Abstract
The most important cephalopod resource in the northwestern area of Mexico is the jumbo squid whose postmortem biochemical behavior has been studied in detail. Adenosine triphosphate (ATP) degradation in this organism is different than the other species because of the fast degradation of adenosine monophosphate (AMP) metabolite in mantle. In this research, AMP deaminase from jumbo squid mantle was partially characterized. The enzyme showed an optimum behavior at 50 °C, the enzyme lost more than 90% of its activity within 15 min from 55 to 60 °C, and the enzyme remained stable for 30 min from 10 to 50 °C. It was also stable within the pH range of 3.0–5.0 and exhibited an optimum activity at pH 4.5. Enzyme was strongly activated by Mg+2 and weakly activated by Ca+2. ATP was an excellent activator even in a low concentration, while adenosine diphosphate (ADP) did it at higher concentrations. These results suggest that enzyme might be regulated by the adenylate energy charge.
El calamar gigante es el cefalópodo más importante en el noroeste de México y su bioquímica posmortem ya ha sido estudiada en detalle. En este organismo la degradación de ATP es diferente respecto a otras especies ya que el metabolito AMP es degradado muy rápidamente en el manto. En la presente investigación la enzima AMP deaminasa del manto de calamar gigante fue parcialmente caracterizada. La enzima mostró un comportamiento óptimo a 50 °C, de 55 a 60 °C perdió más del 90% de su actividad en 15 min y de 10 a 50 °C se mantuvo estable por 30 min. Fue también estable en el rango de pH de 3,0 a 5,0 y mostró una actividad óptima a 4,5. La enzima fue fuertemente activada por Mg+2 y débilmente activada por Ca+2. ATP fue un excelente activador a bajas concentraciones, mientras que ADP lo fue a altas concentraciones. Los resultados sugieren que la enzima puede ser regulada por medio de la carga energética adenilada.
Palabras clave:
Introduction
Jumbo or giant squid (Dosidicus gigas) is the most important cephalopod resource in the northwestern area of Mexico where Mexican fishermen have called it “the alternative fishery”. Because of its relatively new importance as marketable sea species, Marquez-Rios, Moran-Palacio, Lugo-Sanchez, Ocano-Higuera, and Pacheco-Aguilar (2007) have studied in detail the postmortem biochemistry of this organism as well as its relation with quality parameters. Adenosine triphosphate (ATP) quantification and degradation of their products have mainly been focused on getting the K Index (KI) which is a useful parameter to determine the seafood products freshness (Ocano-Higuera, Maeda-Martínez, Lugo-Sanchez, & Pacheco-Aguilar, 2006; Saito, Arai, & Matsuyoshi, Citation1959). ATP degradation of fish during the storage on ice has been studied since the 1950s which increased during the '70s when high performance liquid chromatography (HPLC) methodology appeared making work easier (Howgate, Citation2005). The ATP total molar concentration and related compounds in the muscle, as well as rates and patterns of changes in their levels during storage are species-dependent and muscle-dependent; however, the most common behavior detected is: ATP ->ADP (adenosine diphosphate) -> AMP (adenosine monophosphate) -> IMP (inosine monophosphate) -> HxR (inosine) -> Hx (hypoxanthine). ATP in the muscle rapidly decreases, degrading into IMP or AMP within the first 24 h postmortem. The slow degradation of these metabolites causes a linear increase of K value. Marquez-Rios et al. (Citation2007) studying the ATP degradation in jumbo squid mantle (Dosidicus gigas), detected after 24 h of storage on ice very low levels of AMP and a high level of Hx giving a K value of 74%. This data suggested great activity of enzymes involved in AMP degradation (up to Hx) of this species (Picher, Burch, Hirsh, Spychala, & Boucher, Citation2003); therefore, the squid muscle freshness should not be evaluated by K value because it is not a good indicator in this organism (Saito et al., Citation1959).
Balance between the action of AMP deaminase and 5′-nucleotidase (EC.3.1.3.5) determined if purine ring structure is retained in the nucleotide level as IMP or converted to adenosine, diffusible catabolite with pleitrophic intracellular and extracellular effects (Haas & Sabina, Citation2003). Marquez-Rios et al. (Citation2008) found a high value of K m (13 mM) for AMP deaminase while studying the AMP degradation of jumbo squid mantle muscle. On the other hand, Pacheco-Aguilar et al. (Citation2009) found a Km value of 1.41, which is closer to the AMP concentration in squid mantle, while they were working with 5′-nucleotidase (5′-NT) from jumbo squid. According to previous studies, this enzyme is the main one responsible for the AMP degradation in this organism; thus becoming very important in its biochemical postmortem process.
