Abstract
Several physicochemical parameters were determined to study the behavior of three commercial oils with different chemical composition in deep fat frying at 160°C. Peroxide value seems not to be an accurate tool to control the quality of frying oils. On the contrary, iodine value, conjugated diene, and viscosity gave useful information about the oils' degradation during deep fat frying. Sunflower oil (SO) showed the highest increase in conjugated diene value. The increase in viscosity was lower for olive oil. The fatty acid profile changed throughout the fryings. Levels of palmitic acid increased and levels of linoleic acid decreased, and high correlations between palmitic acid, oleic acid, and linoleic acid in all oils were found. Trans fatty acids increased in all oils with increasing frying cycles. Extra virgin olive oil and high-oleic acid sunflower oil seem to be the more suitable oils for frying at 160°C.
Se determinaron varios parámetros fisicoquímicos (compuestos polares, índice de yodo, índice de peróxidos, dienos conjugados, viscosidad, ácidos grasos y ácidos grasos trans) para estudiar el comportamiento de tres aceites comerciales con diferente composición (aceite de oliva extra virgen, aceite alto oleico de girasol y aceite de girasol) en fritura por inmersión a 160°C. El índice de peróxidos parece no ser una herramienta exacta para el control de los aceites de fritura. Por el contrario, el índice de yodo, los dienos conjugados y la viscosidad proporcionan información útil sobre la degradación del aceite durante la fritura por inmersión. El aceite de girasol mostró el aumento más alto en el valor de dienos conjugados. El incremento de la viscosidad más bajo fue en el aceite de oliva virgen extra. El perfil de ácidos grasos cambió a lo largo de la fritura. Los niveles de ácido palmítico aumentaron y los de ácido linoleico disminuyeron, habiéndose encontrado altas correlaciones entre el ácido palmítico, oleico y linoleico en todos los aceites. Los ácidos grasos trans aumentaron en todos los aceites a medida que aumentaban los ciclos de fritura. Los aceites de oliva virgen extra y de girasol alto oleico parecen ser los más adecuados para fritura a 160°C.
Introduction
Deep fat frying is one of the oldest culinary techniques used in food preparation, especially in the Mediterranean area. This process exerts huge changes in sensorial and nutritive properties of food. By the immersion of food in hot oil, a brown and crispy crust is formed, a certain amount of oil is absorbed by the food, and its flavor is enhanced. The quality of fried foods depends not only on the frying conditions, such as temperature, frying time, or ratio of food:oil, but also on oil and kind of food used (Varela, Citation1994).
Frying not only changes the food, but it also modifies the oil's physical and chemical properties. There are three important factors in oil alteration during frying: the exposure to water (from food), to high temperatures, and to oxygen. Under these conditions, oils undergo oxidation, polymerization, hydrolysis, cyclization, and isomerization (Lalas, Citation2009). The hydrolysis of oils may cause the formation of free fatty acids. In addition, thermo-oxidation of oil could lead to formation of hydro-peroxides and conjugate di-, tri-, and tetradiene groups, and could also cause the loss of unsaturated fatty acids. The polymerization of oils results in an increase in viscosity. All these physical and chemical changes worsen the quality of oil. In commercial and industrial frying, the oil is exposed to high temperature, moisture, and oxygen for a long time. As a result of the chemical reactions described above, a high number or harmful compounds are generated and the quality of the oil changes drastically. Several methods have been proposed in order to measure changes in deep fat frying oils (Lalas, Citation2009; Paul & Mittal, Citation1997; White, Citation1991). Perhaps, the most extended method to control the quality of fried oil is the total polar compounds measurement. Spanish laws and regulations establish amaximum level of polar compounds of 25% for used frying fats and oils (Orden 26/01/1989).
Usually many oils can be used for deep fat frying. Although olive oil has been used traditionally in the Mediterranean area, in the last decades its use has declined in favor of other vegetable oils like sunflower oil (SO) and other seed oils. These oils have a mild flavor and are cheaper, which is a very important parameter in commercial frying, even in small restaurants. Nevertheless, these oils have different physicochemical properties and chemical composition (fatty acids profiles, phenolic compounds, and minor lipid components), which can lead to a different behavior during frying, different stability, and different shelf-life.
