Abstract
The aim of this study was prevention of thermal degradation of blackberry juice anthocyanins through sugar addition. Blackberry juice samples were prepared without and with addition of 10% of different sugars (sucrose, fructose, glucose and trehalose), and heated at 50, 70 and 90 °C for 1 and 2 h. Since degradation of anthocyanins, over the time, followed first-order reaction model, calculation of the reaction rate constant (k), half-lives (t 1/2) and activation energy was conducted. During heating, at all investigated temperatures, degradation of anthocyanins occurred in higher or lesser extent. Addition of trehalose had the highest impact on anthocyanin stability during heating at all investigated temperatures and during both heating times. Samples with trehalose addition had the lowest k values thus the highest t 1/2 values at all investigated temperatures. In conclusion, careful formulation of foods, in our case sugar addition to blackberry juice, can minimise anthocyanins degradation during heating.
El objetivo de este estudio fue la prevención de degradación termal de antocianinas de zumo de mora por la adición de azúcar. Se prepararon muestras de zumo de mora sin y con adición de 10% de diferentes azúcares (sacarosa, fructosa, glucosa y trehalosa) y se calentaron a 50, 70 y 90 °C durante 1 y 2 horas. Puesto que la degradación de antocianinas, durante el tiempo, siguió el modelo de reacción de primer orden, el cálculo de la constante de velocidad de reacción (k), semivida (t1/2) y energía de activación se llevó a cabo. Durante el calentamiento, en todas las temperaturas investigadas, la degradación de antocianinas sucedió en mayor o menor grado. La adición de trehalosa tuvo el mayor impacto en la estabilidad de antocianinas durante el calentamiento a todas las temperaturas investigadas y durante los dos tiempos de calentamiento. Las muestras con adición de trehalosa tuvieron unos valores k más bajos así como los mayors valores t1/2 a todas las temperaturas investigadas. En conclusión, la formulación cuidadosa de comidas, en nuestro caso adición de azúcar a zumo de mora, puede minimizar la degradación de antocianinas durante el calentamiento.
Palabras clave:
Introduction
The attractive red colour of food products, such as jams, jellies and juices is an important quality parameter, which effects consumers' preference and behaviour, especially when it comes to colour from anthocyanins. Retaining a strong and stable colour of fruit and berry products can be a huge problem during processing and storage, since anthocyanins are highly unstable and susceptible to degradation.
Anthocyanins, the biggest group of water-soluble natural pigments, are glycosides of polyhydroxy and polymethoxy derivatives of 2-phenylbenzopyrylium or flavylium salts. They are responsible for attractive colours of flowers, fruits (especially berries) and vegetables, as well as their products (Mazza & Brouillard, Citation1990). It has been recognised that anthocyanin-rich plant extracts might have potential as natural food colorants. Except as colorants, anthocyanins have multiple biological roles, e.g. antioxidant activity, anti-inflammatory action, inhibition of blood platelet aggregation and antimicrobial activity, treatment of diabetic retinopathy and prevention of cholesterol-induced atherosclerosis (Cliflord, Citation2000; Espin, Soler-Rivas, Wichers, & Garcia-Viguera, Citation2000; Mazza & Miniati, Citation1993; Wang, Cao, & Prior, Citation1997). The major problem of anthocyanins use as natural food colourants is their instability, either in simple or in complex food formulations. Anthocyanins are stable under acidic conditions (pH 2), but under normal processing and storage conditions they transform to colourless compounds and subsequently to insoluble brown pigments. A number of factors influence the stability of anthocyanins, such as temperature, pH, light, oxygen, enzymes, presence of ascorbic acid, sugars, sulphite salts, metal ions and copigments (Bąkowska, Kucharska, & Oszmiański, Citation2003; Francis, Citation1989; Gradinaru, Biliaderis, Kallithraka, Kefalas, & Garcia-Viguera, Citation2003; Jackman, Yada, Tung, & Speers, Citation1987; Tsai & Huang, Citation2004). Many studies were conducted with the aim of improving stability of anthocyanins through addition of different additives, such as acids, sugars, salts, hydrocolloids and different phenolic compounds (Bąkowska et al., Citation2003; Baranac, Petranović, & Dimitrić-Marković, Citation1997; Hubbermann, Heins, Stőckmann, & Schwarz, Citation2006; Kopjar et al., Citation2007, Citation2008, Citation2009a; Kopjar, Piližota, Šubarić, & Babić, Citation2009b; Lewis, Walker, & Lancaster, Citation1995; Mazzaracchio, Pifferi, Kindt, Munyaneza, & Barbiroli, Citation2004; Oszmiański, Wojdyło, & Kolniak, Citation2009; Rein & Heinonen, Citation2004; Rizzolo, Nani, Viscardi, Bertolo, & Torreggiani, Citation2003; Sadilova, Stintzing, Kammerer, & Carle, Citation2009; Tsai, Delva, Yu, Huang, & Dufosse, Citation2005; Wilska-Jeszka & Korzuchowska, Citation1996).
