Abstract
The worldwide high consumption of food additives has encouraged to perform research on new species producers of gums or hydrocolloids that can compete with those that are traditionally used, such as flamboyant (Delonix regia) seed gum, a species considered underutilized and of ornamental type. Flamboyant tree seeds were used to obtain a native gum (flamboyant gum (FNG)), which was modified by carboxymethylation (CFG), this reaction was confirmed using Fourier transformed infrared (FTIR) spectroscopy and colorimetric titration. Swelling index and dispersion were evaluated in a range of 30–90 °C. The apparent viscosity (μ) was evaluated with a rheometer. Compression strength of the gel (CSG) was measured with a texture analyzer and calorimetric profile with differential scanning calorimetry (DSC). The CFG had a degree of substitution of 0.33 and the FTIR analysis indicated the presence of carboxy-ether groups in the CFG. The modification of the FNG increased dispersion by 66%. Both gums exhibited shear-thinning behaviors although carboxymethylation caused a decrease in viscosity. The DSC showed a state crystalline in the FNG and a state amorphous in the CFG. The modification of the FNG favored hydrogen bridge interactions with water but decreased intra-catenary interactions in the polysaccharide.
El alto consumo de aditivos alimentarios a nivel mundial incentiva la investigación de nuevas especies productoras de gomas o hidrocoloides que puedan competir con aquéllas usadas tradicionalmente, tal es el caso de la goma de la semilla de flamboyán (Delonix regia), especie subutilizada y considerada de tipo ornamental. Las semillas de flamboyán fueron usadas para obtener goma nativa (FNG), la cual fue modificada por carboximetilación (CFG), esta reacción se confirmó usando espectroscopia de infrarrojo con transformada de Fourier (FTIR) y titulación colorimétrica. El índice de hinchamiento y la dispersión en agua fueron evaluados de 30 a 90 °C. La viscosidad aparente (μ) fue evaluada con reómetro. La fuerza de compresión del gel (CSG) fue medida con un analizador de textura y el perfil calorimétrico con un calorímetro diferencial de barrido (DSC). La CFG tuvo un grado de sustitución de 0,33. La modificación de la FNG incrementó la solubilidad en un 66%. Ambas gomas tuvieron un comportamiento reofluidizante. La carboximetilación ocasiono un decremento de la μ. El DSC mostró un estado cristalino en la FNG y amorfo en la CFG. La modificación de la FNG favoreció las interacciones puentes de hidrógeno con el agua pero disminuyó las interacciones intracatenarias en el polisacárido.
Introduction
Hydrocolloids, or gums, are widely used in the food industry as emulsifying, stabilizing, thickening, gelling, and film-forming agents (Bósquez & Vernon, Citation2005; Lal, O'Connor, & Eyres, Citation2006). The term gum was originally used to refer to the exudation products obtained from some plants and trees, but is now used to refer to a large group of high-molecular weight polysaccharides from seeds, fruits, the exudation of plants, and fermentation products (Srivastava & Kapoor, Citation2005).
Some gums (for example guar) are extracted from the legume seeds, mainly from the endosperm, which are responsible for the water absorption during germination. These gums are constituted generally for heterogeneous polysaccharides known as seed galactomannans. The galactomannan-type structure consists of a linear chain with d-mannopyranose (β−1 → 4 linkages) and ramifications formed by α−1 → 6 linkages with d-galactopyranose units (Srivastava and Kapoor, Citation2005).
In Yucatan, Mexico, there are 206 legume species having a potential use in gum extraction, including the flamboyant tree (Delonix regia) (Niembro, 1987). D. regia seeds contain a galactomannan-type polysaccharide similar to those of guar gum (Cyamopsis tetragonalobus) and locust bean (Prosopis chilensis) gum (Matsuhiro, Presle, Saenz, & Urzua, Citation2006). The few branch regions in flamboyant seed gum consist of α-d-mannose (1 → 4) linkages and α-d-galactose (1 → 6) branches (mannose–galactose 2:1 ratio). Its mannose and galactose proportions are similar to those of guar gum but differ in terms of the OH bond position in the main chain: flamboyant gum (FNG) has α-d-mannose while the guar gum has β-d-mannose (Kapoor, Citation1972). The toxicological research on FNG using rats showed that the intake of this polysaccharide has no effect at upto 50 g/kg daily intake and is therefore a potential additive in food industry applications (Morochi & Shiomi, Citation1999). However, this gum has no ionic charge, which is probably the cause for its low water dispersion at room temperature, uncontrollable viscosity after cooking, and low pH, all properties that limit its use as an additive. Improving its technological functional characteristics requires modification of its chemical structure (Gupta, Sharma, & Soni, Citation2004). Many hydrocolloids have been chemically modified to change of functional properties, for example to increase water dispersion at room temperature (Socaciu, Citation2007; Moslemy, Guiot, & Neufeld, 2008). These modified polysaccharides can be used in the functional food industry (e.g. microencapsulation of bioactive compounds), as well as in the petroleum, pharmaceuticals, water treatment, and cosmetics industries, among others (Reddy & Tammishetti, 2002).