The aim of this work is to evaluate the changes during frying of several oils that are commonly used for frying in Spain: olive oil, SO, and high-oleic acid sunflower oil (HOSO). This could be interesting both to the consumer and the foodservices and food industry, which produces ready meals.
Materials and methods
Materials for frying
Extra virgin olive oil (EVOO) (Arbequina and Empeltre varieties; Aceites Palacio™, Aragón, Spain), refined SO (Koipesol™, Andalucía, Spain), refined HOSO (Titan™, Andalucía, Spain), and frozen pre-fried potatoes (Agristo, Harelbeke, Belgium) were purchased at a local supermarket.
Frying protocol
A domestic deep fat fryer with 3-l vessel (Magefesa III Levante) was used for frying the 300 g of potatoes at 160°C. The fryer was filled with 3000 ml of oil at the beginning of the deep fat frying process, and the oil was used continuously until it ran out. The potatoes were removed from the fryer as soon as they were uniformly cooked without external burnings. Samples of fresh and fried oils, collected from different frying cycles (10 ml), were stored at −20°C in glass bottles until analysis. The oil temperature was monitored with a digital thermometer attached to a steel probe (Crison, Barcelona, Spain) and a handheld digital thermometer (HDT-1, Humanlitech Co., Ltd, Korea).
Polar compound measurements
Measurement of polar compounds (in percentage) was done with a Testo 265 oil sensor at the end of each frying cycle. This rapid method based on dielectric constant measurement has been validated by Dobarganes and Márquez-Ruiz (Citation1995) using thin layer chromatography as reference method.
Peroxide value
The peroxide value was calculated by the modified method of Matissek, Schnepel and Steiner (Citation1998). Olive oil samples (5 g) were placed in a 100-ml flask and dissolved in 30 ml of acetic acid–chloroform solution (3:2). Then, 0.5 ml of saturated solution of KI was added and was stirred for 1 min. It was left to stand in the dark for 5 min and then 30 ml of water was added. The liberated iodine was titrated with 0.01 N Na2S2O3. When the brown color tended to disappear, 1 ml of a solution of soluble starch (1%) was added to give a better control of the end point.
Iodine value
The iodine value was determined according to Wijs as indicated by AOAC Official Methods of Analysis (1984). Olive oil samples (0.5–0.6 g) were placed in a 300-ml flask and dissolved in 15 ml of carbon tetrachlorine. Then, 25 ml of Wijs solution was added and was stirred softly. It was left to stand in the dark for 1 or 2 h, depending on expected iodine value of the sample. Then, 20 ml KI solution (10%) and 150 ml of water were added. The excess iodine was titrated with 0.1 N Na2S2O3 with constant and vigorous shaking. When the yellow color almost disappeared, 1 ml of soluble starch (1%) solution was added until blue color disappeared.
Absorbance spectrum in the UV
The method described by Matissek et al. (Citation1998) was used to determine the presence of isolene and conjugated di-, tri-, and tetradiene groups. Olive oil sample (0.05 g) was placed in a 20-ml volumetric flask and the total volume was adjusted by adding isooctane. A reference solution, consisting of methyl stearate 1% in isooctane, was also prepared. The spectrum of the sample was read against the blank between 220 and 330nm, with a Jasco UV-Vis Spectrometer V-530 (Easton, MD) controlled by a personal computer with the software Spectra Manager.
Viscosity measurements
A rotational cone-plate viscometer Brookfield CAP2000+ was used to measure the different oil viscosities. The apparatus was controlled by a PC using the software CAP-CALC. Temperature (25°C) was controlled by a Peltier device. The viscosity value is automatically calculated on the basis of the speed and the geometry of the probe.
Lipid extraction and analysis
Lipid extraction of fried potatoes was carried out with a SOXTEC extractor (Foss Tecator, Sweden) equipped with six Soxhlet posts. Petroleum ether (40–60°C) was selected as the extraction solvent.