Since anthocyanin content in food products derived from fruit is much lower than in raw material due to manufacturing and processing conditions, formulation of fruit products is very important. Thermal treatment is a necessary step for food preservation; thus, the evaluation of heat-induced anthocyanin degradation is of utmost importance for establishing processes characterized by improved colour retention of the products. Furthermore, anthocyanin degradation products might serve as markers of heat exposure (Sadilova et al., Citation2009). Formulation of fruit products should be governed to applying processing methods or additives that would enhance anthocyanins stability, thus colour of fruit products. During processing, temperature is one of the most important factors influencing anthocyanin stability. Thermal degradation of anthocyanins was extensively studied (Garzon & Wrolstad, Citation2002; Gradinaru et al., Citation2003; Harbourne, Jacquier, Morgan, & Lyng, Citation2008; Kırca & Cemeroğlu, Citation2003; Kırca, Ozkan, & Cemeroglu, Citation2007; Tseng, Chang, & Wu, Citation2006) but there are only few studies on prevention of thermal degradation of anthocyanins by addition of different compounds such as phenolic compounds (Bąkowska et al., Citation2003; Gradinaru et al., Citation2003; Kopjar et al., Citation2009b), sugars (Sadilova et al., Citation2009), and β-cyclodextrin (Mourtzinos et al., Citation2008).
In this study, stability effect of different sugars (sucrose, fructose, glucose and trehalose) on blackberry juice anthocyanins during heating at 50, 70 and 90 °C was investigated.
Materials and methods
Material
Blackberry fruit was bought from local market and kept at −20 °C before sample preparation. Trehalose and fructose were obtained from Merck and sucrose and glucose from Fluka (Germany).
Sample preparation
Blackberry juice was prepared by pressing through cheese cloth and filtered through rough filter paper under the vacuum. Samples of juice were prepared with addition of 10% sugars (sucrose, fructose, glucose and trehalose). Samples were left for 1.5 h to stabilise and then measurements were conducted.
Degradation studies
The thermal stability of anthocyanins in a blackberry juice was studied at 50, 70 and 90 °C. Aliquots of blackberry juice (20 mL) were put into glass tubes, which were well capped for avoiding evaporation of samples. Tubes were put in water bath, which was pre-heated at desired temperature. Samples were heated for 1 and 2 h at a certain temperature, removed from water bath and rapidly cooled in ice-cooled water to the room temperature. After cooling, determination of monomeric anthocyanins was conducted.
Measurement of monomeric anthocyanins
Determination of monomeric anthocyanins was conducted by pH-differential method (Giusti & Wrolstad, Citation2001). Total monomeric anthocyanins were expressed as cyanidin-3-glucoside. Sample absorbance was read against a blank cell containing distilled water. The absorbance (A) of the sample was then calculated according the following formula:
The monomeric anthocyanin pigment content in the original sample was calculated according the following formula:
Calculation of kinetic parameters of anthocyanin degradation
The first-order reaction rate constants (k), half-lives (t 1/2), i.e. the time which is necessary for degradation of 50% of anthocyanins, were calculated by the following equations:
Arrhenius model was applied to describe the temperature dependence of anthocyanin degradation.
According to Arrhenius equation, there is linear relationship between lnk and 1/T:
Statistical analysis
Anthocyanin content was analyzed by the analysis of variance (ANOVA) and Fisher's least significant difference (LSD) with significance defined at P < 0.05. All statistical analyses were carried out using the software program STATISTICA 8 (StatSoft, Inc, USA). The results were expressed as means ± standard deviation.