Carboxymethylation is a chemical modification that incorporates functional groups with a net ionic charge and therefore provides rheological and functional characteristics different from those of a native gum (Jeffery, Subramanian, & Hong, 2002). Reddy and Tammishetti (2002) used sodium chloroacetate (SCA) to carboxymethylated guar gum, thus giving its polysaccharide a net ionic charge and allowing the encapsulation of protein by ionic gellification. In other research, Jie, Wen-ren, Manurung, Ganzeveld, and Heeres, (2004) used 700 g/L of SCA to carboxymethylate the cassava (Manihot esculenta) starch; this modification favored the gelatinization and the water dispersion at room temperature. The objective of the present study was to determine the effect of carboxymethylation on the physicochemical properties of native flamboyant (D. regia) seed gum.
Materials and methods
Flamboyant seeds
Flamboyant tree (D. regia) seed pods were collected randomly from four zones in the city of Merida, Yucatan, Mexico, with the aim of obtaining a representative sample. All seeds were collected from dry seed pods taking into account undamaged physical seeds, and longer than 1 cm in length (∼100 days after the flowering period). A total of 5 kg of seeds were removed from the pods per zone and mixed in a single sample and stored in polyethylene bags at 4 °C until use.
Endosperm flour
The flour was produced from the endosperm of the seeds according to Morochi, San Martin, and Ordoñez, (Citation1999). The seeds were hydrated in distilled water (1:5 w/v) at 70 °C for 10 h. They were then wet milled (Tecator Cemotec 1090 Sample Mill, Höganäs, Sweden) to crack the seeds and free the endosperm, and washed three times with distilled water to eliminate the husk and germ. The resulting endosperm was dried at 55 °C for 24 h in a circulating air oven (Imperial V) and milled (Thomas-Wiley Laboratory Mill Model 4, Swedesboro, NJ) when passing through a 20 mesh (833 μm).
Flamboyant gum extraction
The extraction of FNG was done using the Azero and Andrade (Citation2006) method, with some modifications. Briefly, endosperm flour in water suspension (1:30 w/v, pH 7) was prepared and heated to 50 °C (to preserve the native structure) under constant agitation for 30 min. It was then filtered sequentially through 42 (351 μm) and 100 mesh (147 μm) to separate the fibrous particles from the FNG. The filtered sample was precipitated in 700 g/L ethanol, dried at 55 °C for 24 h in a circulating air oven (Imperial V Lab-Line Model 3476M, Boston, MA), and milled (Thomas-Wiley Laboratory Mill Model 4, Swedesboro, NJ) to an 80 mesh (173 μm) size. The proximate composition (nitrogen 954.01; fat 920.39; ash 923.03; crude fiber 962.09; moisture 925.09 and total carbohydrates were nitrogen free extracts) was determined according to AOAC International (Citation1997) methods.
Carboxymethylation
The FNG was modified by carboxymethylation using SCA under heterogeneous conditions using the method of Bahamdan and Daly (Citation2005), with some modifications. A 70 g sample of FNG was swelled in 400 mL of 2-propanol with nitrogen, and under constant agitation (Caframo RZ-1, Heidolph, Schwabach, Germany). Then, 24.8 g of NaOH solution (400g/L) were added over a period of 20 min and the mixture left to stand for 30 min at room temperature to allow further swelling. Sixty grams of SCA solution (400 g/L) were added over 30 min and the mixture allowed to react for 1 h. The reaction temperature was then raised to 70 °C over 1 h, and the reaction allowed to proceed for 3 h at 70 °C. The mixture was cooled to room temperature and filtered. The resulting solid was washed and soaked in 800 mL/L methanol/water for 30 min to remove inorganic salts. The carboxymethylated flamboyant gum (CFG) was recovered by filtration, washed with bulk methanol and dried overnight in an oven (Imperial V Lab-Line Model 3476M, Boston, MA) at 60 °C.