Many isoforms of 5′-NT have been found in organisms ranging from bacteria through plants to vertebrates. Studies of the cellular distribution of mammalian nucleotidases and their sequences have led to a distinction between cytoplasmic, mitochondrial and membrane-bound (ecto-) forms (Bianchi & Spychala, Citation2003). Soluble cytosolic forms of 5′-NT have not been fully characterized yet and are still a subject of much controversy. There are variants of this enzyme differing in substrate preferences, kinetic and regulatory properties and cellular localization. cN-II is an IMP-preferring form which regulates the cellular nucleotide pool, but its role in adenosine formation is less clear (Sala-Newby, Freeman, Skladanowski, & Newby, Citation2000). 5′-Nucleotidase-I (cN-I), earlier denominated as “AMP selective”, first discovered and characterized in the pigeon heart (Skladanowski & Newby, Citation1990), is widely distributed in mammalian myocardial and endothelial tissue (Darvish & Metting, Citation1993; Tkacz-Stachowska, Lechward, & Skladanowski, Citation2005).
The lack of information about 5′-nucleotidase in jumbo mantle squid and the rapid degradation of adenylated compounds in jumbo squid muscle make the K value not a good index to predict giant squid freshness; therefore, it is important to get more information about enzymes involved in squid freshness to establish the correct index of freshness in giant squid. According to our research group, 5′-nucleotidase enzyme has been purified and partially characterized from jumbo squid muscle by Pacheco-Aguilar et al. (Citation2009), it has a native molecular weight of 107 kDa with subunits of 33 kDa, K m of 1.41 mM and isoelectric point of 3.8. This study continues from the research carried out by Pacheco-Aguilar et al. (Citation2009) who purified this enzyme from jumbo squid, therefore purification procedure is not going to be discussed. With the purpose to learn more about 5′-nucleotidase and thus to understand the faster degradation of AMP in jumbo squid, the aim of this research was partially to characterize the enzyme purified from jumbo squid and to generate more information about a decisive enzyme involved in the rapid AMP degradation in postmortem jumbo squid muscle.
Materials and methods
Experimental organisms
Giant squid specimens were captured away from the coast of Sonora in the Gulf of California. They were carefully gutted and washed with distilled water. For their transportation, mantles were placed in polyethylene bags to be later on combined with layers of ice. Once in the laboratory, mantles were washed with cold distilled water, placed again in polyethylene bags, and stored at −86 °C until they were used.
Enzyme assay
Enzyme activity was measured according to Fiske and Subbarow (Citation1925): 40 μL of enzymatic extract (any solution that is determined by enzyme activity, for example, crude extract or any fraction resulting of chromatographic step) were taken and mixed with 360 μL of 10 mM AMP in 40 mM sodium citrate buffer (pH 4.5), 0.1 mM DTT, 110 mM NaCl, 20 mM MgCl2, and 20 mM CaCl2. 5′-nucleotidase activity was measured by quantification of inorganic phosphorus. The reaction mixture was incubated during 10 min at 50 °C. Reaction was stopped with ammonium molybdate in HCl 0.5 N and 10% of acid ascorbic followed by incubation at 40 °C for 40 min to promote the blue color. Abosrvance820 nm was recorded with a Cary 50-Bio Rad spectrophotometer (Cary 50 Bio Rad). The enzyme activity (UA) was defined as μmoles of free inorganic phosphorous/min/mg of protein.
Purification of 5′-nucleotidase
The purification of 5′-nucleotidase was carried out in accordance with Marseno, Hori, and Miyazawa (Citation1993a). The crude extract was prepared homogenizing 50 g of jumbo squid mantle muscle with four volumes of 40 mM sodium citrate (pH 4.5), 20 mM MgCl2, 25 mM NaCl (buffer A), and 0.1 mM of phenylmethylsulphonyl fluoride (PMSF). The resultant mixture was saturated at 55% with ammonium sulphate. Once the mixture was dissolved, it was centrifuged at 3000g and 4 °C for 20 min, then the supernatant was dialyzed against 40 mM sodium citrate (pH 4.5) containing 1 mM MgCl2, 110 mM NaCl, 1 mM CaCl2 (buffer B), and 0.1 mM of PMSF using a cellulose membrane (ID × length = 2.7 × 30 cm2) to maintain the molecular weights to approximately 12,000 kDa. Dialysis was made twice against 20 volumes of buffer B with a space of 5 h between each change. The dialyzed fraction was loaded into a concanavalin A column at a flow rate of 10 mL/h, then three volumes of buffer C (buffer B plus 0.1% triton X-100) were applied at a flow rate of 20 mL/h until absorbance readings at 280 nm were constant. Next, the enzyme was eluted with 60 mL of methyl α-D-manopiranoside in buffer C at a flow rate of 10 mL/h, collecting fractions every 2 mL. Active fractions, which were obtained by Con A-affinity chromatography, were pooled and dialyzed twice against 30 volumes of buffer D (40 mM TRIS-HCl, pH 7.4 and 0.15 M NaCl) with simultaneous changing of buffer every 5 h. Dialyzed fraction was loaded into 5′-AMP sepharose 4B affinity column (Amersham Pharmacia Biotech, Uppsala, Sweden) at a flow rate of 5 mL/h and run with buffer D (same flow rate) until absorbance readings at 280 nm were constant. Later, the enzyme was eluted with 20 mM AMP in buffer D at a flow rate of 5 mL/h. Protein concentration was carried out in accordance with Bradford (Citation1976). To remove AMP, the active fractions were pooled and dialyzed twice against 30 volumes of buffer D for 10 h (Marseno, Hori, & Miyazawa, Citation1993b).