Fatty acid composition of the oils was determined according to the EU Regulation 796/2002 (Commission Regulation (EC) No 796/2002). Two ml of hexane was added to 0.1 g of sample and vigorously mixed. Then, 0.2 ml of potassium hydroxide methanolic solution (2 N) was added and vigorously mixed; and the solution was left in the dark for 15 min. Then, 0.5 ml of the upper part of the solution (clear) was placed in a vial and 0.02 ml of internal standard was added. Fatty acid methyl esters (FAME) separation was performed in gas chromatograph (GC) (Hewlett-Packard HP5890 series II) with a split/splitless injector and a FID detector, equipped with an HP-88 capillary column of 100 m length, 0.25 mm i.d., and 0.20 μm film thickness (J & W Scientific, Folsom, CA). The temperature program was as follows: initial temperature, 120°C (hold for 1 min); from 120°C to 175°C at 10°C/min (hold for 10 min); from 175°C to 210°C at5°C/min (hold for 5 min); from 210°C to 230°C at 5°C/min, then hold at 230°C for 30 min; run time, 62.50 min. The injector and detector temperatures were 250 and 280°C, respectively. Nitrogen was used as carrier gas at a flow rate of 1.18 ml/min. Split ratio of 43:1 was used and 1.0 μl was injected in GC for the analysis. Data acquisition and processing were performed with an Hewlett-Packard Chemstation software. FAME were identified by comparing their retention times with those for commercial standard mixtures (Supelco 37 component FAME Mix, linoleic acid methyl ester isomer mix, and linolenic acid methyl ester isomer mix; Supelco, Bellefonte, PA, US). Methyl tridecanoate (puriss, GCgrade, Sigma-Aldrich Chemical Co, Steinheim, Germany) was used as an internal standard.
Results and discussion
Peroxide value
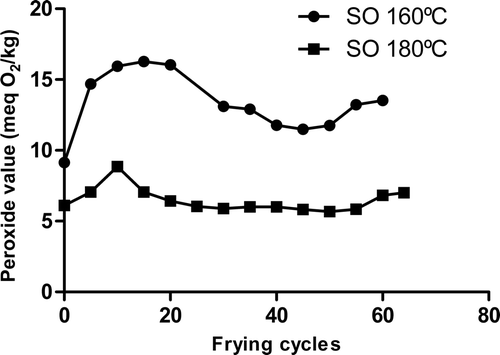
Supplementary shows the evolution of peroxide values of SO in different frying cycles. Similar results were obtained for EVOO and HOSO (data not shown). At 160°C, peroxide values showed a slight increase during the first 20 frying cycles followed by a decrease. At 180°C, peroxide values showed a slight peak at 10 cycles, remaining almost constant after 15 frying cycles. Our results are in accord to those obtained by Casal, Malheiro, Sendas, Oliveira, and Pereira (Citation2010) and by Ndjouenkeu and Ngassoum (Citation2002). Peroxides are transient chemical compounds and they are also unstable under frying conditions. There is equilibrium between peroxide formation rate and peroxide degradation rate. According to Choe and Min (Citation2007), hydroperoxides are decomposed to alkoxy radicals and hydroxyl radicals by homolysis of the peroxide bond. This would explain why at 160°C peroxides increase during the first 20 frying cycles and then decrease. It also explains why, at a higher temperature (180°C), peroxide values are lower: at severe frying conditions, peroxide decomposition rate becomes higher than peroxide formation rate (Velasco, Marmesat, & Dobarganes, Citation2009). Although peroxide value is a classical method to evaluate oil oxidation, it seems not to be an accurate tool for the evaluation of thermal treated oils.
Polar compounds
The polar compounds are product of the oil's oxidation. An increase in polar compounds was observed for the three oils with increasing frying cycles (data not shown). Nevertheless, the maximum limit established by the Spanish legislation for heated fats (25% polar compounds) was not reached in any of the studied conditions.
