most commonly found to have detectable levels of ETU and PTU were foods of plant origin. ETU was even found in highly processed foods, such as pan-fried dumplings, potato chips, tomato paste/ketchup, beer and wine too. Similar to 2016 New Zealand TDS, ETU was also found in some fruit including mango, melons and papaya. Hence, detection of DTC metabolites can provide a better overview of usage of DTCs in food and health risk assessment than analysing CS2. Besides, false positive reports of DTCs in brassica vegetables can be avoided. Moreover, health risk can be assessed with respect to the corresponding ADI. It is suggested that the analysis of metabolites of DTCs should include MITC and DMTU if possible.
Chemicals to be analysed for assessing dietary exposure risk of DTCs
For comprehensive assessment of health risk of DTCs, both DTCs and their toxic metabolites with established ADIs should be analysed, including metam, DMDTC, thiram, EBDTC, PBDTC, dazomet, ETU, PTU, MITC and DMTU. As demonstrated by Vaclavik et al. (Citation2018) PBDTC and PTU can be analysed together by alkaline extraction and then methylated by dimethyl sulphate. Hence, such extraction and derivatisation should also be applicable to metam, DMDTC, EBDTC, ETU, PTU and DMTU as they have similar structures. Thiram was demonstrated to undergo hydrolysis to give DMDTC in such systems by Jafari et al. (Citation2012) and Kakitani et al. (Citation2017). On the other hand, Al-Alam et al. (Citation2017) also showed dazomet can be separated and analysed in such systems. For the remaining metabolite, MITC, it is expected it can be analysed by such systems but needs to be demonstrated to be the case in future studies. In summary, the ideal method to be used for accessing risk from dietary exposure to DTCs is still pending. However, modification of the method developed by Al-Alam et al. (Citation2017) may be able to serve the purpose to an extent.
As methods of analysis for multiclass pesticide residues in food are routinely used by monitoring laboratories and Chung and Lam (Citation2012) demonstrated ETU and PTU can be analysed together with pyrethroids by LC-MS, there is no doubt that DTC metabolites can be analysed simultaneously with other classes of pesticides. Moreover, the JMPR in 1997 concluded that DTCs should be divided into two groups on the basis of toxicity. As the thyroid toxicity of DTCs is mediated by their metabolites, analysing ETU and PTU provides a cost-effective alternative for DTCs. All-in-all, DTC metabolites should be determined together with other pesticides including organophosphorus pesticides, N-methyl carbamates, pyrethroids, etc., by an LC-MS method instead of analysing CS2 by the acid digestion method. If possible, MITC and DMTU should also be analysed.
Conclusions
There is an emerging need to develop a method for quantification of individual DTCs and their toxic metabolites for dietary exposure assessment as DTCs have different toxicological potencies and some metabolites of DTCs are more toxic than the parent fungicide. Since DTCs are reactive and undergo oxidisation and hydrolysis in alkaline and acidic medium respectively, samples are commonly cut into small pieces before extraction instead of homogenisation. Recent studies have shown that comminution of sample can be performed under liquid nitrogen and good recoveries obtained. As decomposition of DTCs is caused by enzymes on the surface section of fruit and vegetables, spike recoveries of DTCs in apple, cucumber and celeriac should be demonstrated.
Sodium hydrogen carbonate buffer containing stabilising agent(s) has been demonstrated to be suitable for extraction of DTCs. Amongst various stabilising agents, Nakazawa et al. (Citation2004) showed TCEP is the best. Moreover, toxic metabolites of DTCs, ETU and PTU, can be extracted simultaneously. After extraction, DTCs can be methylated by iodomethane or dimethyl sulphate. By doing so, extraction of methylated DTCs can be cleaned-up by SPE cartridge or dispersive SPE sorbent to remove matrix compounds.
Both reversed phase and HILIC systems have been used to separate DTCs or their methylated products. However, some HILIC columns give poor separation of DTCs. Therefore, reversed phase systems seem to be more appropriate. Even though there is no reported LC system that can separate all DTCs and their metabolites, it is expected these analytes could be quantified by LC-MS without any difficulty.