Results and discussion
Anthocyanin content
Temperature is one of the most important factors influencing anthocyanin stability and thus prevention of thermal degradation of blackberry juice anthocyanins was studied. Different sugars (sucrose, fructose, glucose and trehalose) in amount of 10% were added in order to increase anthocyanin stability during heating of blackberry juice at 50, 70 and 90 °C for 1 and 2 h. Sample without addition of sugars was used as control sample. Results of anthocyanin content determination are presented in Supplementary Table 1. Anthocynin content of blackberry juice prior heating was 202.91 mg/L. During heating of all samples, degradation of anthocyanins occurred in higher or lesser extent, from 99.32 to 180.60 mg/L, depending on added sugars, applied temperatures and heating time. All blackberry juice samples with addition of sugars heated at 50 °C had higher anthocyanin content in comparison to control sample, regardless of heating time. At this temperature, the highest stability of anthocyanins was achieved with addition of glucose (179.35 and 159.56 mg/L, depending on heating time) and trehalose (180.60 and 160.94 mg/L, depending on heating time). It has been reported that sugar stabilizes the red colour of strawberry anthocyanin with 40% sucrose concentration or at temperatures below 55 °C due to reduced water activity, hyperchromic effect, enzymes inhibition or steric interference (Wrolstad, Skrede, Lea, & Enersen, Citation1990).
Supplementary Table 1. Anthocyanin content (mg/L) of blackberry juice prepared without and with addition of different sugars during heating for 1 and 2 hours at 50, 70 and 90 °C.
Tabla adicional 1. Contenido de antocianinas (mg/L) de zumo de mora preparado sin y co adición de diferentes azúcares durante el calentamiento de 1 y 2 horas a 50, 70 y 90 °C.
At 70 and 90 °C different tendency was observed, showing importance of type of sugar applied for prevention of stabilisation. Blackberry juice samples with addition of sucrose and fructose had lower anthocyanin content in comparison to control sample, without showing stabilisation effect of these two sugars on anthocyanins. In contrast to sucrose and fructose, addition of glucose and trehalose improved anthocyanin stability at those two temperatures. Antocyanin content of blackberry juice heated at 70 °C was 159.04 mg/L and 124.62 mg/L, depending on heating time. At 90 °C, antocyanin content of control sample was 142.65 mg/L and 101.82 mg/L, depending on heating time. Samples with addition of sucrose heated at 70 °C had the lowest anthocyanin content of 156.43 mg/L and 120.24 mg/L, depending on heating time, and that tendency was retained at 90 °C (140.89 mg/L and 99.57 mg/L, depending on heating time). Overall looking, trehalose had the highest effect on anthocyanin stability, especially at higher temperatures. At 70 °C, anthocyanin content of blackberry juice was 163.57 mg/L and 127.50 mg/L, depending on heating time, and at 90 °C the anthocyanin content was 145.66 mg/L and 107.58 mg/L.
Protection of flavylium ring against attack by water is absolutely necessary to maintain intensely coloured solutions. One way of retaining anthocyanin colour is by removal of water and displacement of the hydration/dehydration equilibrium towards the coloured species (i.e. reduce the extent of the hydration reaction) (Brouillard, Citation1983; Lewis et al., Citation1995). It is well known that sugar causes decrease in water activity and sugar molecules are effective at binding water (Coultate, Citation1989); so in this way sugars could protect anthocyanins from water attack, thus increasing their stability. Also, it was suggested that two mechanisms are responsible for thermal degradation of anthocyanins: (a) hydrolysis of the 3-glycoside linkage to form the more labile aglycon; and (b) hydrolytic opening of the pyrilium ring to form a substituted chalcone, which then degrades to a brown insoluble compound of a polyphenolic nature (Simpson, Citation1985). Probably, all sugars at lower temperature (50 °C), and glucose and trehalose at higher temperatures (70 °C and 90 °C), suppress one of these two mechanisms responsible for thermal degradation of anthocyanins.