Temperature effect
The optimum temperature was determined by measuring the activity at temperatures between 30 and 60 °C. The thermal stability was assayed by incubating the enzyme for 60 min at different temperatures from 10 to 60 °C. Aliquots were taken every 15 min and their remaining activity was evaluated.
pH effect
Optimum pH was determined by measuring the activity at different pH levels from 3.0 to 7.5, using buffers such as sodium citrate (3.0–6.5) and TRIS-HCl (7.0–7.5) both at 40 mM concentration. Stability was determined by incubating the enzyme at different pH levels during 60 min and after every 15 min, aliquots of enzyme was taken and tested for activity.
Effect of NaCl, MgCl2, CaCl2, ATP, and ADP
The effect of salt as NaCl was measured by varying its concentration from 0 to 500 mM, MgCl2 and CaCl2 effect were assayed from 0 to 22 mM. The effect of ATP and ADP were evaluated from 0 to 5 mM. All trials were performed in sodium citrate buffer (at 40 mM, pH 4.5, 50 °C) as previously described (Fiske & Subbarow, Citation1925).
Results and discussions
Effects of temperature and pH
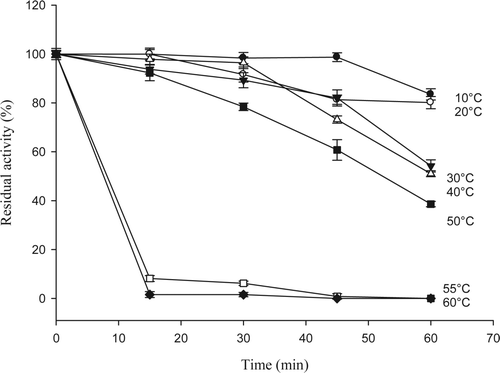
Although the enzyme 5′-nucleotidase has been widely studied because it is important in the cycle of adenylated compounds, there is little information about seafood products. The enzyme was purified from jumbo squid according to the methodology described by Marseno et al. (Citation1993b). shows the effect of temperature on 5′-nucleotidase activity in jumbo squid mantle. Our results showed that 5′-nucleotidase exhibited low enzyme activity at low temperatures and optimum activity at 50 °C, this result is similar to that reported by Marseno et al. (Citation1993a,Citationb) who worked with 5′-nucleotidase from rock fish (Sebastes inermis) finding the optimum temperature at 46 and 47 °C for cytosolic and ecto-enzyme, respectively. Very little information has been published about temperature effects on this enzyme. The majority of the researches have been conducted at 37 °C because 5′-nucleotidase has mostly been studied in warm-blooded organisms. Thermostability results in this study indicated that enzyme remained stable in the range of 10–50 °C () after an incubation period of 30 min, keeping residual activities between 100 and 80%. When temperature exceeded 20 °C, higher incubation periods gave as a result a great decrease of stability. Enzyme was very unstable at incubation temperatures of 55–60 °C, with residual activities of 8.1 and 1.6%, respectively, after 15 min (). Similar results were reported by Marseno et al. (Citation1993a,Citationb) for ecto 5′-nucleotidase and 5′-nucleotidase cytosolic from rock fish, with residual activities of 90 and 80%, respectively, after 30 min of incubation at 35 and 40 °C.
Figure 1. Optimum temperature of enzyme 5′-nucleotidase purified from jumbo squid mantle. Enzyme essay: 40 μL of enzyme extract were taken and mixed with 360 μL of 10 mM AMP in 40 mM sodium citrate (pH 4.5) containing 20 mM MgCl2, 20 mM CaCl2, and 200 mM NaCl. The reaction was carried out for 10 min at 50 °C.
Figura 1. Temperatura óptima de la enzima 5′-nucleotidasa purificada de manto de calamar gigante. Ensayo enzimático: 40 μL de extracto enzimático + 360 μL de AMP 10 mM en citrato de sodio 40 mM (pH 4,5) conteniendo MgCl2 20 mM, CaCl2 20 mM y NaCl 200 mM. Diez min de reacción a 50 °C.