Iodine value
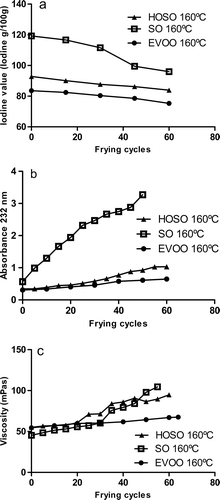
Iodine value is a measure of the number of double bonds in the oil. Supplementary (a) shows the changes in iodine value at 160°C for the studied oils. During frying, a progressive decrease in unsaturation was observed in all oils. This decrease shows the consumption of double bonds by oil oxidation. Our results were also corroborated by the changes in the fatty acid composition of the oils during frying (Supplementary ). Similar results were obtained by Ndjouenkeu and Ngassoum (Citation2002), Sanchez-Gimeno, Negueruela, Benito, Vercet, and Oria (Citation2008b) and Takeoka, Full, and Dao (Citation1997). Although the decrease in iodine value is a result of complex physicochemical changes, this decrease is indicative of the oxidation rate (Lalas, Citation2009) and could be a useful quality parameter to control oil quality during frying.
Conjugated diene
Supplementary (b) shows the conjugated diene formation during frying at 160°C in EVOO, SO, and HOSO. Conjugated diene content increased in the three oils at 160°C. The conjugated diene formation was faster and higher in SO than in the other two studied oils (EVOO and HOSO). Similar results were obtained for SO (Farhoosh & Tavassoli-Kafrani, Citation2011; Smith, King, & Min, Citation2007), HOSO (Smith et al., Citation2007), and EVOO (Sanchez-Gimeno, Benito, Vercet, & Oria, Citation2008a). Conjugated dienes are formed during oxidation of unsaturated fatty acids containing two or more double bonds to achieve a more stable radical (Choe & Min, Citation2007). Our results are according to this hypothesis and to previous results from Smith et al. (Citation2007): the oil with highest content in poly-unsaturated fatty acids (PUFA) (in our study, SO) showed the highest increase in conjugated dienes.
Viscosity
Oils viscosity increased during frying. Supplementary (c) shows the change in viscosity depending on the frying cycle at 160°C for the three studied oils. Viscosities tend to increase with frying cycles, this effect being particularly noticeable after 20 frying cycles and for SOs. On the contrary, EVOO showed the lowest increase in viscosity. Several authors have studied physical changes during frying of several oils with different results (Bansal, Zhou, Barlow, Neo, & Lo, Citation2010; Besbes et al., Citation2005; Lalas, Gortzi, & Tsaknis, Citation2006; Sanchez-Gimeno et al., Citation2008b; Santos, Santos, & Souza, Citation2005; Valdes & Garcia, Citation2006). The increase of oil viscosity is attributed to polymerization and formation of high molecular weight compounds. Dimers and polymers are large molecules with a molecular weight range between 690 and 1600 Daltons and their formation depends on the oil type, frying temperature, and numbers of frying (Choe & Min, Citation2007). Several authors have found that an oil rich in linoleic acid is more easily polymerized during deep fat frying than an oil rich in oleic acid (Takeoka et al., Citation1997; Valdes & Garcia, Citation2006). Our results are in accord to this hypothesis: the oil with the lowest content in linoleic acid (EVOO) showed the lowest increase in viscosity during frying. This test is a useful method to evaluate heat abuse of polyunsaturated oils (Lalas, Citation2009).
Fatty acid methyl esters (FAME)
The major fatty acid in fresh EVOO and HOSO was oleic acid (75.6% and 71.2%), but EVOO had lower linoleic acid (8.1% vs 17.6%) and higher palmitic acid (11.6% vs 4.1%), and linolenic acid (0.97% vs 0.07%) contents than HOSO. In SO, linoleic acid (53.5%) and oleic acid (33.8%) were the major fatty acids followed by palmitic acid (6.6%) and linolenic acid (0.09%).
Changes in ratios among total saturated fatty acids (SFA), mono-unsaturated fatty acids (MUFA), and PUFA at 160°C are shown in Supplementary .
According to Andrikopoulos, Dedoussis, Falirea, Kalogeropoulos, & Hatzinikola (Citation2002), the triglyceride fatty acid content remained relatively constant except for a slight decrease of PUFA/SFA and MUFA/SFA ratios after 10 fryings. However, these ratios decreased progressively with increased number of frying cycles.