The ability of sugar molecules to bind protectively onto the surface of molecular structures has also been ascribed to their ability to form hydrogen bonds, the so-called “water replacement hypothesis”. Unlike most other disaccharides, trehalose has no direct internal hydrogen bonds. All four internal bonds are indirectly connected via the two water molecules, which form part of the native dihydrate structure. This arrangement gives the molecule an unusual flexibility around the disaccharide bond, which may allow it to fit more closely with the irregular surface of macromolecules than other, more rigid disaccharides, in which the rings are directly hydrogen bonded to each other (Colaço & Roser, Citation1995). According to Bordat, Lerbret, Demaret, Affouard, and Descamps (Citation2004) trehalose has superior effects in “destructuring” the network of water and in slowing down its dynamics. This property could play a key role in the understanding of the microscopic mechanisms of bioprotection.
Steric hindrance developed by the disaccharide, which can protect or slow down the nucleophilic attacks of water, was probably more pronounced in samples with trehalose addition than in samples with sucrose addition due to higher stability of trehalose. Trehalose has low free energy of activation of glycosidic bond, so trehalose structure is very stable regarding hydrolysis in comparison to sucrose (Colaço & Roser, Citation1995). Elevated levels of 5-hydroxymethylfurfural (HMF) may effect anthocyanin stability (Sadilova et al., Citation2009), which could also be a reason of anthocyanin stability when glucose and trehalose were added to samples, since Sadilova et al. (Citation2009) reported that samples with addition of glucose had the lowest HMF levels.
Kinetic parameters
Results of calculation of kinetic parameters are presented in Supplementary Table 2. Prevention of anthocyanins from thermal degradation was also proven by calculation of the reaction rate constants and half-lives. The linear relationship indicated that degradation of anthocyanins fitted first-order reaction kinetics both in blackberry juice (R 2 from 0.9650 to 0.9952, depending on temperature) and blackberry juice with addition of sugars (R 2 from 0.9867 to 0.9994, depending on temperature). Previous studies showed that degradation of anthocyanins also followed first-order degradation kinetics (Domínguez-López, Remondetto, & Salvador, Citation2008; Garzon & Wrolstad, Citation2002; Gradinaru et al., Citation2003; Kırca & Cemeroğlu, Citation2003; Mourtzinos et al., Citation2008; Tseng et al., Citation2006; Yue & Xu, Citation2008), thus it was possible to calculate the reaction rate constants and half-life of anthocyanin degradation.
Supplementary Table 2. Kinetic parameters for thermal degradation of anthocyanins of blackberry juice without and with addition of sugars.
Tabla adicional 2. Parámetros cinéticos para la degradación termal de antocianinas de zumo de mora sin y con adición de azúcares.
As expected, the degradation of anthocyanins increased with increased temperature and time. Blackberry juice without addition of sugars had reaction rate constant 0.130 h−1 at 50 °C. Addition of sugars (sucrose, fructose, glucose and trehalose) caused decrease of reaction rate constant from 0.116 h−1 to 0.127 h−1. The lowest reaction rate constant had samples with addition of trehalose. With increase of temperature increase of reaction rate constants occurred, but the tendency at higher temperatures (70 and 90 °C) was different. At 70 °C, samples with sucrose and fructose addition had higher values of reaction rate constants (0.262 h−1 and 0.248 h−1) than sample without sugar addition (0.244 h−1), while samples with addition of glucose and trehalose had lower values of this parameter (0.241 h−1 and 0.232 h−1) in comparison to control sample. At 90°C, this tendency was more perceived, and in samples with sucrose and fructose addition reaction rate constants (0.356 h−1 and 0.357 h−1) increased, while samples with addition of glucose and trehalose had lower values of this parameter (0.338 h−1 and 0.317 h−1) in comparison to sample without sugar addition (0.345 h−1).
Comparison of half-life values revealed that at 50°C, all sugars prevented thermal degradation of anthocyanins. Sample without sugars had value of half-lives 5.345 h, and with sugars addition values increased and ranged from 5.445 h to 5.982 h. The highest half-life value had sample with addition of trehalose. At higher temperatures, a different tendency was observed. As was expected, values of half-lives decreased. At both investigated temperatures, samples with addition of sucrose and fructose had lower half-lives than blackberry juice, while samples with addition of glucose and trehalose had higher values, indicating higher stability of anthocyanins.