Degree of substitution
The degree of substitution (DS) of the CFG was determined by titration using the Bahamdan and Daly (Citation2005) method. Ten grams of CFG were transferred to acid form by slurry in 150 mL ethanol (950 mL/L), 12 mL (70 g/L), HNO3 (1.42 specific gravity) added and the slurry stirred for 20 min. While stirring, the slurry was heated to boiling for 5 min, removed from the heat and stirring continued for 20 min. The mixture was cooled, filtered and the residue was washed with three 150 mL aliquots of 800 mL/L aqueous methanol to remove salts and excess acid. It was then washed with methanol and dried overnight in an oven at 60 °C. One-gram portions of the dried acid form samples produced in the previous step were transferred to 200 mL Erlenmeyer flasks and suspended in distilled water (100 mL) until completely dissolved. An excess of 15 mL 0.5–1.0 N NaOH solution was stirred in, and agitation continued for 15 min before the solution was heated to boiling for 30 min. While still hot, excess NaOH in the solution was back titrated with 0.5 N HCl to a phenolphthalein end point. The amount of acid consumed was recorded and DS calculated as follows (
EquationEquation (1)):
FTIR spectroscopy
Fourier transformed infrared (FTIR) spectroscopy of the FNG and CFG was done according to Grosev, Bozac, and Puppels, (Citation2001) using a Tensor 27 Bruker device with ATR for solid samples and a functional group detection disk. Using 1 mg of lyophilized sample, the FTIR spectrum was determined in a wave λ interval of 800–4000 cm−1 at a 4 cm−1 resolution, with 20 scans.
Differential scanning calorimetry
The DSC profiles for the FNG and CFG were generated by using the method of Cardoso, Simões, Mendes, Teixeira, and Ferreira, (Citation1988). Briefly, 8 mg (db) of sample were weighed out, placed in an aluminum pan for dry samples (Perkin-Elmer, No. 0219-0041) and hermetically sealed. Thermal transitions were then determined in a differential scanning calorimeter (Perkin-Elmer DSC-6, Norwalk, CT) between −20 and 220 °C at a rate of 10 °C/min and a nitrogen flow of 20 mL/min. The thermogram was used to calculate the glass transition (T g), melting temperature (T m), heat capacity (ΔC p) and fusion enthalpy (ΔH m). For the gum, T g was determined as the mid-point of extrapolated tangents in heat capacity change (Reading & Haines, 1995).
Swelling index and water dispersion
Swelling index (SI) and water dispersion (W
DIS) were determined at different temperatures using a modification of the method of Adikwu, Ezeabasili, and Esimone, (Citation2001). Gum dispersions with 10 g/L (dry matter) FNG or CFG were placed in centrifuge tubes and heated in a water bath at 30, 50, 70, and 90 °C for 30 min. To prevent the gum swell sedimentation during heating, middle stirring was applied periodically using magnetic stirrers. All tubes were covered with plastic covers to prevent the water loss. After heating, the samples were centrifuged (2120g, 15 min) in an ultracentrifuge (DS-15R, Beckman, Coulter, Fullerton, CA), the precipitated paste separated from the supernatant and weighed (W
p). Both phases were dried at 105 °C for 4 h and dry solids were calculated for the precipitated paste (W
ps) and supernatant (W
s). SI was calculated (
EquationEquation (2)) as the ratio of the weight of the hydrated gum after centrifugation (g) to its dry mass (g).
The W DIS is the percentage of dry mass in supernatant to the dry mass of whole gum sample (W o):
Apparent viscosity
Apparent viscosity (μ) for the FNG and CFG was determined using a modification of the method of Mothé and Rao (1999), using a rheometer (AR-2000, TA Instrument, New Castle, DE) with a 20 mm diameter cone and plate geometry, a 4° angle, within the shear rate range from 1 to 1500 s−1. The samples were dispersed to 50 g/L (db) in water (pH 7), heated to 90 °C for 2 min and left to cool to 25 °C before analysis. The results were fixed to an Ostwald-de Waele model (σ = k × y n) to determine the consistency index (k) and flow behavior index (n).
Compression strength of gel
The compression strength of gels (CSG) produced with FNG and CFG was analyzed using the method of Olatunji, Huang, and Dai, (Citation1997). A 50 g/L gum–water suspension was prepared, heated to 80 °C for 1 h, poured into 20 × 40 mm cylindrical Pyrex molds coated with mineral oil and cooled to 4 °C for 24 h. The resulting gels were compressed in a Universal Testing Machine (Instron Model 4411, Canton, MA,USA) at 5 kg compression force and a rate of 5 mm/min. The load resistance was calculated and the data processed with the IX ver 11061 software.