To conduct cost-effective dietary exposure studies, especially TDS, DTC toxic metabolites can be simultaneously analysed with other LC-amendable pesticides. By doing so, it would only underestimate the dietary exposure of parent DTCs from fruit.
| Abbreviations | ||
| ADI | = | acceptable daily intake |
| APCI | = | atmospheric pressure chemical ionization |
| C18 | = | octadecyl |
| CS2 | = | carbon disulfide |
| DMDTC | = | dimethyldithiocarbamate |
| DMTU | = | N,N’-dimethylthiourea |
| DTC | = | dithiocarbamate |
| dSPE | = | dispersive solid-phase extraction |
| EBDTC | = | ethylenebisdithiocarbamate |
| EC | = | electrochemical |
| EDTA | = | ethylenediaminetetraacetic acid |
| EFSA | = | European Food Safety Authority |
| ESI | = | electrospray ionization |
| ETU | = | ethylene thiourea |
| FAPAS | = | Food Analysis Performance Assessment Scheme |
| GPC | = | gel permeation chromatography |
| HILIC | = | hydrophilic interaction chromatography |
| JMPR | = | The Joint Meeting on Pesticide Residues |
| LC | = | liquid chromatography |
| LOD | = | limit of detection |
| LOQ | = | limit of quantification |
| MDTC | = | methyldithiocarbamate |
| MITC | = | methyl isothiocyanate |
| MS | = | mass spectrometer |
| PBDTC | = | propylenebisdithiocarbamate |
| PSA | = | primary and secondary amine |
| PTU | = | propylene thiourea |
| SPE | = | solid-phase extraction |
| TDS | = | total diet study |
| TCEP | = | tri(2-carboxyethyl)phosphine |
| UV | = | ultraviolet |
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Al-Alam J, Bom L, Chbani A, Fajloun Z, Millet M. 2017. Analysis of dithiocarbamate fungicides in vegetable matrices using HPLC-UV followed by atomic absorption spectrometry. J Chromatogr Sci. 55(4):429–435. doi:10.1093/chromsci/bmw198
- Arslan S, Mert ID, Yiğitkaya S, Dagaşan O, Sakallı FN, Oztürk S. 2019. The false positive effect of residue of sulphur sources on dithiocarbamate analysis based on CS2 measurement. Food Addit Contam A. 36(1):131–140. doi:10.1080/19440049.2018.1562235
- Bardarov V, Zaikov C, Mitewa M. 1989. Application of high-performance liquid chromatography with spectrophotometric and electrochemical detection to the analysis of alkylenebis(dithiocarbamates) and their metabolites. J Chromatogr. 479:97–105. doi:10.1016/S0021-9673(01)83320-3
- Blasco C, Font G, Pico Y. 2004. Determination of dithiocarbamates and metabolites in plants by liquid chromatography–mass spectrometry. J Chromatogr A. 1028(2):267–276. doi:10.1016/j.chroma.2003.12.002
- Brewin S, Miller C, Khoshab A. 2008. The LC-MS/MS ethylene bisdithiocarbamate (EBDC) analytical method – a comparison with the CS2 analysis technique on wheat samples generated from field trials. 7th European Pesticide Residues Workshop (EPRW), Book of Abstracts, Berlin, Germany, 2008, p. 142.
- Charoenkitamorn K, Chailapakul O, Siangproh W. 2015. Development of gold nanoparticles modified screen-printed carbon electrode for the analysis of thiram and their derivative in food using ultra-high performance liquid chromatography. Talanta 132:416–423. doi:10.1016/j.talanta.2014.09.020
- Chung SWC, Lam CH. 2012. Development and validation of a method for determination of residues of 15 pyrethroids and two metabolites of dithiocarbamates in foods by ultra-performance liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem. 403(3):885–896. doi:10.1007/s00216-012-5882-1
- Crnogorac G, Schwack W. 2007. Determination of dithiocarbamate fungicide residues by liquid chromatography/mass spectrometry and stable isotope dilution assay. Rapid Commun Mass Spectrom. 21(24):4009–4016. doi:10.1002/rcm.3312
- Crnogorac G, Schwack W. 2009. Residue analysis of dithiocarbamate fungicides. Trends Anal Chem. 28(1):40–50. doi:10.1016/j.trac.2008.10.008
- Crnogorac G, Schwack W, Schmauder S. 2008. Trace analysis of dithiocarbamate fungicide residues on fruits and vegetables by hydrophilic interaction liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 22(16):2539–2546. doi:10.1002/rcm.3646
- de Silva RC, Wickert C, Pizzutti IR, de Kok A. 2021. Clean-up strategy for dithiocarbamate fungicide determination in soybean by GC-ITD-MS and GC-PFPD: Method development and validation. J Agric Food Chem. 69(38):11485–11493. doi:10.1021/acs.jafc.1c01870
- Deutsche Forschungsgemeinschaft (DFG). 1987. Pesticides Commission, Manual of Pesticide Residue Analysis. vol. I, S 21: Ethylene and propylene bisdithiocarbamate fungicides, VCH, Weinheim, Germany, p. 407.