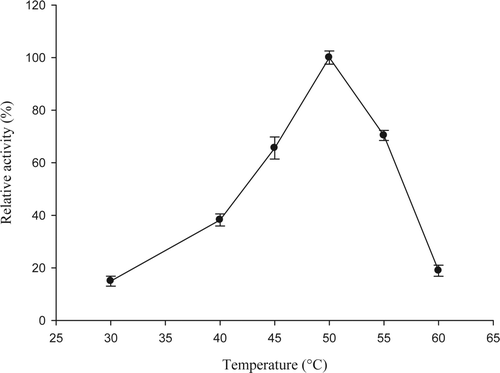
Figure 2. Temperature stability of enzyme 5′-nucleotidase purified from jumbo squid mantle. Enzyme essay: 40 μL of enzyme extract were taken and mixed with 360 μL of 10 mM AMP in 40 mM sodium citrate (pH 4.5) containing 20 mM MgCl2, 20 mM CaCl2, and 200 mM NaCl. The reaction was carried out for 10 min at 50 °C.
Figura 2. Estabilidad a diferentes temperaturas de la enzima 5′-nucleotidasa purificada de manto de calamar gigante. Ensayo enzimático: 40 μL de extracto enzimático + 360 μL de AMP 10 mM en citrato de sodio 40 mM (pH 4,5) conteniendo MgCl2 20 mM, CaCl2 20 mM, y NaCl 200 mM. Diez min de reacción a 50 °C.
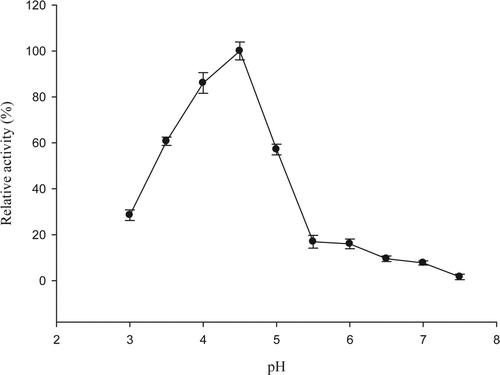
Optimum pH was detected at 4.5 (), a lower value than those reported by others. Kuda, Matsumoto, and Yano (Citation2002) found that the optimum pH for mackerel (Trachurus japonicus) was 5.0. Echetebu (Citation1980) reported an optimum of 5.2 for 5′-nucleotidase isolated from lizard, whereas Burger and Lowenstein (Citation1970) found an optimum pH of 5.4 while they were working with smooth muscle from thin intestine. On the other hand, the optimum pH for 5′-nucleotidase from rock fish was 8.3 (Marseno et al., Citation1993b), for pollock Pacific it has been reported to be 7.6 (Taar, Gardner, & Ingram, Citation1969), whereas for human heart it has been 7.0 (Skladanowski & Newby, Citation1990) and for dog heart the optimum reported was 7.5 (Darvish & Metting, Citation1993). These differences can be attributed to the wide variety of primary structure reported by this enzyme. 5′-nucleotidase is a multimeric enzyme of varied molecular weight and subunit numbers, in jumbo squid mantle it was found to be a homotrimeric enzyme (Pacheco-Aguilar et al., Citation2009), which differ from other enzyme sources in the literature reported. Fini et al. (Citation2003) as well as Darvish and Metting (Citation1993) worked with bull plasma and dog heart they reported a trimmer with native MW of 200 kDa and 66 kDa subunits and a tetramer of 166 kDa with 43 kDa subunits, respectively. On the other hand, Höglund and Reichard (Citation1990) reported a native MW of 45 kDa with subunits of 22 kDa, suggesting a homodimeric nucleotidase in human placenta.
Figure 3. Optimum pH of enzyme 5′-nucleotidase purified from jumbo squid mantle. Enzyme essay: 40 μL of enzyme extract were taken and mixed with 360 μL of 10 mM AMP in 40 mM sodium citrate or cacodylate at different pHs, containing 20 mM MgCl2, 20 mM CaCl2, and 200 mM NaCl. The reaction was carried out for 10 min at 50 °C.
Figura 3. pH óptimo de la enzima 5′-nucleotidasa purificada de manto de calamar gigante. Ensayo enzimático: 40 μL de extracto enzimático + 360 μL de AMP 10 mM en citrato de sodio o cacodilato 40 mM a diferentes pHs, conteniendo MgCl2 20 mM, CaCl2 20 mM, y NaCl 200 mM. Diez min de reacción a 50 °C.
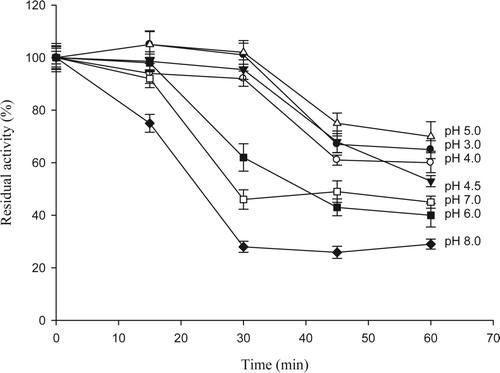
Results about pH stability are shown in . The enzyme was stable for 30 min in pH range from 3 to 5, while enzymatic activity was only 62, 46, and 28% at pH 6, 7, and 8, respectively. It was noticed that after 60 min of incubation at different pH levels, the enzyme lost its activity from 35 to 71%, when the pH was 8 the residual activity was only 29%. Marseno et al. (Citation1993a) studied rock fish, they found good stability of this enzyme at the pH range 7.0–9.0 for 30 min. At pH 6.0, its residual activity dramatically dropped to 8% whereas the remaining activity at pH less than 6.0 was negligible. These results suggest that 5′ nucleotdiase from giant squid has different pH stability. This discrepancy can be attributed to a great variation between 5′-nucleotidases isolated from different sources.