Statistical analysis
A student t-test was applied to compare the means of functional properties for the CFG and FNG, and a two-way analysis of variance (ANOVA) was used for the factorial design. A 5% significance level was used in all cases and the analyses were done with the Statgraphics plus ver. 5.1 statistics software as described by Montgomery (Citation2004).
Results and discussion
Gum extraction and carboxymethylation
Native gum (FNG) extraction produced 400 g/kg, with a proximate content of 21.6 g/kg protein, 1.9 g/kg ash, 5.4 g/kg fat, 18.0 g/kg crude fiber, and 953.1 g/kg total carbohydrates. The carboxymethylation of the FNG resulted in a DS of 0.33, equivalent to 11% carboxymethyl substitution. This DS of CFG is lower than those reported for gums with a similar number of mannose–galactose ratio (2:1) in their structure, also guar gum (Cyamopsis tetragonolobus) with 0.41 (Bahamdan & Daly, Citation2005) and 0.42 for locust bean (Prosopis chilensis) (Matsuhiro et al., Citation2006). These results could be explained for the beta position anomeric carbon linear chain of the guar gum and locust bean differs from the alpha position of the FNG (Morochi & Shiomi, Citation1999), which may have caused a steric impediment in the latter, consequently reducing its carboxymethyl DS. In modified works with polysaccharides with structures linear (α−1 → 4 linkages in the main chain) DS between 0.25 and 0.40 for amylose (Kittipongpatana, Chaitep, Charumanee, & Kittipongpatana, Citation2006) and DS of 0.1 to 2.1 in cashew gum was reported (Silva et al., 2004). These results were similar to that the FNG in study. However, in linear polysaccharides with β−1 → 4 glucosidic linkages, like carboxymethyl cellulose, have reported values of DS greater than 1.00 (Zhao, Cheng, Li, & Zhang, Citation2003). This is because they have no steric impediment in the reactive centers susceptible to esterification, and due to the polysaccharide's anomeric linkage type.
FTIR spectroscopy
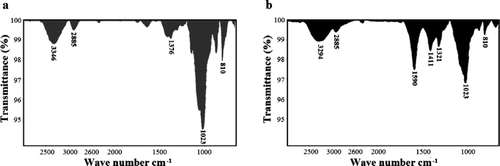
The absorption peaks were observed at different intensities for FNG and CFG in the 810 cm−1 (anomeric) region, suggesting the presence of mannose (Lim et al., Citation2005) (). A low-intensity band was also observed in the 2885 cm−1 region which corresponds to symmetrical and asymmetrical stretching of the methylic and methylenic C‒H bonds. Both gums exhibited absorption bands in the 3500–3000 cm−1 region corresponding to vibrations in the ‒OH bond. However, the CFG presented a wider peak than to FNG, probably because more water molecules are joined to CFG by the presence of their ionic groups (Skoog, West, Holler, & Crouch, 2005). This modification may also have caused disappearance of an alcohol ‒C‒O bond overtone in the FNG at 2359 cm−1 and appearance of medium-intensity bands at 1590, 1411, and 1321 cm−1 corresponding to symmetrical and asymmetrical stretching of the carboxylate anion (in electronic resonance or ionized state). There was also a decrease in the ‒C‒O stretch band (900–1150 cm−1), which, due to carboxymethylation, was asymmetrically coupled with the ‒C‒C bond stretch of the carbohydrate backbone. This region normally exhibits typical absorption bands that correspond to asymmetrical stretching vibrations of the aliphatic ether groups, usually of weak intensity and at 1125 cm−1. In the present case, these would have been hidden by the signals of the medium-intensity vibration modes of the ‒C‒C stretch of the carbohydrate carbonated backbone overlapped with the ‒C‒O group vibration modes. The FTIR spectrum of the CFG shows the partial substitution of the ‒OH in the FNG, which is consistent with the absorption band observed when comparing both spectra.
Differential scanning calorimetry
The FNG exhibited an endothermic peak at 95.2 ± 1 °C, attributable to a first-order transition or T m, with a ΔH m of 306 ± 1 J g−1 (). These and the lack of any other thermal transitions illustrate the FNG's crystalline state or organized nature (Grosev et al., Citation2001). Other native gums exhibit endothermic peaks in a similar range (80–97 °C) attributable to polymer T m: karaya (Sterculia urens) (Babu et al., Citation2002); chitosan and xanthan (Xanthomonas campestris) (Phaechamud & Ritthidej, Citation2007). However, the T m for the FNG was lower than the 124 °C reported for copal (Bursera bipinnata) resin gum and the 142 °C reported for damar (Shorea wiesneri) resin gum (Morkhade, Fulzele, Satturwar, & Joshi, Citation2006), meaning the FNG changes state at a lower temperature than resin gums.