Sadilova et al. (Citation2009) investigated influence of sucrose, fructose and glucose on black carrot, elderberry and strawberry anthocyanin stability during heating at 95 °C and obtained t 1/2 values from 1.54 h to 3.16 h depending on sugar type and source of anthocyanins. The highest t 1/2 values had samples with glucose addition. Our results are in accordance to their findings; samples with addition of sucrose and fructose had lower values of t 1/2 than samples with addition of glucose and trehalose.
Kopjar et al. (Citation2009b) investigated prevention of thermal degradation of red currant juice anthocyanins by addition of different phenolic compounds. Since the reaction rate constants were lower and half-lives higher when phenolic compounds were added to juice, stabilization of anthocyanins at high temperatures was achieved. Mourtzinos et al. (2008) investigated thermal stability of anthocyanin extract of Hibiscus sabdariffa L. in the presence of β-cyclodextrin, and found out that β-cyclodextrin decreased reaction rate constant and increased half-lives at all investigated temperatures (60, 70, 80 and 90 °C); thus their results indicated that anthocyanins were more stable in the presence of β-cyclodextrin.
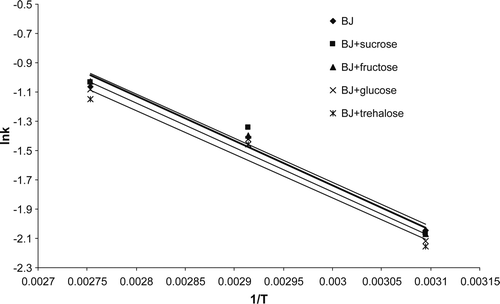
The activation energies (Supplementary Table 2 and Supplementary Figure 1) of degradation in the presence of sugars, as calculated by the Arrhenius plot, were higher (from 24.72 kJ/mol to 25.44 kJ/mol) than in absence of sugars (23.96 kJ/mol). Mourtzinos et al. (Citation2008) with calculation of E a observed that sample without and with β-cyclodextrin had similar values, 54.05 kJ/mol and 54.02 kJ/mol, and they concluded that degradation mechanism of anthocyanins inside the β-cyclodextrin and of the free anthocyanins were the same. Since in our case E a was higher when sugars were added, degradation of anthocyanins when sugars were added did not follow the same mechanism as degradation of anthocyanins without sugar addition.
Conclusions
Protection of blackberry juice anthocyanins from thermal degradation by addition of sugars (sucrose, fructose, glucose and trehalose) was investigated. Our study showed that at 50 °C all investigated sugars improved anthocyanin stability, while at higher temperatures (70 and 90 °C) stabilisation effect was only observed when glucose and trehalose were added to blackberry juice.
Results of our study could attribute to stability of anthocyanins at high temperature and help food technologist in formulation of food products choosing the sugar that is the most appropriate for achieving the desired food quality properties.
Supplementary material
The supplementary material for this article is available online at http://dx.doi.org/10.1080/19476337.2010.522735
tcyt_a_522735_sup_21461530.pdf
Download PDF (483.2 KB)Acknowledgement
This work was supported by the Ministry of Science, Education and Sports of the Republic of Croatia.
References
- Bąkowska , A. , Kucharska , A. Z. and Oszmiański , J. 2003 . The effects of heating, UV irradiation, and storage on stability of the anthocyanin-polyphenol copigment complex . Food Chemistry , 81 : 349 – 355 .
- Baranac , J. M. , Petranović , N. A. and Dimitrić-Marković , J. M. 1997 . Spectrophotometric study of anthocyan copigmentation reactions 2. Malvin and the nonglycosidized flavone quercetin . Journal of Agricultural and Food Chemistry , 45 : 1694 – 1697 .
- Bordat , P. , Lerbret , A. , Demaret , J.-P. , Affouard , F. and Descamps , M. 2004 . Comparative study of trehalose, sucrose and maltose in water solutions by molecular modelling . Europhysics Letters , 65 : 41 – 47 .
- Brouillard , R. 1983 . The in vivo expression of anthocyanin colour in plants . Phytochemistry , 22 : 1311 – 1365 .
- Cliflord , M. N. 2000 . Chlorogenic acids and other cinnamatesnat-nature, occurrence, dietary burden, absorption and metabolism . Journal of Science of Food and Agriculture , 80 : 1033 – 1043 .