After 60 fryings, the PUFA/SFA ratios decreased strongly and, according to Andrikopoulos et al. (Citation2002), the reduction was proportional to the oil poly-unsaturation (SO > HOSO > EVOO). The MUFA/SFA ratios also decreased strongly. The reduction was more prolonged in the oils rich in oleic acid (HOSO and EVOO) than in SO. The MUFA/PUFA ratio increased slightly in HOSO and SO and was almost unaffected in EVOO. This fact should be related to the higher amounts of linoleic acid initially found in HOSO and SO that is preferentially oxidized in frying (Romero, Cuesta, & Sánchez-Muniz, Citation2003).
The results showed that the reduction of PUFA/SFA ratios was due mainly to the decreased levels of linoleic acid (from 53.49% to 42.65% in SO, from 17.60% to 14.98% in HOSO, and from 8.11% to 7.44% in EVOO) and the increased levels of palmitic acid (from 6.55% to 14.25% in SO, from 4.13% to 10.96% in HOSO, and from 11.58% to 17.36% in EVOO). However, the increased levels of palmitic acid were more important than the decreased levels of oleic acid (from 71.77% to 67.17% in HOSO and from 75.56% to 69.91% in EVOO) or increased oleic acid (from 33.80% to 36.25% in SO) in reducing the MUFA/SFA ratio.
Romero, Cuesta and Sánchez-Muniz (Citation1998) and Romero, Sánchez-Muniz and Cuesta (Citation2000b) also observed a decrease of linoleic acid and an increase of palmitic and oleic acids in SO after 20 fryings, and Pozo-Diez, Masoud-Musa, Pérez-Camino and Dobarganes (Citation1995) also reported a decrease of linoleic and oleic acids, and an increase of palmitic acid in HOSO after 15 fryings. On the contrary, Romero et al. (Citation1998) reported an increase of linoleic acid in HOSO.
The fatty acid modifications that occur during repeated fryings are difficult to explain because various factors are involved: exchange of fatty acids between the bath oil and the food, and unsaturated fatty acids degradation.
The increase of palmitic acid throughout the fryings should be explained in part by the exchange of fatty acids between the bath oil and the pre-fried potatoes. Romero, Cuesta and Sánchez-Muniz (Citation2000a) and Pozo-Diez et al. (Citation1995) reported that frozen pre-fried potatoes fried in SO and HOSO release 55–70% and 80% of fat to the batch fryers, respectively. According to Romero et al. (Citation1998), a part of the increased levels of palmitic acid in the saturated fraction of all oils (7.70% in SO, 6.83% in HOSO, and 6.25% in EVOO) should come from palm oil often used to make these pre-fried potatoes. Fat extracted from the frozen pre-fried potatoes contained 45.03% of palmitic acid, 4.52% of stearic acid, 39.1% of oleic acid, and 8.71% of linoleic acid. This fatty acid profile corresponds to the palm oil reported by Belitz, Grosch and Schieberle (Citation2004). The palmitic acid decreased in the fat of the frozen pre-fried potatoes throughout the fryings, reaching a final value of 12.99% after 60 fryings.
Relationship between palmitic acid, oleic acid, and linoleic acid is shown in Supplementary . The behavior of the oils rich in oleic acid (EVOO, HOSO) was different from the oil rich in linoleic acid (SO). In the first case, a high correlation was found between the increase of palmitic acid and the decrease of oleic acid and linoleic acid, whereas a high correlation was found between the decrease of both oleic acid and linoleic acid. In the second case, high correlations were also found, but correlations were positive between palmitic acid and oleic acid, and negative between oleic acid and linoleic acid.
Trans fatty acids were not detected in fresh EVOO. The major trans fatty acid isomer found in fresh refined oils (HOSO and SO) was 9-cis, 12-trans C18:2, followed by 9-trans, 12-cis C18:2 and trans C18:1. Trans isomers of C18:3 were not found in any oil.
Romero et al. (Citation2000a) reported similar results for EVOO, whereas they only found trans isomers of linoleic acid in HOSO and SO. Bansal, Zhou, Tang, Neo and Lo (Citation2009) have reported results similar to Romero et al. (Citation2000a) for SO.