Figure 2. Differential scanning calorimetry (DSC) profile of flamboyant native gum (FNG) and carboxymethylated flamboyant gum (CFG).
Figura 2. Calorimetría de barrido difrencial (CBD) de la goma nativa (FNG) y carboximetilada (CFG) de flamboyán.
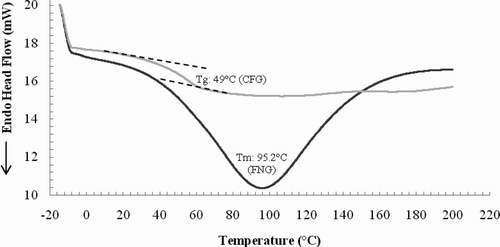
The CFG exhibited only a single thermal transition corresponding to the T g, indicating a condition change from the glass-rubber transition and that the modified gum is in the amorphous state. The amorphous state could be found also in the FNG as it is a polymer graft. The T g of CFG was 49 ± 0.5 °C (a moisture gum 90 g/kg) with a ΔC p of 1.4 ± 0.05 J g−1 C−1), meaning that the structural conformation of the CFG differs from that of the FNG due to addition of ionically charged groups, producing electrostatic repulsive between the carboxyl groups, thereby, possibly change from an organized structure to a disorganized structure. The T g of the CFG was similar to the 50 °C and ΔC p < 0.5 J g−1 C−1 reported for peach gum, which also has carboxyl groups in its structure (Xie, Zhou, & Qian, Citation2005). The CFG had a T g and a carboxymethyl substitution efficiency lower than reported for polyvinyl alcohol carboxymethyl with 74–84 °C, and 12–31%, respectively (Nam, Chun, & Lee, 1999).
Swelling index and water dispertion
Increased system temperature affected (p < 0.05) SI and water dispersion () in the FNG with a linear increase in both parameters between 30 and 70 °C. This is related to T m in the DSC profile, which showed FNG to change from a crystalline solid state to a disorganized fluid state beginning at 49 °C, leading to increased interaction with water. Both SI and W DIS in the CFG decreased at temperatures higher than 70 °C. As the gel formed, water was lost because of an association of inter- and intra- catenary linear chains in the gel, which became increasingly rigid and produced loss of the liquid phase (García-Ochoa & Casas, Citation2006).
Figure 3. Swelling index (a) and water dispersion (b) of flamboyant native gum (FNG) (•) and carboxymethylated flamboyant gum (CFG) (▴) at different temperatures. a–dDifferent letters indicate statistical difference (p < 0.05).
Figura 3. Índice de hinchamiento (a) y dispersión en agua (b) de la goma nativa (FNG) (•) y carboximetilada (CFG) (▴) de flamboyán a diferentes temperaturas. a–dLetras diferentes indica diferencia estadística (p < 0.05).
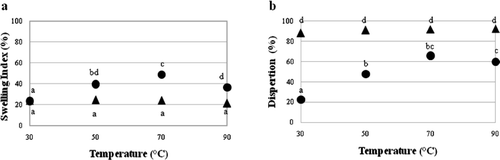
Similar behavior to that of W DIS in the FNG has been reported for carob (Ceratonia siliqua), in which W DIS increased as temperature increased from 5 to 85 °C, and dispersable gum increased from 50 to 90% (Pollard et al., Citation2006). Other hydrocolloids such starch, pectin and carrageenan native and modified have also exhibited similar behavior to the FNG, with maximum SI of 58 g/g water and W DIS of 22.5% at 85 °C (Babic et al., Citation2006).
In contrast to the FNG, increased temperature (30 to 90 °C) had no effect (p > 0.05) on SI and W DIS in the CFG (). However, the gum–temperature interaction for SI and W DIS had an effect (p < 0.05). This was caused by electrostatic repulsive interactions between the carboxyl groups facilitating the formation of hydrogen bonds with water (Choi et al., Citation2003). Higher SI than that of the CFG has been reported for carboxymethylated gums such as carboxymethylcellulose with β(1 → 4) linkages (Rácz & Borsa, Citation1997).