Figure 4. pH stability of enzyme 5′-nucleotidase purified from jumbo squid mantle. Enzyme essay: 40 μL of enzyme extract were taken and mixed with 360 μL of 10 mM AMP in 40 mM sodium citrate or cacodylate at different pHs, containing 20 mM MgCl2, 20 mM CaCl2, and 200 mM NaCl. The reaction was carried out for 10 min at 50 °C.
Figura 4. Estabilidad a diferentes pH de la enzima 5′-nucleotidasa purificada de manto de calamar gigante. Ensayo enzimático: 40 μL de extracto enzimático + 360 μL de AMP 10 mM en citrato de sodio o cacodilato 40 mM a diferentes pHs, conteniendo MgCl2 20 mM, CaCl2 20 mM, y NaCl 200 mM. Diez min de reacción a 50 °C.
Effect of NaCl, MgCl2,and CaCl2
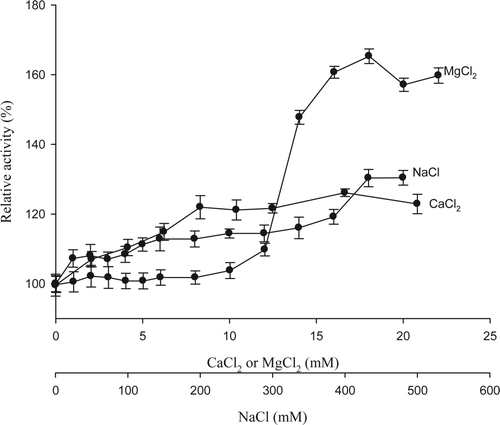
The NaCl as a source of monovalent cation on the 5′-nucleotidase activity showed a positive effect on the enzyme activity increasing it about 130% when NaCl concentration was 400 mM (). Skladanowski, Hoffmann, Krass, Jastorff, and Makarewicz (Citation1996) detected a biphasic behavior in purified enzyme of pigeon heart, 5′-nucleotidase was activated in the range from 0 to 500 mM with a max peak of 50 mM. Willadsen, Nielsen, and Riding (Citation1989) reported 70% of residual activity in presence of 200 mM NaCl on 5′-nucleotidase isolated from Boophilus microplus. A wide variety of enzymes need mono or divalent cations for their activity. One of these is MgCl2 that showed positive effect increasing the enzyme activity about 165% at 18 mM of MgCl2 (). Activator or inhibitor effect depends on 5′-nucleotidase source, even though MgCl2 generally acts as an activator. Similar results were reported by Naito and Lowenstein (Citation1981), who evaluated MgCl2 effect on 5′-nucleotidase from rat heart at concentration range from 0 to 50 mM, getting up to 140% of activity with 20 mM of MgCl2. Moreover, Marseno et al. (Citation1993a) evaluated the effect of the same salt (from 0 to 10 mM) on 5′-nucleotidase purified of rock fish, noticing an increase of 200% at concentration of 10 mM. These results indicated that NaCl and MgCl2 activate the 5′-nucleotidase purified from giant squid. Although the CaCl2 showed an activator effect on 5′-nucleotidase activity, the action was lower than MgCl2. When 20 mM of this cation was evaluated, CaCl2 increased the relative activity about 129% (). Research of 5′-nucleotidase from rock fish (Marseno et al., Citation1993a), observed that Mg+2 worked as activator on the enzyme activity, while Ca+2 showed no inhibitory or activator effect. Darvish and Meeting (1993) assessed the effect of these divalent cations on purified 5′-nucleotidase of dog heart, where MgCl2 showed a maximum peak of activity at a concentration of 3.5 mM, whereas CaCl2 did not present activator or inhibitor effect. In electric ray (Torpedo marmorata) both cations presented a very similar activator effect when they were evaluated within the range from 0 to 20 mM, increasing the relative activity of the enzyme up to 135 and 142% with CaCl2 and MgCl2, respectively (Grondal & Zimmermann, Citation1987).
Figure 5. Effect of NaCl, MgCl2, and CaCl2 on enzyme activity of 5′-nucleotidase purified from jumbo squid mantle. Enzyme essay: 40 μL of enzyme extract were taken and mixed with 360 μL of 10 mM AMP in 40 mM sodium citrate at pH 4.5. The reaction was carried out for 10 min at 50 °C.