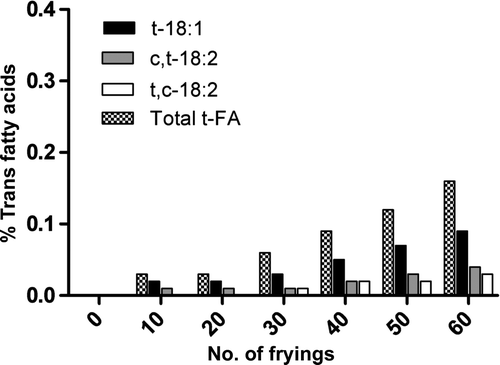
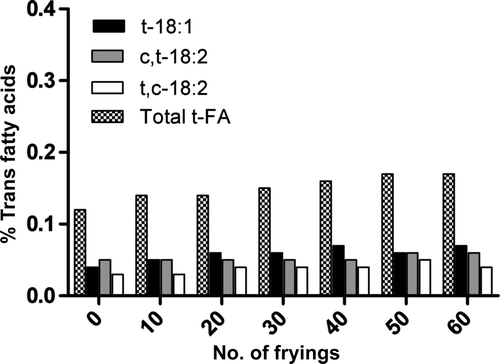
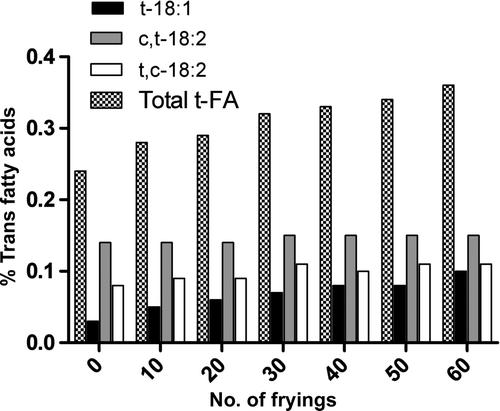
Supplementary , 4, and 5 show the evolution of trans fatty acids in the three oils at 160°C. Trans fatty acids increased with increasing number of frying cycles in all oils, from 0% to 0.16% in EVOO, from 0.12% to 0.17% in HOSO, and from 0.24% to 0.36% in SO, after 60 frying cycles. The SO showed the highest final amount of trans fatty acids. Trans C18:1 was the most increased trans fatty acid in all oils after 60 frying cycles, being similar to the results reported by Romero et al. (Citation1998) in EVOO, HOSO, and SO after 20 frying cycles. The two mono-trans isomers of linoleic acid hardly increased after 60 frying cycles in HOSO. The results also showed that the formation of trans fatty acids is delayed in EVOO compared with the other two oils.
Romero et al. (Citation2000a) reported higher amount of trans fatty acids after 20 frying cycles at 180°C in EVOO, HOSO, and SO without replenishment of fresh oil (4.09 mg/g, 4.23 mg/g, 4.83 mg/g). This fact should be due to various factors as different temperature, nature of fried foods, and variable food/oil ratio. Bansal et al. (Citation2009) found a final amount of total trans fats of 20.08 mg/g after 40 frying cycles with pre-fried French fries at 180–185°C.
Conclusions
Peroxide value seems not to be an accurate tool to control the quality of frying oils. On the contrary, iodine value, conjugated diene, and viscosity give useful information about oil's degradation during deep fat frying. SO showed the highest increase in conjugated diene value. Increase in viscosity was lower for EVOO. Part of the changes in fatty acid profile of fried oils could be explained by the migration of the fatty acids from the pre-fried potatoes to the bath oil. The amount of trans fatty acids formed during deep fat frying in the three oils studied is irrelevant.
Therefore, in our experimental conditions, EVOO and HOSO seem to be the more suitable oils for frying at 160°C. Both oils could be used until 60 frying cycles according to Spanish regulations, but 30 frying cycles would be appropriate taking into account all the parameters studied. EVOO might be a good choice for domestic use and for small restaurants. HOSO might be a good and cheaper alternative to catering and food industries that use large volumes of oil for frying.
tcyt_a_601817_sup_27125824.pdf
Download PDF (756.5 KB)Acknowledgments
The authors are grateful to the Spanish Ministerio de Ciencia e Innovacion (MICIN) for funding this work as a part of the Project AGL 2007-64254 ALI.