A behavior similar to CFG was reported for Billa and Yuen (Citation2000) in xantham gum with carboxylic groups. These authors indicated that the SI doesn't change when increasing the temperature between 60 and 80 °C at the same water content. However, higher values were obtained (70–80 g/g) for IS than IF that the CFG.
Apparent viscosity
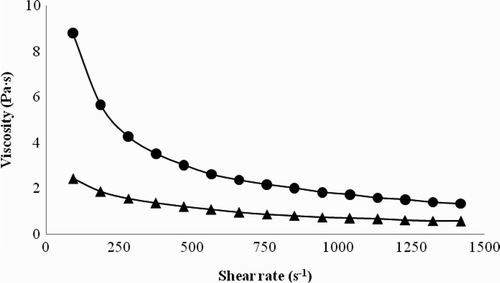
For both FNG (pH 7 in dispersion) and CFG (pH 10.3 dispersion), viscosity exhibited a non-Newtonian, shear-thinning type behavior given that it decreased as deformation rate (γ) increased (). This shear-thinning behavior is similar to that reported for galactomannans such as guar and locust bean, and gums with carboxyl groups, such as xanthan and carboxymethylcellulose (Casas, Mohedano, & García-Ochoa, Citation2000). At the same deformation rate, the CFG had a lower viscosity than the FNG, due to the addition of ionically charged groups (FNG-CHCOO−+Na) indicated in FTIR, which provided the gum a better W DIS and a slight tendency towards Newtonian behavior. The lower viscosity in the FNG caused by carboxymethylation was reflected in a decrease in the consistency index (k) from 264.85 to 26.30 Pa s, and an increase in the fluidity index (n) from 0.26 to 0.48 according to the Ostwald-de Waele model. Zhao et al. (Citation2003) reported similar behavior for carboxymethylation of cellulose, with a decrease in viscosity after modification. They attributed this to possible degradation of the cellulose structure due to the alkali and to the etherifying agent, like this case.
Compression strength of gel
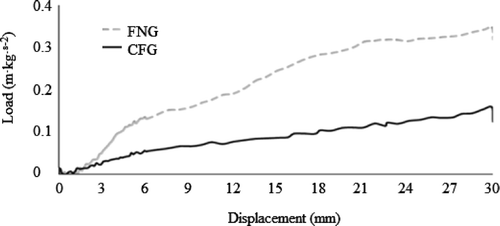
The CSG for the FNG (0.32 m kg s−2) was higher than that for the CFG (0.13 m kg s−2) (), which is directly related to viscosity and SI since modification of the FNG chemical structure affected both characteristics. Modification causes a decrease in gum mechanical and rheological properties and produces a weaker gel (García-Ochoa & Casas, Citation2006). However, both the FNG and CFG didn't show resistance to the rupture by mechanical compression of the gel, they behaved like elastic gels. Burkus and Temelli (Citation1999) reported CSG's higher than that of the FNG for condor gum (3.5 m kg s−2) and barley β-glucans (6.0 m kg s−2). Both these gums are linearly structured, allowing better interaction between their chains and formation of a more resistant gel. Although the FNG also has a linear structure, it is slightly branched, consequently lowering its CSG (Srivastava & Kapoor, Citation2005).
Conclusions
The DS in the carboxymethylated FNG was lower than in commercial carboxymethylated gums such as guar and cellulose. The FTIR analysis indicated that carboxymethyl substitution in the flamboyant native gum occurred only in sterically available secondary alcohols, that the main substituting agent was sodium ether-carboxylate, and that it modified the native structure through presence of more voluminous groups (‒COO−) with a different electronic density. This modification caused structural changes reflected in the transition from the crystalline structure of the FNG to the amorphous structure of the CFG. It also increased the water dispersion, and decreased SI, apparent viscosity and CSG, causing changes in gum functionality that make it potentially versatile in the food industry.
References
- Adikwu , M. U. , Ezeabasili , S. I. and Esimone , C. O. 2001 . Evaluation of the physico-chemical properties of a new polysaccharide gum from prosopis africana . Bolletino Chimico Farmaceutico , 140 : 40 – 45 .
- AOAC International . 1997 . Official methods of analysis of AOAC International , Vol. 17 , Gaithersburg, MD : Author .
- Azero , E. G. and Andrade , C. T. 2006 . Characterization of Prosopis juliflora seed gum and the effect of its addition to κ-carrageenan systems . Journal of the Brazilian Chemical Society , 17 : 844 – 850 .