Figura 5. Efecto del NaCl, MgCl2, y CaCl2 sobre la actividad enzimática de 5′-nucleotidasa purificada del manto de calamar gigante. Ensayo enzimático: 40 μL de extracto enzimático + 360 μL de AMP 10 mM en citrato de sodio 40 mM (pH 4,5). Diez min de reacción a 50 °C.
Effect of ATP and ADP
The 5′-nucleotidase enzyme plays an important role in regulating the AMP levels in the muscle depending on the physiological status of the animal. 5′-nucleotidase enzyme is regulated by mono and divalent cations, AMP analogues like ATP and ADP, as well as the adenylate energy charge (AEC). The AEC describes the energy status of an organism depending on the molar concentrations of ATP, ADP, and AMP. Therefore, the AMP analogues have a strong impact on the enzyme regulation. shows the effect of ATP on 5′-nucleotidase activity detecting an emphasized activator effect at low concentrations of ATP. The enzyme activity increased 100% from 0.09 ± 0.01 U/mg protein to 0.18 ± 0.01 U/mg protein, then the activator effect decreased when ATP concentration increased over 1 mM. This is the normal behavior in resting animal where the ATP levels are high and as a consequence, 5′-nucleotidase action is not necessary within the cell at that moment; however this metabolite can be a good activator when ATP levels are low. Skladanowski and Newby (Citation1990) detected an increase from 5 to 15 U/mL in the activity of 5′-nucleotidase from rat muscle when evaluated ATP effect in the range from 0 to 1 mM. Likewise, the human 5′-nucleotidase enzyme showed an increase activity from 1 U/mg to 6 U/mg protein, in the range of ATP concentration from 0 to 2 mM (Hunsucker, Spychala, & Mitchell, Citation2001). However, Orford and Saggerson (Citation1996) as well as Ipata (Citation1968) detected an inhibitor effect on 5′-nucleotidase activity from other sources.
Figure 6. ATP and ADP effect on enzyme activity of 5′-nucleotidase purified from jumbo squid mantle. Enzyme essay: 40 μL of enzyme extract were taken and mixed with 360 μL of 10 mM AMP in 40 mM sodium citrate (pH 4.5) containing 20 mM MgCl2, 20 mM CaCl2, and 200 mM NaCl. The reaction was carried out for 10 min at 50 °C.
Figura 6. Efecto del ATP y ADP sobre la actividad enzimática de 5′-nucleotidasa purificada del manto de calamar gigante. Ensayo enzimático: 40 μL de extracto enzimático + 360 μL de AMP 10 mM en citrato de sodio 40 mM (pH 4,5) conteniendo MgCl2 20 mM, CaCl2 20 mM, y NaCl 200 mM. Diez min de reacción a 50 °C.
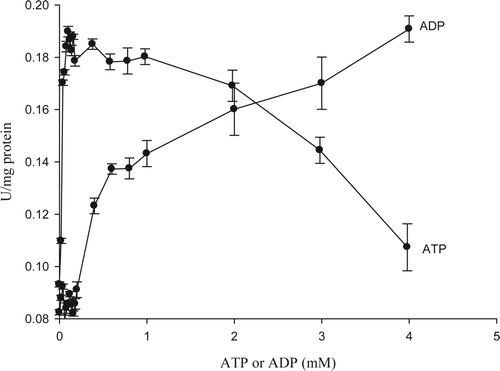
The results of this study showed that ATP is a key metabolite that can act as a good activatory ranging from 0.02 to 4 mM. Considering that ATP concentration in newly harvested squid mantle was 6.54 μM/g (Marquez-Rios et al., Citation2007), and moisture content was 80%, the ATP concentration should be 8.17 mM. Therefore, it is assumed that in newly harvested squid there are no suitable ATP levels to act as positive effectors of 5′-nucleotidase. However, after 15 h of storage on ice, the ATP levels dramatically declined (Marquez-Rios et al., Citation2007) causing appropriate levels that could act as positive effectors.