References
- Andrikopoulos , N. K. , Dedoussis , G. V.Z. , Falirea , A. , Kalogeropoulos , N. and Hatzinikola , H. S. 2002 . Deterioration of natural antioxidant species of vegetable edible oils during the domestic deep-frying and pan-frying of potatoes . International Journal of Food Sciences and Nutrition , 53 : 351 – 363 .
- AOAC. 1984 . Official methods of analysis , Washington : Association of Official Analytical Chemists .
- Bansal , G. , Zhou , W. , Barlow , P.-J. , Neo , F.-L. and Lo , H.-L. 2010 . Performance of palm olein in repeated deep frying and controlled heating processes . Food Chemistry , 121 : 338 – 347 .
- Bansal , G. , Zhou , W. , Tang , T.-W. , Neo , F.-L. and Lo , H.-L. 2009 . Analysis of trans fatty acids in deep frying oils bythree different approaches . Food Chemistry , 116 : 535 – 541 .
- Belitz , H.-D. , Grosch , W. and Schieberle , P. 2004 . Food chemistry , Germany : Springer .
- Besbes , S. , Blecker , C. , Deroanne , C. , Lognay , G. , Drira , N. E. and Attia , G. 2005 . Heating effects on some quality characteristics of date seed oil . Food Chemistry , 91 : 469 – 476 .
- Casal , S. , Malheiro , R. , Sendas , A. , Oliveira , B. P. and Pereira , J. A. 2010 . Olive oil stability under deep-frying conditions . Food and Chemical Toxicology , 48 : 2972 – 2979 .
- Choe , E. and Min , D. B. 2007 . Chemistry of deep-fat frying oils . Journal of Food Science , 72 : 77 – 86 .
- 2002 . Commission Regulation (EC) No. 796/2002 of 6 May 2002 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-pomace oil and on the relevant methods of analysis . Official Journal of the European Communities, 2002 , L128 : 8 – 28 .
- Dobarganes , M. C. and Márquez-Ruiz , G. 1995 . Control de calidad de las grasas de fritura. Validez de los ensayos rápidos en sustitución de la determinación de compuestos polares . Grasas y aceites , 46 : 196 – 201 .
- Farhoosh , R. and Tavassoli-Kafrani , M. H. 2011 . Simultaneous monitoring of the conventional qualitative indicators during frying of sunflower oil . Food Chemistry , 125 : 209 – 213 .
- Lalas , S. 2009 . “ Quality of frying oil ” . In Advances in deep-fat frying of foods , Edited by: Sahin , S. and Sumnu , S. G. 57 – 80 . Boca Raton , Florida : CRC Press .
- Lalas , S. , Gortzi , O. and Tsaknis , J. 2006 . Frying stability in Moringa stenopetala seed oil . Plants Food for Human Nutrition , 61 : 162 – 166 .
- Matissek , R. , Schnepel , F. M. and Steiner , G. 1998 . Análisis de los alimentos , Zaragoza : Acribia .
- Ndjouenkeu , R. and Ngassoum , M. 2002 . Etude comparative de la valeur en friture de quelques huiles vegetales . Journal of Food Engineering , 52 : 121 – 125 .
- 1989 . Orden 26/01/1989 por la que se aprueba la norma de calidad de los aceites y grasas calentados . Boletín Oficial del Estado , BOE núm. 26 : 2665 – 2667 .
- Paul , S. and Mittal , G. S. 1997 . Regulating the use of degraded oil/fat in deep-fat/oil food frying . Critical Reviews in Food Science and Nutrition , 37 : 635 – 662 .
- Pozo-Diez , R. M. , Masoud-Musa , T. A. , Pérez-Camino , M. C. and Dobarganes , M. C. 1995 . Intercambio lipídico durante la fritura de patatas fritas congeladas en aceite de girasol alto oleico . Grasas y Aceites , 46 : 85 – 91 .
- Romero , A. , Cuesta , C. and Sánchez-Muniz , F. J. 1998 . Effect of oil replenishment during deep-fat frying of frozen foods in sunflower oil and high-oleic acid sunflower oil . Journal of the American Oil Chemists' Society , 75 : 161 – 167 .