- Deutsche Forschungsgemeinschaft (DFG) 1987. Pesticides Commission, Manual of Pesticide Residue Analysis, Vol. I, S 15: Dithiocarbamate and Thiuram Disulfide Fungicides, VCH, Weinheim, Germany, p. 353.
- European Food Safety Authority (EFSA). 2019. Review of the existing maximum residue levels for metam according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 17(1):5561–5576. doi:10.2903/j.efsa.2019.5561
- Food Standards Australia and New Zealand (FSANZ). 2001. The 19th Australian Total Diet Survey. [accessed 2022 Apr 18]. https://www.foodstandards.gov.au/publications/documents/19th%20ATDS.pdf.
- French agency for food, environmental and occupational health and safety (ANSES). 2011. Second French Total Diet Study Report 2. [accessed 2022 Apr 18]. https://www.anses.fr/en/system/files/PASER2006sa0361Ra2EN.pdf.
- Gustafsson KH, Fahlgren CH. 1983. Determination of dithiocarbamate fungicides in vegetable food stuffs by high-performance liquid chromatography. J Agric Food Chem. 31(2):461–463. doi:10.1021/jf00116a074
- Hayama T, Takada M. 2008. Simple and rapid method for the determination of ethylenebisdithiocarbamate fungicides in fruits and vegetables using liquid chromatography with tandem mass spectrometry. Anal Bioanal Chem. 392(5):969–976. doi:10.1007/s00216-008-2346-8
- Heise S, Weber H, Alder L. 2000. Reasons for the decomposition of the fungicide thiram during preparation of fruit and vegetable samples and consequences for residue analysis. Fresenius J Anal Chem. 366(8):851–856. doi:10.1007/s002160051584
- IPCS. 1988. Dithiocarbamate Pesticides, Ethylenethiourea and Propylenethiourea: A General Introduction. Environmental Health Criteria 78. Geneva: WHO.
- Jafari A, Shoeibi S, Amini M, Amirahmadi M, Rastegar H, Ghaffarian A, Ghazi-Khansari M. 2012. Monitoring dithiocarbamate fungicide residues in greenhouse and non-greenhouse tomatoes in Iran by HPLC-UV. Food Addit Contam Part B Surveill. 5(2):87–92. doi:10.1080/19393210.2012.657693
- Jensen BH, Andersen JH, Petersen A, Christensen T. 2008. Dietary exposure assessment of Danish consumers to dithiocarbamate residues in food: a comparison of the deterministic and probabilistic approach. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 25(6):714–721. doi:10.1080/02652030701858262
- Kakitani A, Yoshioka T, Nagatomi Y, Harayama K. 2017. A rapid and sensitive analysis of dithiocarbamate fungicides using modified QuEChERS method and liquid chromatography–tandem mass spectrometry. J Pesticide Sci. 42(4):145–150. doi:10.1584/jpestics.D17-025
- Kim HY, Choi HJ, Eom JY, Seo EC, Choi SH, Cheong SY, Choi SH, Lee HJ, Choi JC. 2010. Determination of dithiocarbamates in agricultural products circulated in Korea Korean. J Food Sci Technol. 42:1–7.
- Li J, Dong C, Yang Q, An WJ, Zheng ZT, Jiao BN. 2019. Simultaneous Determination of Ethylenebisdithiocarbamate (EBDC) and Propylenebisdithiocarbamate (PBDC) Fungicides in Vegetables, Fruits, and Mushrooms by Ultra-High-Performance Liquid Chromatography Tandem. Food Anal Methods. 12(9):2045–2055. doi:10.1007/s12161-019-01538-z
- Ministry for Primary Industries (MPI), New Zealand. 2018. 2016 New Zealand Total Diet Study. [accessed 2022 Apr 18]. https://www.mpi.govt.nz/dmsdocument/43177-2016-NZ-Total-Diet-Study-with-Appendices-report.