- Colaço , C. A.L.S. and Roser , B. 1995 . “ Trehalose-a multifunctional additive for food preservation ” . In Food packaging and preservation , Edited by: Mathlouthi , M. (pp. 123–140). London : Blackie Professional .
- Coultate , T. P. 1989 . Food: The chemistry of its components , London : Royal Society of Chemistry .
- Domínguez-López , A. , Remondetto , G. E. and Salvador , N.-G. 2008 . Thermal kinetic degradation of anthocyanins in a roselle (Hibiscuss abdariffa L. cv. ‘Criollo’) infusion . International Journal of Food Science and Technology , 43 : 322 – 325 .
- Espin , J. C. , Soler-Rivas , C. , Wichers , H. and Garcia-Viguera , C. 2000 . Anthocyanin-based natural colorants: A new source of antiradical activity for food stuff . Journal of Agricultural and Food Chemistry , 48 : 1588 – 1592 .
- Francis , F. J. 1989 . Food colorants: Anthocyanins . Critical Reviews of Food Science and Nutrition , 28 : 273 – 314 .
- Garzon , G. A. and Wrolstad , R. E. 2002 . Comparision of the stability of pelargonidin based anthocyanins in strawberry juice and concentrate . Journal of Food Science , 67 : 1288 – 1299 .
- Giusti , M. M. and Wrolstad , R. E. 2001 . Characterization and measurement of anthocyanins by UV-visible spectroscopy. Unit F1.2, in Current Protocols in Food Analytical Chemistry , New York, NY : John Wiley & Sons, Inc .
- Gradinaru , G. , Biliaderis , C. G. , Kallithraka , S. , Kefalas , P. and Garcia-Viguera , C. 2003 . Thermal stability of Hibiscus sabdariffa L. anthocyanins in solution and in solid state: Effects of copigmentation and glass transition . Food Chemistry , 83 : 423 – 436 .
- Harbourne , N. , Jacquier , J. D. , Morgan , D. M. and Lyng , J. G. 2008 . Determination of the degradation kinetics of anthocyanins in a model juice system using isothermal and non-isothermal methods . Food Chemistry , 111 : 204 – 208 .
- Hubbermann , E. M. , Heins , A. , Stőckmann , H. and Schwarz , K. 2006 . Influence of acids, salt, sugars and hydrocolloides on the colour stability of anthocyanins rich blackcurrant and elderberry concentrates . European Food Research and Technology , 223 : 83 – 90 .
- Jackman , R. L. , Yada , R. Y. , Tung , M. A. and Speers , R. A. 1987 . Anthocyanins as food colorants-review . Journal of Food Biochemistry , 11 : 201 – 247 .
- Kırca , A. and Cemeroğlu , B. 2003 . Thermal degradation of blood orange anthocyanins . Food Chemistry , 81 : 583 – 587 .
- Kırca , A. , Ozkan , M. and Cemeroglu , B. 2007 . Effects of temperature, solid content and pH on the stability of black carrot anthocyanins . Food Chemistry , 101 : 212 – 218 .
- Kopjar , M. , Piližota , V. , Hribar , J. , Simčič , M. , Zlatič , E. and Nedić Tiban , N. 2008 . Influence of trehalose addition and storage conditions on the quality of strawberry cream filling . Journal of Food Engineering , 87 : 341 – 350 .
- Kopjar , M. , Piližota , V. , Nedić Tiban , N. , Šubarić , D. , Babić , J. and Ačkar , Đ. 2007 . Effect of different pectin addition and its concentration on colour and textural properties of raspberry jam . Deutsche Lebensmittel-Rundschau , 103 : 164 – 168 .
- Kopjar , M. , Piližota , V. , Nedić Tiban , N. , Šubarić , D. , Babić , J. , Ačkar , Đ. and Sajdl , M. 2009a . Strawberry jams: Influence of different pectins on colour and textural properties . Czech Journal of Food Science , 27 : 20 – 28 .
- Kopjar , M. , Piližota , V. , Šubarić , D. and Babić , J. 2009b . Prevention of thermal degradation of red currant juice anthocyanins by phenolic compounds addition . Croatian Journal of Food Science and Technology , 1 : 24 – 30 .