- Romero , A. , Cuesta , C. and Sánchez-Muniz , F.J. 2000a . Trans fatty acid production in deep fat frying of frozen foods with different oils and frying modalities . Nutrition Research , 20 : 599 – 608 .
- Romero , A. , Cuesta , C. and Sánchez-Muniz , F. J. 2003 . Cyclic FA monomers in high-oleic acid sunflower oil and extra virgin olive oil used in repeated frying of fresh potatoes . Journal of the American Oil Chemists' Society , 80 : 437 – 442 .
- Romero , A. , Sánchez-Muniz , F. J. and Cuesta , C. 2000b . Deep fat frying of frozen foods in sunflower oil. Fatty acid composition in fryer oil and frozen prefried potatoes . Journal of the Science of Food and Agriculture , 80 : 2135 – 2141 .
- Sanchez-Gimeno , A. C. , Benito , M. , Vercet , A. and Oria , R. 2008a . Aceite de oliva virgen extra del Somontano: evaluación de las modificaciones físico-químicas tras la fritura doméstica de patatas prefritas congeladas . Grasas y Aceites , 59 : 57 – 61 .
- Sanchez-Gimeno , A. C. , Negueruela , A. I. , Benito , M. , Vercet , A. and Oria , R. 2008b . Some physical changes in Bajo Aragon extra virgin olive oil during the frying process . Food Chemistry , 110 : 654 – 658 .
- Santos , J. C.O. , Santos , I. M.G. and Souza , A. G. 2005 . Effect of heating and cooling on rheological parameters of edible vegetable oils . Journal of Food Engineering , 67 : 401 – 405 .
- Smith , S. A. , King , R. E. and Min , D. B. 2007 . Oxidative and thermal stabilities of genetically modified high oleic sunflower oil . Food Chemistry , 102 : 1208 – 1213 .
- Takeoka , G. R. , Full , G. H. and Dao , L. T. 1997 . Effect of heating on the characteristics and chemical composition of selected frying oils and fats . Journal of Agricultural and Food Chemistry , 45 : 3244 – 3249 .
- Valdes , A. F. and Garcia , A. B. 2006 . A study of the evolution of the physicochemical and structural characteristics of olive and sunflower oils after heating at frying temperatures . Food Chemistry , 98 : 214 – 219 .
- Varela , G. 1994 . La fritura degli alimenti in olio d'oliva , Madrid : Consiglio Oleicolo Internazionale .
- Velasco , J. , Marmesat , S. and Dobarganes , C. 2009 . “ Chemistry of frying ” . In Advances in deep-fat frying of foods , Edited by: Sahin , S. and Sumnu , S. G. 33 – 56 . Boca Raton , Florida : CRC Press .
- White , P. 1991 . Methods for measuring changes in deep-fat frying oils . Food Technology , 45 : 75 – 80 .
Supplementary material
Supplementary Table 1. Changes in percentage of fatty acids in oils with the number of fryings at 160°C.
Tabla adicional 1. Cambio del porcentaje de ácidos grasos en los aceites con el número de frituras a 160°C.
Supplementary Table 2. Pearson correlations between palmitic acid, oleic acid, and linoleic acid.
Tabla adicional 2. Correlación de Pearson entre los ácidos palmítico, oleico y linoleico.
Supplementary Figure 1. Changes occurred in SO peroxide values in the number of frying cycles at 160°C and 180°C.
Figura adicional 1. Cambios en el índice de peróxidos del SO con el número de ciclos de fritura a 160°C y a 180°C.
Supplementary Figure 2. Evolution of iodine values (a), conjugated dienes (b), and viscosity (c) in SO, EVOO, and HOSO with the number of frying cycles at 160°C.
Figura adicional 2. Evolución de los índices de yodo (a), los dienos conjugados (b) y la viscosidad (c) en SO, EVOO Y HOSO con el número de ciclos de fritura a 160°C.
Supplementary Figure 3. Evolution of trans fatty acids in EVOO with the number of frying cycles at 160°C.
Figura adicional 3. Evolución de los ácidos grasos trans en EVOO con el número de ciclos de fritura a 160°C.