- Babic , J. , Šubaric , D. , Ackar , D. , Pilizota , V. , Kopjar , M. and Tiban , N. N. 2006 . Effects of pectin and carrageenan on thermophysical and rheological properties of tapioca starch . Czech Journal of Food Sciences , 24 : 275 – 282 .
- Babu , G. V. , Kumar , N. R. , Sankar , K. H. , Ram , B. J. , Kumar , N. K. and Murthy , K. V. 2002 . In vivo evaluation of modified gum karaya as a carrier for improving the oral bioavailability of a poorly water-soluble drug, nimodipine . AAPS Pharmaceutical Science Technology , 3 : 1 – 9 .
- Bahamdan , A. and Daly , W. 2005 . Poly(mxyalkylene) grafts/guar gum with applications in hydraulic fracturing fluids . Polymers for Advanced Technologies , 17 : 679 – 681 .
- Billa , N. and Yuen , K. H. 2000 . Formulation variables affecting drug release from xanthan gum matrices at laboratory scale and pilot scale . AAPS PharmSciTech , 1 : 1 – 8 .
- Bósquez , M. E. and Vernon , C. E. 2005 . Efecto de plastificantes y calcio en la permeabilidad al vapor de agua de películas a base de goma de mezquite y cera de candelilla . Revista Mexicana de Ingeniería Química , 4 : 157 – 162 .
- Burkus , Z. and Temelli , F. 1999 . Gelation of barley ß-glucan concentrate . Journal of Food Science , 64 : 198 – 201 .
- Cardoso , R. S. , Simões , A. L. , Mendes , L. C. , Teixeira , S. C. and Ferreira , A. A. 1988 . HDPE/ROSIN blends: I-morphology, termal and dynamic-mechanical behavior . Polymer Bulletin , 40 : 787 – 793 .
- Casas , J. A. , Mohedano , A. F. and García-Ochoa , F. 2000 . Viscosity of guar gum and xanthan/guar gum mixture solutions . Journal of the Science of Food and Agriculture , 80 : 1722 – 1727 .
- Choi , S. W. , Park , J. M. , Yongsu , C. , Yoon , J. Y. , Seungjoo , H. , Kim , J. H. and Kim , W. S. 2003 . Effect of electrostatic repulsive force on the permeate flux and flux modeling in the microfiltration of negatively charged microspheres . Separation and Purification Technology , 30 : 69 – 77 .
- Dawson , J. C. , Subramanian , K. and Hong , V. L. 2002 . US patent No. 6387853B1 , Houston, TX : BJ Services Company .
- García-Ochoa , F. and Casas , J. A. 2006 . Viscosity of locust bean (Ceratonia siliqua) gum solutions . Journal of the Science of Food and Agriculture , 59 : 97 – 100 .
- Grosev , V. M. , Bozac , R. and Puppels , G. J. 2001 . Vibrational spectroscopic characterization of wild growing mushrooms and toadstools . Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy , 57 : 2815 – 2829 .
- Gupta , S. , Sharma , P. and Soni , P. L. 2004 . Carboxymethylation of Cassia occidentalis seed gum . Journal of Applied Polymer Science , 94 : 1606 – 1611 .
- Jie , Y. , Wenren , C. , Manurung , R. , Ganzeveld , K. and Heeres , H. 2004 . Exploratory studies on the carboxymethylation of cassava starch in water-miscible organic media . Starch/Stärke , 56 : 100 – 107 .
- Kapoor , V. P. 1972 . A galactomannan from the seeds of Delonix regia . Phytochemistry , 11 : 1129 – 1132 .
- Kittipongpatana , O. S. , Chaitep , W. , Charumanee , S. and Kittipongpatana , N. 2006 . Effects of amylose content on the physicochemical properties of sodium carboxymethyl rice starches . Chiang Mai University Journal , 5 : 199 – 207 .
- Lal , S. , O'Connor , C. J. and Eyres , L. 2006 . Application of emulsifiers/stabilizers in dairy products of high rheology . Advances in Colloid and Interface Science , 123 : 433 – 437 .
- Lim , J. M. , Joo , J. H. , Kim , H. O. , Kim , H. M. , Kim , S. X. and Yun , J. W. 2005 . Structural analysis and molecular characterization of exopolysaccharides produced by submerged mycelial culture of Collybia maculata TG-1 . Carbohydrate Polymers , 61 : 296 – 303 .
- Matsuhiro , B. , Presle , L. C. , Saenz , C. and Urzua , C. C. 2006 . Structural determination and chemical modifications of the polysaccharide from seeds of prosopis chilensis mol. (stuntz) . Journal of Chilensis Chemical Society , 51 : 1 – 6 .