The ADP effect on 5′-nucleotidase greatly differed than observed for ATP. Low concentration of ADP did not provide visible changes in the enzymatic activity; however when ADP concentration increased to values near 0.6 mM its activity greatly increased (). Tkacz-Stachowska et al. (Citation2005) evaluated the ADP effect on cytosol 5′-nucleotidase from pigeon muscle; they detected an activator effect reducing its K m value from 1.85 to 0.29 in presence of 1 mM of ADP. Likewise, human 5′-nucleotidase activity was affected by ADP in range concentrations from 0 to 2 mM, increasing its specific activity from 1 to 38 μM Pi/min/mg protein (Hunsucker et al., Citation2001). On the other hand, when Skladanowski et al. (Citation1996) were working with 5′-nucleotidase from rat they observed an ADP activator effect in range from 0 to 1 mM, in which its specific activity increased from 0.1 to 0.3 U/mg. Nevertheless, both ATP and ADP may also act as inhibitors depending on the enzyme source (Naito & Lowenstein, Citation1981). Our results assume that 5′-nucleotidase enzyme from squid mantle was activated when squid declined the ATP content; therefore ADP and AMP levels started to accumulate inside the cell. It is an expected behavior because 5′-nucleotidase is a key enzyme involved in AMP levels in the muscle. The ADP molar concentration in newly harvested squid was reported to be about 0.49 μM/g muscle (Marquez-Rios et al., Citation2007) which considering a moisture content of 80% in squid mantle it would correspond to a molar concentration of 0.61 mM, assuming this way that there are appropriate ADP levels to act as positive effectors of 5′-nucleotidase in jumbo squid mantle.
Conclusion
Optimal temperature and pH for 5′-nucleotidase enzyme from giant squid mantle were established. The 5′-nucleotidase enzyme played an important role during the regulation of adenylate compounds in this organism. According to these results, we can suggest that 5′-nucleotidase enzyme regulation inside the cell is carried out by mono and divalent cations, where the salt MgCl2 seems to be an important regulator. We also detected that ATP and ADP are two important nucleotides in regulation of 5′-nucleotidase enzyme from giant squid. The enzyme increased its activity when ATP level significantly decreased; therefore, ADP levels was increased to suitable concentrations for a good enzyme action. In addition, when ADP was increased in the cell, the AMP molar concentrations increased as well, thus increasing the 5′-nucleotidase activity to keep appropriate AMP levels and regulate the pool of these metabolites in the cell. The enzyme seems to be regulated by the AEC, which is inactive at high AEC (high ATP concentrations and low ADP and AMP concentrations) and it is active when the AEC decreases (low ATP concentrations and high ADP and AMP concentrations). Hence, the importance of this enzyme in adenylated compounds pool as well as in the energetic biochemistry of the muscle.
Additional information
Notes on contributors
E. Marquez-Rios †
†Current address: Departamento de Investigación y Posgrado en Alimentos, Universidad de Sonora, Encinas y Rosales S/N, Hermosillo, Sonora 83000, México.References
- Bianchi , V. and Spychala , J. 2003 . Mammalian 5′-nucleotidases . Journal of Biological Chemistry , 278 : 46195 – 46198 .
- Bradford , M. 1976 . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of dye binding . Analytical Biochemistry , 46 : 248 – 254 .
- Burger , R. M. and Lowenstein , J. M. 1970 . Preparation and properties of 5′-nucleotidase from smooth muscle of small intestine . Journal of Biological Chemistry , 245 : 6274 – 6280 .
- Darvish , A. and Metting , P. J. 1993 . Purification and regulation of an AMP-specific cytosolic 5′-nucleotidase from dog heart . American Journal of Physiology , 264 : 1528 – 1534 .
- Echetebu , C. O. 1980 . Intestinal 5′-nucleotidase of the Lizard agama agama . Comparative Biochemistry and Physiology , 67 : 345 – 349 .
- Fini , C. , Talamo , F. , Cherri , S. , Coli , M. , Floridi , A. Ferrara , L. 2003 . Biochemical and mass spectrometric characterization of soluble ecto-5′-nucleotidase from bull seminal plasma . Biochemical Journal , 372 : 443 – 451 .
- Fiske , C. H. and Subbarow , Y. 1925 . The colorimetric determination of phosphorous . Journal of Biological Chemistry , 66 : 375 – 400 .
- Grondal , E. J.M. and Zimmermann , H. 1987 . Purification, characterization and cellular localization of 5′-nucleotidase from torpedo electric organ . Biochemistry Journal , 245 : 805 – 810 .
- Haas , A. M. and Sabina , R. L. 2003 . N-terminal extensions of the human AMPD2 polypeptide influence ATP regulation of isoform L . Biochemical and Biophysical Research Communications , 305 : 421 – 427 .
- Howgate , P. 2005 . A review of the kinetics of degradation of inosine monophosphate in some species of fish during chilled storage . International Journal of Food Science & Technology , 40 : 1 – 13 .
- Höglund , L. and Reichard , P. 1990 . Cytoplasmic 5′(3′)-nucleotidase from human placenta . Journal of Biological Chemistry , 265 : 6589 – 6595 .
- Hunsucker , S. A. , Spychala , J. and Mitchell , B. S. 2001 . Human cytosolic 5′-nucleotidase I. Characterization and role in nucleoside analog resistence . American Society for Biochemistry and Molecular Biology , 276 : 10498 – 10504 .
- Ipata , P. L. 1968 . Sheep brain 5′-nucleotidase. Some enzymic properties and allosteric inhibition by nucleoside triphosphates . Biochemistry , 7 : 507 – 515 .