- Nakamura M, Noda S, Kosugi M, Ishiduka N, Mizukoshi K, Taniguchi M, Nemoto S. 2010. Determination of dithiocarbamates and milneb residues in foods by gas chromatography-mass spectrometry. Shokuhin Eiseigaku Zasshi. 51(5):213–219. doi:10.3358/shokueishi.51.213
- Nakazawa H, Tsuda Y, Ito K, Yoshimura Y, Kubo H, Homma H. 2004. Determination of dithiocarbamate fungicides by reversed-phase ion-pair liquid chromatography with chemiluminescence detection. J Liq Hromatogr Related Technol. 27(4):705–713. doi:10.1081/JLC-120028258
- Oellig C, Schwack W. 2017. Comparison of HILIC columns for residue analysis of dithiocarbamate fungicides. J Liq Chromatogr Relat Technol. 40(8):415–418. doi:10.1080/10826076.2017.1315724
- Perz RC, Schwack W. 2003. High performance ion pair chromatography as a routine-compliant tool for surveilling residues of dithiocarbamate fungicides in fruits and vegetables. Deutsche Lebensmittel-Rundschau. 99(4):137–142.
- Perz RC, van Lishaut H, Schwack W. 2000. CS2 Blinds in Brassica Crops: false positive results in the dithiocarbamate residue analysis by the acid digestion method. J Agric Food Chem. 48(3):792–796. doi:10.1021/jf9905323
- Pflugmacher J, Ebing W. 1980. A new rapid method for determination of alkylenebis-dithiocarbamate fungicide residues | [Eine neue Schnellmethode zur Bestimmung von Alkylen-bis-dithiocarbamat-Fungicid-Rückständen. Z Lebensm Unters Forch. 170(5):349–354. doi:10.1007/BF01042972
- Ringli D, Schwack W. 2013. Selective determination of thiram residues in fruit and vegetables by hydrophilic interaction LC-MS. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 30(11):1909–1917. doi:10.1080/19440049.2013.833669
- Roussev M, Lehotay SJ, Pollaehne J. 2019. Cryogenic sample processing with liquid nitrogen for effective and efficient monitoring of pesticide residues in foods and feeds. J Agric Food Chem. 67(33):9203–9209. doi:10.1021/acs.jafc.9b04006
- SANTÉ. 2021. Analytical quality control and method validation procedures for pesticide residues analysis in food and feed 11312/2021. [accessed 2022 Apr 18]. https://ec.europa.eu/food/system/files/2022-02/pesticides_mrl_guidelines_wrkdoc_2021-11312.pdf.
- Schmidt B, Christensen HB, Petersen A, Sloth JJ, Poulsen ME. 2013. Method validation and analysis of nine dithiocarbamates in fruits and vegetables by LC-MS/MS. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 30(7):1287–1298. doi:10.1080/19440049.2013.801083
- Song S, Wei J, Chen Z, Lei Y, Zhang Y, Deng C, Tan H, Li X. 2018. Determination of propineb and its metabolites propylenethiourea and propylenediamine in banana and soil using gas chromatography with flame photometric detection and LC–MS/MS analysis. J Environ Sci Health B. 53(3):153–160. doi:10.1080/03601234.2017.1399765
- Vaclavik L, Shippar JJ, Koesukwiwat U, Mastovska K. 2018. Method development and validation for low-level propineb and propylenethiourea analysis in baby food, infant formula and related matrices using liquid chromatography-tandem mass spectrometry. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 35(12):2387–2399. doi:10.1080/19440049.2018.1539529
- van Lishaut H, Schwack W. 2000. Selective trace determination of dithiocarbamate fungicides in fruits and vegetables by reversed-phase ion-pair liquid chromatography with ultraviolet and electrochemical detection. J AOAC Int. 83(3):720–727.
- Wong WWK, Yau ATC, Chung SWC, Lam C, Ma S, Ho YY, Xiao Y. 2014. Dietary exposure of Hong Kong adults to pesticide residues: results of the first Hong Kong total diet study. Food Addit Contam Part A. 31(5):852–871. doi:10.1080/19440049.2014.900573
- Zhou L, Xu J, Luan L, Ma J, Gong Y, Qin D, Pan C. 2013. Optimization and validation of a method based on derivatization with methylating agent followed by HPLC–DAD for determining dithiocarbamates residues. Acta Chromatogr. 25(4):613–625. doi:10.1556/AChrom.25.2013.4.2