- Montgomery , D. C. 2004 . Diseño y análisis de experimentos , México, DF : Limusa-Wiley .
- Morkhade , D. M. , Fulzele , S. V. , Satturwar , P. M. and Joshi , S. B. 2006 . Gum copal and gum damar: Novel matrix forming materials for sustained drug delivery . Indian Journal of Pharmaceutical Sciences , 68 : 53 – 58 .
- Morochi , D. , San Martin , E. and Ordoñez , D. 1999 . Estudio sobre la extracción y caracterización de la goma de flamboyán (Delonix regia) . Revista Ciencia y Tecnología de los Alimentos – Universidad Agraria de la Molina , 1 : 59 – 61 .
- Morochi , D. and Shiomi , N. 1999 . Identificación de los Componentes del Polisacárido de la Goma Flamboyán (Delonix regia) y su Estudio Toxicológico . Revista Ciencia y Tecnología de los Alimentos – Universidad Agraria de la Molina , 1 : 11 – 16 .
- Moslemy , P. , Guiot , S. R. and Neufeld , R. J. 2008 . “ Encapsulation of bacteria for biodegradation of gasoline hydrocarbons ” . In Methods in Biotechnology. Immobilization of enzymes and cells , (2nd ed.) , Totowa, NJ : J.M. Guisan-Human Press Inc. .
- Mothé , C. G. and Rao , M. A. 1999 . Rheological behavior of aqueous dispersions of cashew gum and gum arabic: Effect of concentration and blending . Food Hydrocolloids , 3 : 501 – 506 .
- Nam , S. Y. , Chun , H. J. and Lee , Y. M. 1999 . Pervaporation separation of water-isopropanol mixture using carboxymethylated poly(vinyl alcohol) composite membranes . Journal of Applied Polymer Science , 72 : 241 – 249 .
- Niembro , A. 1986 . Árboles y arbustos útiles de México. Universidad Autónoma Chapingo , México, DF : Limusa .
- Olatunji , G. , Huang , Y. and Dai , Q. 1997 . New derivatives of ethyl cellulose . Cellulose , 4 : 247 – 253 .
- Phaechamud , T. and Ritthidej , G. C. 2007 . Sustained-release from layered matrix system comprising chitosan and xanthan gum . Drug Development and Industrial Pharmacy , 33 : 595 – 605 .
- Pollard , M.A. , Kelly , R. , Wahl , C. , Fischer , P. , Windhab , E. , Eder , B. and Amadó , R. 2006 . Investigation of equilibrium solubility of a carob galactomannan . Food Colloids , 21 : 683 – 692 .
- Rácz , I. and Borsa , J. 1997 . Swelling of carboxymethylated cellulose fibres . Cellulose , 4 : 293 – 303 .
- Reading , M. and Haines , P. J. 1995 . “ Thermomechanical, dynamic mechanical and associated methods ” . In Thermal methods of analysis: Principles, applications and problems , Edited by: Haines , J.P. 123 – 160 . Glasgow : Blackie .
- Reddy , T. and Tammishetti , S. 2002 . Gastric resistant microbeads of metal ion cross-linked carboxymethyl guar gum for oral drug delivery . Journal of Microencapsulation , 19 : 311 – 318 .
- Silva , D. A. , de Paula , C. M. , Feitosa , P. A. , de Brito , C. F. , Maciel , J. S. and Paula , C. B. 2004 . Carboxymethylation of cashew tree exudate polysaccharide . Carbohydrate Polymers , 58 : 163 – 171 .
- Skoog , D. A. , West , D. M. , Holler , F. J. and Crouch , S. R. 2005 . Fundamentos de química analítica , Vol. 8 , España Madrid : Thomson Paraninfo Inc .
- Socaciu , C. 2007 . New techonolgies to synthesize, extract and encapsulate natural food colorants (review) . Bulletin USAMV-CN , 63–64 : 1 – 7 .
- Srivastava , M. and Kapoor , V. 2005 . Seed galactomannans, an overview . Chemistry and Biology , 2 : 295 – 317 .
- Xie , Y. L. , Zhou , H. M. and Qian , H. F. 2005 . Effect of addition of peach gum on physicochemical properties of gelatin-based microcapsule . Journal of Food Biochemistry , 30 : 302 – 312 .
- Zhao , H. , Cheng , F. , Li , G. and Zhang , J. 2003 . Optimization of a process for carboxymethyl cellulose (CMC) preparation in mixed solvents . International Journal of Polymeric Materials , 52 : 749 – 759 .