- Lewis , C. E. , Walker , J. R.L. and Lancaster , J. E. 1995 . Effect of polysaccharides on the colour of the anthocyanins . Food Chemistry , 54 : 315 – 319 .
- Mazza , G. and Brouillard , R. 1990 . The mechanism of copigmentation of anthocyanins in aqueous solutions . Phytochemistry , 29 : 1097 – 1102 .
- Mazza , G. and Miniati , E. 1993 . Anthocyanins in fruits, vegetables and grains , Boca Raton, FL : CRC Press .
- Mazzaracchio , P. , Pifferi , P. , Kindt , M. , Munyaneza , A. and Barbiroli , G. 2004 . Interactions between anthocyanins and organic food molecules in model systems . International Journal of Food Science and Technology , 39 : 53 – 59 .
- Mourtzinos , I. , Makkis , D. P. , Yannakopoulou , K. , Kalogeropoulos , N. , Michali , I. and Karathanos , V. T. 2008 . Thermal stability of Anthocyanin extract of Hibiscuss abdariffa L. in the presence of β-cyclodextrin . Journal of Agricultural and Food Chemistry , 56 : 10303 – 10310 .
- Oszmiański , J. , Wojdyło , A. and Kolniak , J. 2009 . Effect of l-ascorbic acid, sugar, pectin and freeze-thaw treatment on polyphenol content of frozen strawberries . LWT , 42 : 581 – 586 .
- Rein , M. J. and Heinonen , M. 2004 . Stability and enhancement of berry juice color . Journal of Agricultural and Food Chemistry , 52 : 3106 – 3114 .
- Rizzolo , A. , Nani , R. C. , Viscardi , D. , Bertolo , G. and Torreggiani , D. 2003 . Modification of glass transition temperature through carbohydrates addition and anthocyanin and soluble phenol stability of frozen blueberry juices . Journal of Food Engineering , 56 : 229 – 231 .
- Sadilova , E. , Stintzing , F. C. , Kammerer , D. R. and Carle , R. 2009 . Matrix dependent impact of sugar and ascorbic acid addition on color and anthocyanin stability of black carrot, elderberry and strawberry single strength and from concentrate juices upon thermal treatment . Food Research International , 42 : 1023 – 1033 .
- Simpson , K. L. 1985 . “ Chemical changes in food during processing ” . In Chemical changes in natural food pigments , Edited by: Richardson , T. and Finley , J. W. New York, NY : Van Nostrand Reinhold .
- Tsai , P. J. , Delva , L. , Yu , T. Y. , Huang , Y. T. and Dufosse , L. 2005 . Effect of sucrose on the anthocyanin and antioxidant capacity of mulberry extract during high temperature heating . Food Research International , 38 : 1059 – 1065 .
- Tsai , P. J. and Huang , H. P. 2004 . Effect of polymerization on the antioxidant capacity of anthocyanins in roselle . Food Research International , 37 : 313 – 318 .
- Tseng , K.-C. , Chang , H.-M. and Wu , J. S.-B. 2006 . Degradation kinetics of anthocyanins in ethanolic solutions . Journal of Food Processing Preservation , 30 : 503 – 514 .
- Wang , H. , Cao , G. and Prior , R. 1997 . Oxygen radical absorbing capacity of anthocyanins . Journal of Agricultural of Food Chemistry , 45 : 304 – 309 .
- Wilska-Jeszka , J. and Korzuchowska , A. 1996 . Anthocyanins and chlorogenic acid copigmentation. Influence on the color of strawberry and chokeberry juices . Zeitschrift für Lebensmitteluntersuchung und –Forschung A , 203 : 38 – 42 .
- Wrolstad , R. E. , Skrede , G. , Lea , P. and Enersen , G. 1990 . Influence of sugar on anthocyanin pigment stability in frozen strawberries . Journal Food Science , 55 : 1064 – 1065 .
- Yue , X. and Xu , Z. 2008 . Changes of anthocyanins, anthocyanidins and antioxidant activity in bilberry extract during drying heating . Journal of Food Science , 73 : C494 – C499 .
Supplementary Figure 1. Relationship lnk vs 1/T for blackberry juice without and with addition of sugars (BJ, blackberry juice).
Figura adicional 1. Relación entre lnk y 1/T para zumo de mora sin y con adición de azúcares (BJ – zumo de mora).