- Kuda , T. , Matsumoto , C. and Yano , T. 2002 . Changes in acid and alkaline phosphatase activities during the spoilage of raw muscle from horse mackerel Trachurus japonicus and gurnard Lepidotriga microptera . Food Chemistry , 76 : 443 – 447 .
- Marquez-Rios , E. , Morán-Palacio , E. F. , Lugo-Sánchez , M. E. , Ocano-Higuera , V. M. and Pacheco-Aguilar , R. 2007 . Postmortem biochemical behaviour of giant squid (Dosidicus gigas) mantle muscle stored in ice and its relation with quality parameters . Journal of Food Science , 72 : 356 – 362 .
- Marquez-Rios , E. , Pacheco-Aguilar , R. , Castillo-Yañez , F. J. , Figueroa-Soto , C. G. , Ezquerra-Brauer , J. M. and Gollas-Galvan , T. 2008 . Isolation and properties of AMP deaminase from jumbo squid flying (Dosidicus gigas) mantle muscle from the Gulf of California, Mexico . Food Chemistry , 110 : 69 – 75 .
- Marseno , D. W. , Hori , K. and Miyazawa , K. 1993a . Purification and properties of cytosol 5′-nucleotidase from black rockfish (Sebastes inermis) muscle . Journal of Agricultural and Food Chemistry , 41 : 1208 – 1212 .
- Marseno , D. W. , Hori , K. and Miyazawa , K. 1993b . Purification and properties of membrane-bound 5′-nucleotidase from black rockfish (Sebastes inermis) muscle . Journal of Agricultural and Food Chemistry , 41 : 863 – 869 .
- Naito , Y. and Lowenstein , M. 1981 . 5′-nucleotidase from rat heart . Biochemistry , 20 : 5188 – 5194 .
- Ocano-Higuera , V. M. , Maeda-Martínez , A. N. , Lugo-Sánchez , M. E. and Pacheco-Aguilar , R. 2006 . Postmortem biochemical and textural changes in the adductor muscle of catarina scallop stored at 0 °C . Journal of Food Biochemistry , 30 : 373 – 389 .
- Orford , M. R. and Saggerson , D. 1996 . A low-Km 5′-nucleotidase from rat brain cytosolic fraction: purification, kinetic properties, and description of regulation by a novel factor that increase sensitivity to inhibition by ATP and ADP . Journal of Neurochemistry , 67 : 795 – 804 .
- Pacheco-Aguilar , R. , Ramirez-Suarez , J. C. , Castillo-Yañez , F. J. , Peña-Ramos , E. A. , Valenzuela-Soto , E. M. and Marquez-Rios , E. 2009 . Isolation and properties of 5′-nucleotidase isolated from jumbo squid (Dosidicus gigas) mantle muscle from the Gulf of California, Mexico . Food Chemistry , 112 : 880 – 884 .
- Picher , M. , Burch , L. H. , Hirsh , A. J. , Spychala , J. and Boucher , R. C. 2003 . Ecto 5′-nucleotidase and nonspecific alkaline phosphatase. Two AMP-hidrolyzin ectoenzimes with distinct roles in human airways . Journal of Biological Chemistry , 278 : 13468 – 13479 .
- Saito , T. , Arai , K. and Matsuyoshi , M. 1959 . A new method for estimating the freshness of fish . Bulletin of the Japanese Society of Scientific Fisheries , 24 : 749 – 752 .
- Sala-Newby , G. B. , Freeman , N. V.E. , Skladanowski , A. C. and Newby , A. C. 2000 . Distinct roles for recombinant cytosolic 5′-nucleotidase-I and -II in AMP and IMP catabolism in COS-7 and H9c2 rat myoblast cell lines . Journal of Biological Chemistry , 275 : 11666 – 11671 .
- Skladanowski , A. C. , Hoffmann , C. , Krass , J. , Jastorff , B. and Makarewicz , W. 1996 . Structure–activity relationship of cytoplasmic 5′-nucleotidase sites . Biochemical Journal , 314 : 1001 – 1007 .
- Skladanowski , A. C. and Newby , A. C. 1990 . Partial purification and properties of an AMP-specific soluble 5′-nucleotidase from pigeon heart . Biochemical Journal , 268 : 117 – 122 .
- Taar , H. L.A. , Gardner , L. J. and Ingram , P. 1969 . Pacific cod muscle 5′-nucleotidase . Journal of Food Science , 34 : 637 – 640 .
- Tkacz-Stachowska , K. , Lechward , K. and Skladanowski , A. C. 2005 . Isolation and characterization of pigeon breast muscle cytosolic 5′-nucleotidase-I (cN-I) . Acta Biochimica Polonica , 52 : 789 – 796 .
- Willadsen , P. , Nielsen , J. M. and Riding , G. A. 1989 . Purification and properties of a novel nucleotide-hydrolysing enzyme (5′-nucleotidase) from Boophilus microplus . Biochemical Journal , 258 : 79 – 85 .